SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies
et al., The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227, Jan 2026
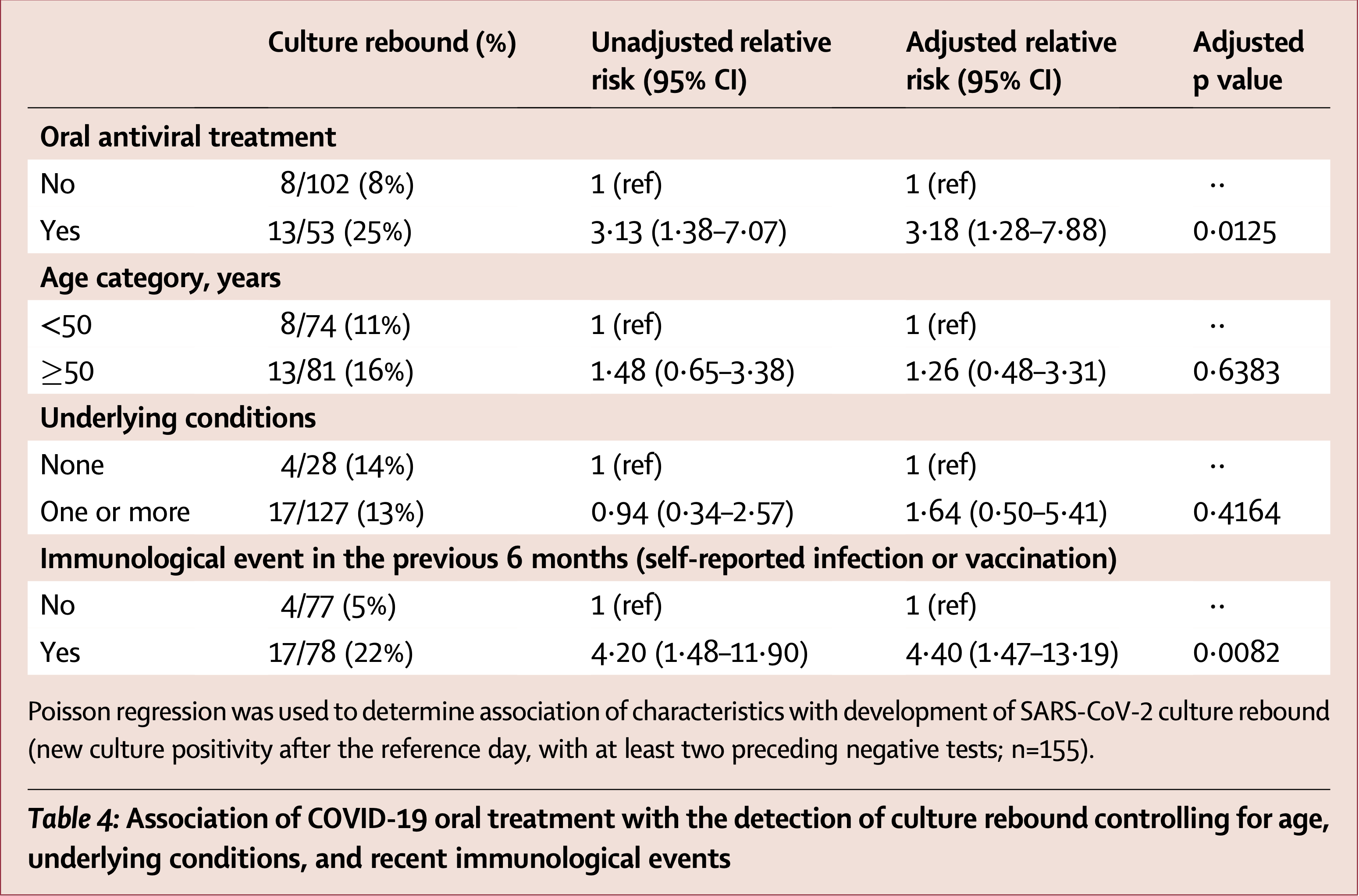
Prospective study of 160 non-hospitalized adults at high risk for severe COVID-19 showing paxlovid or molnupiravir associated with increased risk of viral rebound with infectious virus. Among treated participants, 25% experienced culture rebound (indicating potential infectiousness) compared to 8% of untreated participants. However, among participants without viral RNA rebound, treated individuals had shorter duration of infectious viral shedding (4 vs 6 days) and antigen positivity (5 vs 8 days) compared to untreated participants.
