Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology
et al., Endocrine and Metabolic Science, doi:10.1016/j.endmts.2024.100163, Jan 2024
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
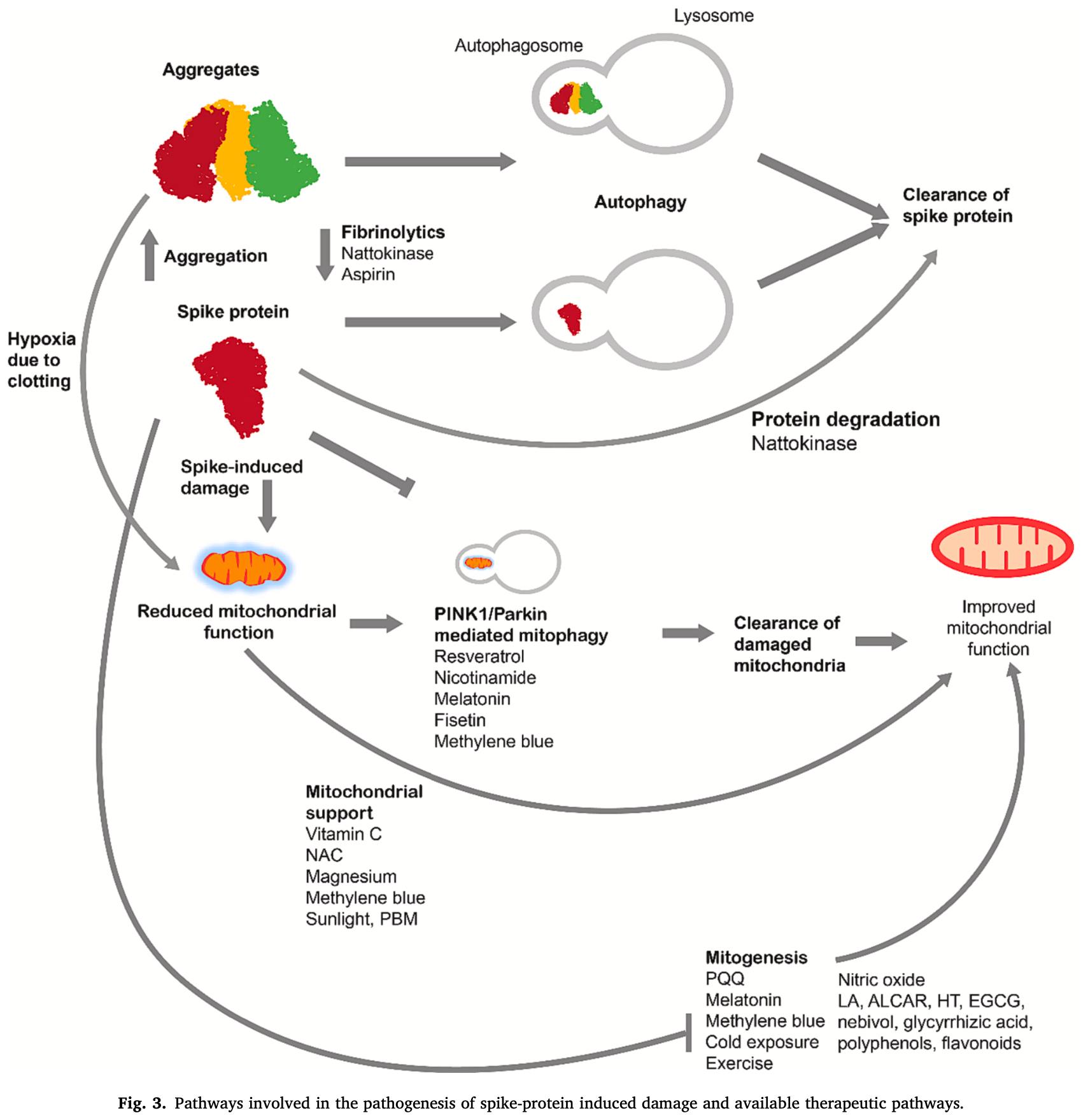
Review of autophagy pathways for treating SARS-CoV-2 spike protein-related pathology. Authors note that the spike protein is implicated in long COVID and post-vaccination syndrome through mechanisms like inflammation, vascular damage, and mitochondrial dysfunction. Authors discuss the mechanisms by which fasting and other interventions can upregulate autophagy to clear spike protein aggregates and improve mitochondrial function.
