Comparative Effectiveness of Combination Therapy with Nirmatrelvir-Ritonavir and Molnupiravir versus Monotherapy with Molnupiravir or Nirmatrelvir-Ritonavir in Hospitalised COVID-19 Patients: A Target Trial Emulation Study
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1812, Jan 2026
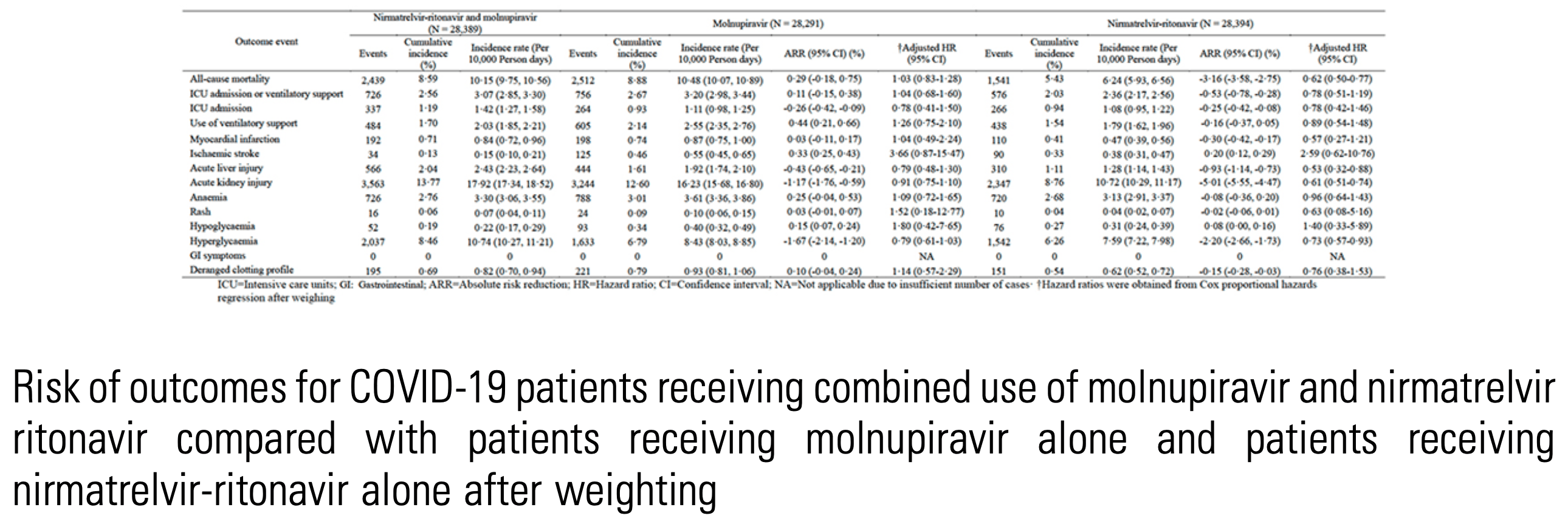
IPTW retrospective target trial emulation of 28,355 hospitalized COVID-19 patients in Hong Kong showing no benefit and potential harm (higher mortality) with combined nirmatrelvir-ritonavir and molnupiravir compared to nirmatrelvir-ritonavir monotherapy. Results are subject to potential unadjusted confounding by indication if combination therapy was preferentially given to patients at higher risk. Additive toxicity from the combined drugs may also be a factor.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments25.
This study is excluded in the after exclusion results of meta-analysis:
substantial unadjusted confounding by indication possible.
Study covers molnupiravir and paxlovid.
|
risk of death, 61.3% higher, HR 1.61, p < 0.001, treatment 28,389, control 28,394, inverted to make HR<1 favor treatment, paxlovid+molnupiravir vs. paxlovid, propensity score weighting.
|
|
risk of mechanical ventilation, 12.4% higher, HR 1.12, p = 0.66, treatment 28,389, control 28,394, inverted to make HR<1 favor treatment, paxlovid+molnupiravir vs. paxlovid, propensity score weighting.
|
|
risk of ICU admission, 28.2% higher, HR 1.28, p = 0.44, treatment 28,389, control 28,394, inverted to make HR<1 favor treatment, paxlovid+molnupiravir vs. paxlovid, propensity score weighting.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Hong Choi et al., 11 Jan 2026, retrospective, China, peer-reviewed, 3 authors, study period 16 March, 2022 - 31 March, 2024.