Adjusted results are only provided for the combined group including both paxlovid and molnupiravir.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers molnupiravir and paxlovid.
|
culture rebound, 218.0% higher, RR 3.18, p = 0.01, treatment 54, control 106, adjusted per study, combined treatments.
|
|
culture rebound, 657.1% higher, RR 7.57, p = 0.002, treatment 4 of 7 (57.1%), control 8 of 106 (7.5%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Shah et al., 9 Jan 2026, prospective, USA, peer-reviewed, median age 50.0, 42 authors, study period 1 January, 2022 - 10 June, 2023.
SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies
The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227
Background The effect of COVID-19 oral antivirals on the duration of SARS-CoV-2 infectious viral shedding and viral rebound remains uncertain. This study aimed to examine the association of oral antivirals with viral dynamics, shedding of infectious virus, and SARS-CoV-2 rebound. Methods A prospective case-ascertained household study design was used. Participants were non-hospitalised adults older than 18 years with symptomatic SARS-CoV-2 enrolled in one of two prospective household transmission studies (University of California, San Francisco, FindCOVID and Respiratory Virus Transmission Network-Sentinel [RVTN-S]) conducted in the USA across six states at academic institutions from Jan 1, 2022 to June 10, 2023. We excluded those reporting receipt of multiple COVID-19 outpatient medications, treatment with remdesivir, a treatment duration of more than 6 days, and those from whom fewer than five daily anterior nasal swabs were collected during their illness. Participants were considered to be at high risk of severe COVID-19 (and, therefore, eligible for SARS-CoV-2 oral antiviral treatment) if they were aged 50 years or older or were aged 18 years or older and reporting at least one underlying condition. Study procedures included frequent selfcollected nasal swabs (daily for 14 days after symptom onset and then every 3 days until day 28 after symptom onset in FindCOVID and two nasal swabs daily for 10 days from enrolment in RVTN-S) and viral testing by quantitative reverse transcriptase PCR (qRT-PCR), at-home antigen testing, and viral culture. Treatment was defined as self-reported receipt of an oral antiviral (nirmatrelvir-ritonavir or molnupiravir). The primary analysis compared viral detection by qRT-PCR, antigen test positivity, and culture positivity and assessed SARS-CoV-2 viral rebound (viral RNA, antigen, culture, and symptom rebound) in untreated and treated participants at high risk of severe outcomes. We used multivariable Poisson regression to assess associations between treatment, duration of test positivity, and the presence of viral culture rebound, adjusting for age, underlying conditions, and recent immunological events. Findings Between Jan 1, 2022, and June 10, 2023, 160 individuals with symptomatic COVID-19 and at high risk of severe outcomes were included in FindCOVID and RVTN-S. There was no significant difference in the duration of viral detection between treated and untreated participants at high risk of severe COVID-19 by antigen test positivity (6 days [IQR 5-11] vs 8 days [5-10]; adjusted relative risk [RR] 1⋅07, 95% CI 0⋅24-4⋅81) or viral culture (7 days [4-11] vs 6 days [5-9]; 2⋅21, 0⋅45-10⋅79). Among 122 participants without viral RNA rebound, the last day of antigen test positivity and detection of culturable virus post-symptom onset was earlier in treated participants than in untreated participants (5 days [4-8] vs 8 days [5-10] for antigen test positivity; 0⋅19, 0⋅04-0⋅79; 4 days [3-6] vs 6 days [4-9]; 0⋅18,..
Respiratory Virus Transmission Network-Sentinel Study Group Jessica E Biddle, Meredith Davis-Gardner, Son McLaren, Joshua Petrie, Kathleen Pryor, Yuwei Zhu.
UCSF FindCOVID Study Group Khamal Anglin, Jessica Y Chen, Elizabeth Jump, Ana Martinez, Karen Pfister, Carolina Torres, Badri Viswanathan, Amethyst Zhang.
Contributors MMS, GRA, SL, MBH, CMM, AMM, JDK, and SES-J contributed to the conceptualisation of the study. SL, SAG, JP-R, SGD, JNM, MJP, RA, and MG-K, and members of the University of California, San Francisco FindCOVID Study Group AZ, AM, JYC, BV, KA, CT, KP, and EJ contributed to data curation for FindCOVID. SES-J, CGG, HKT, JS, KL, KDE, MelSSt, ES, HQN, SR, EJA, MehSS, JEB, MD-G, SM, JP, KP, and YZ contributed to data curation for the Respiratory Virus Transmission Network─Sentinel (RVTN─Sentinel) study. GRA, MMS, CMM, JDK, SES-J, and AMM contributed to formal data analysis. Overall study supervision and oversight was provided by JDK, MBH, MMS, CMM, HLK, and SES-J. MMS, GRA, and SES-J wrote the initial draft, with substantial review and editing provided by all coauthors. MMS and GRA accessed and verified the data from both RVTN and FindCOVID and accept responsibility for the decision to submit for publication. All authors reviewed the and approved the final version for publication. MMS and GRA had full access to all data for the analysis. All authors reviewed the data analysed, provided critical input the manuscript, and approved of submission for publication.