1.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
2.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
3.
Duan et al., Bioactive compounds,quercetin, curcumin and β-glucan,regulate innate immunity via the gut-liver-brain axis, Trends in Food Science & Technology, doi:10.1016/j.tifs.2024.104864.
4.
Rajak et al., Antiallergic Implications of Curcumin During COVID-19: Current Status and Perspectives, Biotechnology of Medicinal Plants with Antiallergy Properties, doi:10.1007/978-981-97-1467-4_4.
5.
Kali et al., Curcumin as a Promising Therapy for COVID-19: A Review, Global Journal of Medical, Pharmaceutical, and Biomedical Update, doi:10.25259/GJMPBU_78_2023.
6.
Vajdi et al., Effect of polyphenols against complications of COVID-19: current evidence and potential efficacy, Pharmacological Reports, doi:10.1007/s43440-024-00585-6.
7.
Yong et al., Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases, International Journal of Nanomedicine, doi:10.2147/ijn.s451206.
8.
Halma et al., Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology, Endocrine and Metabolic Science, doi:10.1016/j.endmts.2024.100163.
9.
Arab et al., Immunoregulatory effects of nanocurcumin in inflammatory milieu: Focus on COVID-19, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2024.116131.
10.
Daskou et al., The Role of the NRF2 Pathway in the Pathogenesis of Viral Respiratory Infections, Pathogens, doi:10.3390/pathogens13010039.
11.
Law et al., Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review, Pharmaceuticals, doi:10.3390/ph17010034.
12.
Donzelli, A., Neglected Effective Early Therapies against COVID-19: Focus on Functional Foods and Related Active Substances. A Review, MDPI AG, doi:10.20944/preprints202312.1178.v1.
13.
Hulscher et al., Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination, Cureus, doi:10.7759/cureus.49204.
14.
Hegde et al., Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far?, ACS Omega, doi:10.1021/acsomega.2c07326.
Halma et al., 30 Jan 2024, peer-reviewed, 3 authors.
Contact: matt@worldcouncilforhealth.org, pmarik@flccc.net, dr.saleeby@carolinaholisticmedicine.com.
Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology
Endocrine and Metabolic Science, doi:10.1016/j.endmts.2024.100163
Fasting, a practice with historical roots in various cultures, has recently garnered significant interest in the field of medicine. In this article, we delve into the mechanisms underlying fasting-induced autophagy and its therapeutic applications for spike protein associated pathology. We explore the therapeutic potential of fasting on spike protein-related pathology and the role of interventions to upregulate autophagy, including compounds like spermidine, resveratrol, rapamycin, and metformin. In conclusion, fasting, coupled with an understanding of its nuances, holds promise as a therapeutic intervention for SARS-CoV-2 spike protein related diseases; with broad implications for human health. This review presents the therapeutic possibility of using autophagy to treat spike protein related diseases, and details the interventions to deploy this therapeutic modality.
Institutional review board statement Not applicable.
CRediT authorship contribution
Declaration of competing interest M.T.J.H. is a member of the World Council for Health, a non-profit health advocacy organization. P.E.M. is the founder of the Frontline Covid-19 Critical Care Alliance (FLCCC). Y.M.S. is the medical director for Carolina Holistic Medicine and a member of the FLCCC.