Abstract: unclear. Preclinical studies and case reports suggest combining these antivirals may
reduce viral shedding and enhance survival.
1
1 1
Quinnipiac University
Risk of outcomes for COVID-19 patients receiving combined use of molnupiravir and nirmatrelvir
ritonavir compared with patients receiving molnupiravir alone and patients receiving
nirmatrelvir-ritonavir alone after weighting
Methods. This target trial emulation study evaluated the safety and efficacy of
combined molnupiravir and nirmatrelvir-ritonavir versus monotherapy in hospitalised COVID-19 adults in Hong Kong. Data were extracted from electronic health records of patients aged 18 and older treated within five days of hospital admission
between March 16, 2022, and March 31, 2024. Inverse probability of treatment weighting (IPTW) was used to balance baseline characteristics. Outcomes, including allcause mortality, Intensive Care Unit (ICU) admission, and ventilatory support,
were assessed using Cox proportional hazards models.
Abstract citation ID: ofaf695.1812
P-1636. Comparative Effectiveness of Combination Therapy with
Nirmatrelvir-Ritonavir and Molnupiravir versus Monotherapy with Molnupiravir
or Nirmatrelvir-Ritonavir in Hospitalised COVID-19 Patients: A Target Trial
Emulation Study
Ming Hong Choi, MBBS1; Eric Yuk Fai Wan, PhD2; Fan Ngai Ivan Hung, MD2;
1
Queen Mary Hospital, Hong Kong, Hong Kong; 2The University of Hong Kong,
Hong Kong, Not Applicable, Hong Kong
Session: 219. COVID-2019 and Co-Infections
Wednesday, October 22, 2025: 12:15 PM
Background. While molnupiravir and nirmatrelvir-ritonavir have demonstrated
efficacy in reducing hospitalisation and mortality among unvaccinated, high-risk
COVID-19 patients in outpatient settings, their impact on hospitalised adults remains
S1022 • OFID 2026:13 (Suppl 1) • Poster Abstracts
Study flow diagram
1
Frank H. Netter School of Medicine, Hamden, CT
Baseline characteristics of eligible COVID-19 patients after the inverse probability of treatment
weighting (IPTW)
SMD=Standardised mean difference; SD=Standard deviation; IQR = interquartile range;
CCI=Charlson Comorbidity Index; ICU=Intensive
care units;
†SMD<0.1 indicates balance between groups·
†† Level 1: Hospitalised patients with no oxygen therapy; Level 2: Hospitalised patients with
oxygen by mask, nasal prongs, non-invasive
ventilation or high flow; Level 3: Hospitalised patients with intubation and mechanical ventilation, vasopressors, dialysis, or extracorporeal
membrane oxygenation
90-day cumulative incidence of outcomes in recipients of combination treatment with
nirmatrelvir-ritonavir and molnupiravir compared to recipients of molnupiravir monotherapy
and recipients of nirmatrelvir-ritonavir
Shared area refers to the 95% confidence interval for the cumulative incidence. The P
values indicate the overall P values of the Log-rank test comparing the three treatment
groups for each outcome
1
2
1
2
3 1
3
3
Chang Gung University, Taoyuan, Taoyuan, Taiwan
(Republic of China) 2Chang gung university, Taoyuan, Taoyuan, Taiwan 3Chang
Gung Memorial Hospital, Taoyuan, Taoyuan, Taiwan
2
3
2
4 1
Yale School of
Medicine, West Hollywood, California 2Connecticut Department of Correction,
3
Wethersfield, Connecticut Yale School of Public Health/Oswaldo Cruz Foundation/
Brazilian Ministry of Health, New Haven, Connecticut 4Boston University School..