..
References
Aggarwal, Molina, Beaty, Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study, Lancet Infect Dis
Anderson, Caubel, Rusnak, Investigators, Nirmatrelvir-ritonavir and viral load rebound in Covid-19, N Engl J Med
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge, N Engl J Med
Bajema, Berry, Streja, Effectiveness of COVID-19 treatment with nirmatrelvir-Ritonavir or molnupiravir among U.S. veterans: target trial emulation studies with one-month and sixmonth outcomes, Ann tntern Med
Caraco, Crofoot, Moncada, Phase 2/3 trial of molnupiravir for treatment of Covid-19 in nonhospitalized adults, NEJM Evid
Deyoe, Kelly, Grijalva, Association of culturable-virus detection and household transmission of SARS-CoV-2, California and Tennessee, 2020-2022, J Infect Dis
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system: a population-based cohort study, Ann Intern Med
Edelstein, Boucau, Uddin, SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy: an observational study, Ann Intern Med
Ganatra, Dani, Ahmad, Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with coronavirus disease 2019 (COVID-19), Clin Infect Dis
Garcia-Knight, Anglin, Tassetto, Infectious viral shedding of SARS-CoV-2 delta following vaccination: a longitudinal cohort study, PLoS Pathog
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Harrington, Cong, Troy, Evaluation of SARS-CoV-2 RNA rebound after nirmatrelvir/ritonavir treatment in randomized, doubleblind, placebo-controlled trials-United States and international sites, 2021-2022, Morb Mortal Wkly Rep
Hay, Kissler, Fauver, Quantifying the impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound: a retrospective cohort study, Elife
Ke, Martinez, Smith, Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness, Nat Microbiol
Kelly, Lu, Anglin, Magnitude and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) household transmission: a longitudinal cohort study, Clin Infect Dis
Lewnard, Malden, Hong, Effectiveness of nirmatrelvirritonavir against hospital admission: a matched cohort study in a large US healthcare system, medRxiv, doi:10.1101/2022.10.02.22280623v2
Perelson, Ribeiro, Phan, An explanation for SARS-CoV-2 rebound after paxlovid treatment, medRxiv, doi:10.1101/2023.05.30.23290747v1
Rolfes, Talbot, Morrissey, Reduced risk of SARS-CoV-2 infection among household contacts with recent vaccination and past COVID-19 infection: results from two multi-site case-ascertained household transmission studies, Am J Epidemiol
Se, Biddle, Talbot, Symptoms, viral loads, and rebound among COVID-19 outpatients treated with nirmatrelvir/ritonavir compared with propensity score-matched untreated individuals, Clin Infect Dis
Shah, Joyce, Plumb, Paxlovid associated with decreased hospitalization rate among adults with COVID-19-United States, April-September 2022, Morb Mortal Wkly Rep
Smith, Lambrou, Patel, SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals, Morb Mortal Wkly Rep
Takahashi, Ishikane, Ujiie, Duration of infectious virus shedding by SARS-CoV-2 omicron variant-infected vaccinees, Emerg Infect Dis
Wang, Berger, Davis, Kaelber, Volkow et al., COVID-19 rebound after paxlovid and molnupiravir during January-June 2022, medRxiv, doi:10.1101/2022.06.21.22276724v1
Wong, Lau, Au, Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study, Lancet Infect Dis
Xie, Choi, Al-Aly, Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition, JAMA Intern Med
DOI record:
{
"DOI": "10.1016/j.lanmic.2025.101227",
"ISSN": [
"2666-5247"
],
"URL": "http://dx.doi.org/10.1016/j.lanmic.2025.101227",
"alternative-id": [
"S2666524725001557"
],
"article-number": "101227",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Microbe"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.lanmic.2025.101227"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Shah",
"given": "Melisa M",
"sequence": "first"
},
{
"affiliation": [],
"family": "Abedi",
"given": "Glen R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia-Knight",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pineda-Ramirez",
"given": "Jesus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldberg",
"given": "Sarah A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grijalva",
"given": "Carlos G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Keipp Talbot",
"given": "H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schmitz",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lutrick",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ellingson",
"given": "Katherine D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stockwell",
"given": "Melissa S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sano",
"given": "Ellen",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2725-6439",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nguyen",
"given": "Huong Q",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rao",
"given": "Suchitra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asturias",
"given": "Edwin J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suthar",
"given": "Mehul S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mellis",
"given": "Alexandra M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deeks",
"given": "Steven G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martin",