References
Abramczyk, Brozek-Pluska, Beton, Decoding COVID-19 mRNA Vaccine Immunometabolism in Central Nervous System: Human Brain Normal Glial and Glioma Cells by Raman Imaging
Adzigbli, Sokolov, Wimmers, Sokolova, Ponsuksili, Effects of hypoxia and reoxygenation on mitochondrial functions and transcriptional profiles of isolated brain and muscle porcine cells, Sci. Rep, doi:10.1038/s41598-022-24386-0
Ajaz, Mcphail, Singh, Mujib, Trovato et al., Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19, Am. J. Phys. Cell Phys, doi:10.1152/ajpcell.00426.2020
Al-Kuraishy, Al-Gareeb, Kaushik, Kujawska, Batiha, Hemolytic anemia in COVID-19, Ann. Hematol, doi:10.1007/s00277-022-04907-7
Alamdari, Moghaddam, Amini, Keramati, Zarmehri et al., Application of methylene blue -vitamin C-N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2020.173494
Albosta, Bakke, Intermittent fasting: is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians, Clinical Diabetes and Endocrinology, doi:10.1186/s40842-020-00116-1
Ali, Poortvliet, Strömberg, Yngve, Polyamines in foods: development of a food database, Food Nutr, doi:10.3402/fnr.v55i0.5572
Alirezaei, Kemball, Flynn, Wood, Whitton et al., Shortterm fasting induces profound neuronal autophagy, Autophagy, doi:10.4161/auto.6.6.12376
Altay, Arif, Li, Yang, Aydın et al., Combined metabolic activators accelerates recovery in mild-to-moderate COVID-19, Adv. Sci, doi:10.1002/advs.202101222
Alturaiki, Alkadi, Alamri, Awadalla, Alfaez et al., Association between the expression of toll-like receptors, cytokines, and homeostatic chemokines in SARS-CoV-2 infection and COVID-19 severity, Heliyon, doi:10.1016/j.heliyon.2022.e12653
Anand, Kunnumakkara, Newman, Aggarwal, Bioavailability of curcumin: problems and promises, Mol. Pharm, doi:10.1021/mp700113r
Ancona, Alagna, Alteri, Palomba, Tonizzo et al., Gut and airway microbiota dysbiosis and their role in COVID-19 and long-COVID, Front. Immunol
Anton, Moehl, Donahoo, Marosi, Lee et al., Flipping the metabolic switch: understanding and applying the health benefits of fasting, Obesity (Silver Spring), doi:10.1002/oby.22065
Aparicio-Trejo, Reyes-Fermín, Briones-Herrera, Tapia, León-Contreras et al., Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2018.11.005
Arndt, Rogon, Höhfeld, To be, or not to bemolecular chaperones in protein degradation, Cell. Mol. Life Sci, doi:10.1007/s00018-007-7188-6
Assimakopoulos, Aretha, Komninos, Dimitropoulou, Lagadinou et al., N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study, Infect. Dis. Ther, doi:10.1080/23744235.2021.1945675
Atkuri, Mantovani, Herzenberg, Herzenberg, Nacetylcysteine-a safe antidote for cysteine/glutathione deficiency, Curr. Opin. Pharmacol, doi:10.1016/j.coph.2007.04.005
Attinà, Leggeri, Paroni, Pivari, Dei Cas et al., Fasting: how to guide, Nutrients, doi:10.3390/nu13051570
Avolio, Carrabba, Milligan, Kavanagh Williamson, Beltrami et al., The SARS-CoV-2 spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease, Clin. Sci, doi:10.1042/CS20210735
Banerjee, Baidya, Adhikari, Ghosh, Jha, Glycyrrhizin as a promising kryptonite against SARS-CoV-2: clinical, experimental, and theoretical evidences, J. Mol. Struct, doi:10.1016/j.molstruc.2022.134642
Barbagallo, Veronese, Dominguez, Magnesium in aging, health and diseases, Nutrients, doi:10.3390/nu13020463