DOI record:
{
"DOI": "10.1093/ofid/ofaf695.1812",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofaf695.1812",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>While molnupiravir and nirmatrelvir-ritonavir have demonstrated efficacy in reducing hospitalisation and mortality among unvaccinated, high-risk COVID-19 patients in outpatient settings, their impact on hospitalised adults remains unclear. Preclinical studies and case reports suggest combining these antivirals may reduce viral shedding and enhance survival.Baseline characteristics of eligible COVID-19 patients after the inverse probability of treatment weighting (IPTW)SMD=Standardised mean difference; SD=Standard deviation; IQR = interquartile range; CCI=Charlson Comorbidity Index; ICU=Intensivecare units;†SMD&lt;0.1 indicates balance between groups·†† Level 1: Hospitalised patients with no oxygen therapy; Level 2: Hospitalised patients with oxygen by mask, nasal prongs, non-invasiveventilation or high flow; Level 3: Hospitalised patients with intubation and mechanical ventilation, vasopressors, dialysis, or extracorporealmembrane oxygenationRisk of outcomes for COVID-19 patients receiving combined use of molnupiravir and nirmatrelvir ritonavir compared with patients receiving molnupiravir alone and patients receiving nirmatrelvir-ritonavir alone after weighting</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This target trial emulation study evaluated the safety and efficacy of combined molnupiravir and nirmatrelvir-ritonavir versus monotherapy in hospitalised COVID-19 adults in Hong Kong. Data were extracted from electronic health records of patients aged 18 and older treated within five days of hospital admission between March 16, 2022, and March 31, 2024. Inverse probability of treatment weighting (IPTW) was used to balance baseline characteristics. Outcomes, including all-cause mortality, Intensive Care Unit (ICU) admission, and ventilatory support, were assessed using Cox proportional hazards models.Study flow diagram90-day cumulative incidence of outcomes in recipients of combination treatment with nirmatrelvir-ritonavir and molnupiravir compared to recipients of molnupiravir monotherapy and recipients of nirmatrelvir-ritonavirShared area refers to the 95% confidence interval for the cumulative incidence. The P values indicate the overall P values of the Log-rank test comparing the three treatment groups for each outcome</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Among 28,355 patients (combination: 1,081; molnupiravir: 8,416; nirmatrelvir-ritonavir: 18,858), IPTW-adjusted analyses showed that nirmatrelvir-ritonavir monotherapy was associated with a significantly lower risk of mortality (HR: 0.62; 95% CI 0.50-0.77; ARR: -3.16%) compared to combination therapy. Risks of ICU admission and ventilatory support were similar across all groups. Patients receiving nirmatrelvir-ritonavir monotherapy also showed lower risks of acute liver injury (HR: 0.53 [95% CI 0.32-0.88]), acute kidney injury (HR: 0.61 [95% CI 0.51-0.74]), and hyperglycaemia (HR 0·73 [95% CI 0.57- 0.93]).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Combining nirmatrelvir-ritonavir and molnupiravir does not significantly reduce mortality, ICU admissions, or ventilatory support needs in hospitalised COVID-19 adults. Further randomised controlled trials are needed to confirm these findings.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Disclosures</jats:title>\n <jats:p>All Authors: No reported disclosures</jats:p>\n </jats:sec>",
"article-number": "ofaf695.1812",
"author": [
{
"affiliation": [
{
"name": "Queen Mary Hospital , Hong Kong ,",
"place": [
"Hong Kong"
]
}
],
"family": "Hong Choi",
"given": "Ming",
"sequence": "first"
},
{
"affiliation": [
{
"name": "The University of Hong Kong , Hong Kong, Not Applicable ,",
"place": [
"Hong Kong"
]
}
],
"family": "Fai Wan",
"given": "Eric Yuk",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The University of Hong Kong , Hong Kong, Not Applicable ,",
"place": [
"Hong Kong"
]
}
],
"family": "Ivan Hung",
"given": "Fan Ngai",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T07:29:12Z",
"timestamp": 1768202952000
},
"deposited": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T07:41:18Z",
"timestamp": 1768203678000
},
"indexed": {
"date-parts": [
[
2026,
1,
13
]
],
"date-time": "2026-01-13T10:46:32Z",
"timestamp": 1768301192693,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "Supplement_1",
"issued": {
"date-parts": [
[
2026,
1
]
]
},
"journal-issue": {
"issue": "Supplement_1",
"published-print": {
"date-parts": [
[
2026,
1,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 11,
"start": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T00:00:00Z",
"timestamp": 1768176000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/article-pdf/13/Supplement_1/ofaf695.1812/66354845/ofaf695.1812.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/13/Supplement_1/ofaf695.1812/66354845/ofaf695.1812.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2026,
1
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
11
]
]
},
"published-other": {
"date-parts": [
[
2026,
1
]
]
},
"published-print": {
"date-parts": [
[
2026,
1,
11
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofaf695.1812/8422000"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "P-1636. Comparative Effectiveness of Combination Therapy with Nirmatrelvir-Ritonavir and Molnupiravir versus Monotherapy with Molnupiravir or Nirmatrelvir-Ritonavir in Hospitalised COVID-19 Patients: A Target Trial Emulation Study",
"type": "journal-article",
"volume": "13"
}