"given": "Jeffrey N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peluso",
"given": "Michael J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andino",
"given": "Raul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rolfes",
"given": "Melissa A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kirking",
"given": "Hannah L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith-Jeffcoat",
"given": "Sarah E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Midgley",
"given": "Claire M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hagen",
"given": "Melissa Briggs",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7616-0321",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kelly",
"given": "J Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Biddle",
"given": "Jessica E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davis-Gardner",
"given": "Meredith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McLaren",
"given": "Son",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petrie",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pryor",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Yuwei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anglin",
"given": "Khamal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Jessica Y",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jump",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martinez",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pfister",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Viswanathan",
"given": "Badri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Amethyst",
"sequence": "additional"
}
],
"container-title": "The Lancet Microbe",
"container-title-short": "The Lancet Microbe",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T20:04:07Z",
"timestamp": 1767989047000
},
"deposited": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T21:10:58Z",
"timestamp": 1767993058000
},
"funder": [
{
"DOI": "10.13039/100000030",
"award": [
"75D30120C08009"
],
"award-info": [
{
"award-number": [
"75D30120C08009"
]
}
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000030",
"id-type": "DOI"
}
],
"name": "Centers for Disease Control and Prevention"
}
],
"indexed": {
"date-parts": [
[
2026,
1,
10
]
],
"date-time": "2026-01-10T04:02:12Z",
"timestamp": 1768017732152,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2026,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
1
]
],
"date-time": "2026-01-01T00:00:00Z",
"timestamp": 1767225600000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
1
]
],
"date-time": "2026-01-01T00:00:00Z",
"timestamp": 1767225600000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
13
]
],
"date-time": "2025-08-13T00:00:00Z",
"timestamp": 1755043200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2666524725001557?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2666524725001557?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101227",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2026,
1
]
]
},
"published-print": {
"date-parts": [
[
2026,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/EVIDoa2100043",
"article-title": "Phase 2/3 trial of molnupiravir for treatment of Covid-19 in nonhospitalized adults",
"author": "Caraco",
"doi-asserted-by": "crossref",
"journal-title": "NEJM Evid",
"key": "10.1016/j.lanmic.2025.101227_bib1",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lanmic.2025.101227_bib2",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge",
"author": "Arbel",
"doi-asserted-by": "crossref",
"first-page": "790",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lanmic.2025.101227_bib3",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7148e2",
"article-title": "Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April–September 2022",
"author": "Shah",
"doi-asserted-by": "crossref",
"first-page": "1531",
"journal-title": "Morb Mortal Wkly Rep",
"key": "10.1016/j.lanmic.2025.101227_bib4",
"volume": "71",
"year": "2022"
},
{
"article-title": "Effectiveness of nirmatrelvir-ritonavir against hospital admission: a matched cohort study in a large US healthcare system",
"author": "Lewnard",
"journal-title": "medRxiv",
"key": "10.1016/j.lanmic.2025.101227_bib5",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00011-7",
"article-title": "Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "696",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.lanmic.2025.101227_bib6",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.7326/M22-3565",
"article-title": "Effectiveness of COVID-19 treatment with nirmatrelvir–Ritonavir or molnupiravir among U.S. veterans: target trial emulation studies with one-month and six-month outcomes",
"author": "Bajema",
"doi-asserted-by": "crossref",
"first-page": "807",
"journal-title": "Ann tntern Med",
"key": "10.1016/j.lanmic.2025.101227_bib7",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.7326/M22-2141",
"article-title": "Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system: a population-based cohort study",
"author": "Dryden-Peterson",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.lanmic.2025.101227_bib8",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac673",
"article-title": "Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with coronavirus disease 2019 (COVID-19)",
"author": "Ganatra",
"doi-asserted-by": "crossref",