Barbara, Clavario, De Marzo, Lotti, Guglielmi et al., Effects of exercise rehabilitation in patients with long coronavirus disease 2019, Eur. J. Prev. Cardiol, doi:10.1093/eurjpc/zwac019
Bauer, Laksono, Vrij, De, Kushner et al., The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2, Trends Neurosci, doi:10.1016/j.tins.2022.02.006
Begum, Calaza, Kam, Salt, Hogg et al., Near-infrared light increases ATP, extends lifespan and improves mobility in aged drosophila melanogaster, Biol. Lett, doi:10.1098/rsbl.2015.0073
Bellavite, Ferraresi, Isidoro, Immune response and molecular mechanisms of cardiovascular adverse effects of spike proteins from SARS-CoV-2 and mRNA vaccines, Biomedicines, doi:10.3390/biomedicines11020451
Bento, Renna, Ghislat, Puri, Ashkenazi et al., Mammalian autophagy: how does it work?, Annu. Rev. Biochem, doi:10.1146/annurev-biochem-060815-014556
Bertholet, Delerue, Millet, Moulis, David et al., Mitochondrial fusion/ fission dynamics in neurodegeneration and neuronal plasticity, Neurobiol. Dis, doi:10.1016/j.nbd.2015.10.011
Bettuzzi, Gabba, Cataldo, Efficacy of a polyphenolic, standardized green tea extract for the treatment of COVID-19 syndrome: a proof-of-principle study, COVID, doi:10.3390/covid1010002
Bhargavan, Kanmogne, SARS-CoV-2 spike proteins and cell-cell communication inhibits TFPI and induces thrombogenic factors in human lung microvascular endothelial cells and neutrophils: implications for COVID-19 coagulopathy pathogenesis, Int. J. Mol. Sci, doi:10.3390/ijms231810436
Block, Kuo, Rationale for nicotinamide adenine dinucleotide (NAD+) metabolome disruption as a pathogenic mechanism of post-acute COVID-19 syndrome, Clin Med Insights Pathol, doi:10.1177/2632010X221106986
Booth, Ruegsegger, Toedebusch, Yan, Chapter six -endurance exercise and the regulation of skeletal muscle metabolism, Progress in Molecular Biology and Translational Science, Molecular and Cellular Regulation of Adaptation to Exercise
Brasche, Bischof, Daily time spent indoors in German homesbaseline data for the assessment of indoor exposure of German occupants, Int. J. Hyg. Environ. Health, doi:10.1016/j.ijheh.2005.03.003
Bruchfeld, The COVID-19 pandemic: consequences for nephrology, Nat. Rev. Nephrol, doi:10.1038/s41581-020-00381-4
Buzhdygan, Deore, Baldwin-Leclair, Bullock, Mcgary et al., The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier, Neurobiol. Dis, doi:10.1016/j.nbd.2020.105131
Camell, Yousefzadeh, Zhu, Prata, Huggins et al., Senolytics reduce coronavirus-related mortality in old mice, Science, doi:10.1126/science.abe4832
Cardinali, Brown, Pandi-Perumal, Possible application of melatonin in long COVID, Biomolecules, doi:10.3390/biom12111646
Carr, Maggini, Vitamin C and immune function, Nutrients, doi:10.3390/nu9111211
Carrubba, Veronese, Di Bella, Cusumano, Di Prazza et al., Prognostic value of magnesium in COVID-19: findings from the COMEPA study, Nutrients, doi:10.3390/nu15040830
Catenacci, Pan, Ostendorf, Brannon, Gozansky et al., A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity, Obesity
Cattadori, Di Marco, Baravelli, Picozzi, Ambrosio, Exercise training in post-COVID-19 patients: the need for a multifactorial protocol for a multifactorial pathophysiology, J. Clin. Med, doi:10.3390/jcm11082228
Changeux, Amoura, Rey, Miyara, A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications, C. R. Biol, doi:10.5802/crbiol.8
Chatam, Chapnik, Froy, Resveratrol induces the fasting state and alters circadian metabolism in hepatocytes, Plant Foods Hum. Nutr, doi:10.1007/s11130-022-00954-7
Cheema, Sohail, Fatima, Shahid, Shahzil et al., Quercetin for the treatment of COVID-19 patients: a systematic review and meta-analysis, Rev. Med. Virol, doi:10.1002/rmv.2427
Chen, Mcgowan, Ren, Lal, Nassif et al., Nattokinase: a promising alternative in prevention and treatment of cardiovascular diseases, Biomark. Insights, doi:10.1177/1177271918785130
Chen, Yang, Wang, Huang, Yu et al., Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer's disease, J. Pineal Res, doi:10.1111/jpi.12774
Chen, Zhao, Shu, Xing, Wang et al., Effect of resveratrol on intestinal tight junction proteins and the gut microbiome in high-fat diet-fed insulin resistant mice, Int. J. Food Sci. Nutr, doi:10.1080/09637486.2020.1754351
Cheng, Adams, Perin, Wei, Zhou et al., Prolonged fasting reduces IGF-1/ PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression, Cell Stem Cell, doi:10.1016/j.stem.2014.04.014
Cheng, Chen, Sun, Ahmadian, Ardalan et al., Natural phytochemicals that affect autophagy in the treatment of oral diseases and infections: a review, Oxidative Med. Cell. Longev, doi:10.1155/2021/4946711
Cheng, Luo, Wang, Zhang, Wang et al., Kidney disease is associated with in-hospital death of patients with COVID-19, Kidney Int, doi:10.1016/j.kint.2020.03.005
Choi, Lee, Seo, Kim, Jeon et al., Myocarditis-induced sudden death after BNT162b2 mRNA COVID-19 vaccination in Korea: case report focusing on histopathological findings, J. Korean Med. Sci, doi:10.3346/jkms.2021.36.e286
Choi, Park, Jo, Kim, Koh, SARS-CoV-2 spike S1 subunit protein-mediated increase of beta-secretase 1 (BACE1) impairs human brain vessel cells, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2022.07.113
Chourasia, Boland, Macleod, Mitophagy and cancer, Cancer & Metabolism, doi:10.1186/s40170-015-0130-8
Chowdhury, Richardson, Tsintzas, Thompson, Betts, Effect of extended morning fasting upon ad libitum lunch intake and associated metabolic and hormonal responses in obese adults, Int. J. Obes, doi:10.1038/ijo.2015.154
Chuang, Papp, Kuczmog, Eells, Condor Capcha et al., Methylene blue is a nonspecific protein-protein interaction inhibitor with potential for repurposing as an antiviral for COVID-19, Pharmaceuticals, doi:10.3390/ph15050621
Chung, Park, Lim, The effects of exercise and cold exposure on mitochondrial biogenesis in skeletal muscle and white adipose tissue, J. Exerc. Nutr. Biochem, doi:10.20463/jenb.2017.0020
Clifton, Ma, Fontana, Peterson, Intermittent fasting in the prevention and treatment of cancer, CA Cancer J. Clin, doi:10.3322/caac.21694
Clough, Inigo, Chandra, Chaves, Reynolds et al., Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: implications for neuro-COVID, J. NeuroImmune Pharmacol, doi:10.1007/s11481-021-10015-6
Cnubben, Rietjens, Wortelboer, Van Zanden, Van Bladeren, The interplay of glutathione-related processes in antioxidant defense, Environ. Toxicol. Pharmacol, doi:10.1016/S1382-6689(01)00077-1
Cohen, Native American medicine, Altern. Ther. Health Med
Cosentino, Marino, Understanding the pharmacology of COVID-19 mRNA vaccines: playing dice with the spike?, Int. J. Mol. Sci, doi:10.3390/ijms231810881
Coto-Montes, Boga, Rosales-Corral, Fuentes-Broto, Tan et al., Role of melatonin in the regulation of autophagy and mitophagy: a review, Mol. Cell. Endocrinol, doi:10.1016/j.mce.2012.04.009