"first-page": "563",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.lanmic.2025.101227_bib9",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.7326/M23-1756",
"article-title": "SARS-CoV-2 virologic rebound with nirmatrelvir–ritonavir therapy: an observational study",
"author": "Edelstein",
"doi-asserted-by": "crossref",
"first-page": "1577",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.lanmic.2025.101227_bib11",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciad696",
"article-title": "Symptoms, viral loads, and rebound among COVID-19 outpatients treated with nirmatrelvir/ritonavir compared with propensity score–matched untreated individuals",
"author": "Smith-Jeffcoat",
"doi-asserted-by": "crossref",
"first-page": "1175",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.lanmic.2025.101227_bib12",
"volume": "78",
"year": "2024"
},
{
"DOI": "10.15585/mmwr.mm7251a2",
"article-title": "Evaluation of SARS-CoV-2 RNA rebound after nirmatrelvir/ritonavir treatment in randomized, double-blind, placebo-controlled trials—United States and international sites, 2021–2022",
"author": "Harrington",
"doi-asserted-by": "crossref",
"first-page": "1365",
"journal-title": "Morb Mortal Wkly Rep",
"key": "10.1016/j.lanmic.2025.101227_bib13",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7251a1",
"article-title": "SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals",
"author": "Smith",
"doi-asserted-by": "crossref",
"first-page": "1357",
"journal-title": "Morb Mortal Wkly Rep",
"key": "10.1016/j.lanmic.2025.101227_bib14",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00873-8",
"article-title": "Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "683",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.lanmic.2025.101227_bib15",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2205944",
"article-title": "Nirmatrelvir–ritonavir and viral load rebound in Covid-19",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "1047",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lanmic.2025.101227_bib16",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac545",
"article-title": "Magnitude and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) household transmission: a longitudinal cohort study",
"author": "Kelly",
"doi-asserted-by": "crossref",
"first-page": "S193",
"issue": "suppl 2",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.lanmic.2025.101227_bib17",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1371/journal.ppat.1010802",
"article-title": "Infectious viral shedding of SARS-CoV-2 delta following vaccination: a longitudinal cohort study",
"author": "Garcia-Knight",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Pathog",
"key": "10.1016/j.lanmic.2025.101227_bib18",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1093/aje/kwae334",
"article-title": "Reduced risk of SARS-CoV-2 infection among household contacts with recent vaccination and past COVID-19 infection: results from two multi-site case-ascertained household transmission studies",
"author": "Rolfes",
"doi-asserted-by": "crossref",
"first-page": "1603",
"journal-title": "Am J Epidemiol",
"key": "10.1016/j.lanmic.2025.101227_bib19",
"volume": "194",
"year": "2025"
},
{
"DOI": "10.3201/eid2805.220197",
"article-title": "Duration of infectious virus shedding by SARS-CoV-2 omicron variant–infected vaccinees",
"author": "Takahashi",
"doi-asserted-by": "crossref",
"first-page": "998",
"journal-title": "Emerg Infect Dis",
"key": "10.1016/j.lanmic.2025.101227_bib22",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"article-title": "Association of treatment with nirmatrelvir and the risk of post–COVID-19 condition",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "554",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.lanmic.2025.101227_bib23",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.1038/s41564-022-01105-z",
"article-title": "Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness",
"author": "Ke",
"doi-asserted-by": "crossref",
"first-page": "640",
"journal-title": "Nat Microbiol",
"key": "10.1016/j.lanmic.2025.101227_bib25",
"volume": "7",
"year": "2022"
},
{
"article-title": "An explanation for SARS-CoV-2 rebound after paxlovid treatment",
"author": "Perelson",
"journal-title": "medRxiv",
"key": "10.1016/j.lanmic.2025.101227_bib27",
"year": "2023"
},
{
"DOI": "10.7554/eLife.81849",
"article-title": "Quantifying the impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound: a retrospective cohort study",
"author": "Hay",
"doi-asserted-by": "crossref",
"journal-title": "Elife",
"key": "10.1016/j.lanmic.2025.101227_bib24",
"volume": "11",
"year": "2022"
},
{
"article-title": "COVID-19 rebound after paxlovid and molnupiravir during January–June 2022",
"author": "Wang",
"journal-title": "medRxiv",
"key": "10.1016/j.lanmic.2025.101227_bib28",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiad018",
"article-title": "Association of culturable-virus detection and household transmission of SARS-CoV-2, California and Tennessee, 2020–2022",
"author": "Deyoe",
"doi-asserted-by": "crossref",
"first-page": "1343",
"journal-title": "J Infect Dis",
"key": "10.1016/j.lanmic.2025.101227_bib29",
"volume": "227",
"year": "2023"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2666524725001557"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy"
}