Couzin-Frankel, The mystery of the pandemic's 'happy hypoxia, Science, doi:10.1126/science.368.6490.455
Coyle, Physiological determinants of endurance exercise performance, J. Sci. Med. Sport, doi:10.1016/S1440-2440(99)80172-8
Cuervo, Knecht, Terlecky, Dice, Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation, Am. J. Phys. Cell Phys, doi:10.1152/ajpcell.1995.269.5.C1200
Dabholkar, Gorantla, Dubey, Alexander, Taliyan et al., Repurposing methylene blue in the management of COVID-19: mechanistic aspects and clinical investigations, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.112023
Davidovich, Kearney, Martin, Inflammatory outcomes of apoptosis. necrosis and necroptosis, Biol Chem, doi:10.1515/hsz-2014-0164
Denaro, Haloush, Hsiao, Orgera, Osorio et al., COVID-19 and neurodegeneration: the mitochondrial connection, Aging Cell, doi:10.1111/acel.13727
Deore, Tran, Andrews, Ramirez, Galie, SARS-CoV-2 spike protein disrupts blood-brain barrier integrity via RhoA activation, J. NeuroImmune Pharmacol, doi:10.1007/s11481-021-10029-0
Devaux, Camoin-Jau, Molecular mimicry of the viral spike in the SARS-CoV-2 vaccine possibly triggers transient dysregulation of ACE2, leading to vascular and coagulation dysfunction similar to SARS-CoV-2 infection, Viruses, doi:10.3390/v15051045
Di, He, Zhao, Huang, Wu et al., Methylene blue reduces acute cerebral ischemic injury via the induction of mitophagy, Mol. Med, doi:10.2119/molmed.2015.00038
Dice, Chaperone-mediated autophagy, Autophagy, doi:10.4161/auto.4144
Domi, Hoxha, Kolovani, Tricarico, Zappacosta, The importance of nutraceuticals in COVID-19: what's the role of resveratrol?, Molecules, doi:10.3390/molecules27082376
Dusabimana, Kim, Kim, Park, Kim, Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3amediated autophagy and mitochondrial biogenesis, Exp. Mol. Med, doi:10.1038/s12276-019-0245-z
Díaz-Resendiz, Benitez-Trinidad, Covantes-Rosales, Toledo-Ibarra, Ortiz-Lazareno et al., Loss of mitochondrial membrane potential (ΔΨm) in leucocytes as post-COVID-19 sequelae, J. Leukoc. Biol, doi:10.1002/JLB.3MA0322-279RRR
Díaz-Resendiz, Covantes-Rosales, Benítez-Trinidad, Navidad-Murrieta, Razura-Carmona et al., Effect of fucoidan on the mitochondrial membrane potential (ΔΨm) of leukocytes from patients with active COVID-19 and subjects that recovered from SARS-CoV-2 infection, Mar. Drugs, doi:10.3390/md20020099
Díez, Iglesias, García, Martín-Casasempere, Bernabéu-Andréu, Serum calcium, magnesium, and phosphorus levels in patients with COVID-19: relationships with poor outcome and mortality, Horm. Metab. Res, doi:10.1055/a-1899-8862
Dörre, Time-activity-patterns of some selected small groups as a basis for exposure estimation: a methodological study, J. Expo. Anal. Environ. Epidemiol
Falconer, Skouras, Carter, Greenway, Paisley, Preoperative fasting: current practice and areas for improvement, Updat. Surg, doi:10.1007/s13304-013-0242-z
Fazel, Medical implications of controlled fasting, J. R. Soc. Med
Fernandes, Demetriades, The multifaceted role of nutrient sensing and mTORC1 signaling in physiology and aging, Frontiers in Aging
Fernández-Galilea, Pérez-Matute, Prieto-Hontoria, Houssier, Burrell et al., α-Lipoic acid treatment increases mitochondrial biogenesis and promotes beige adipose features in subcutaneous adipocytes from overweight/obese subjects, Biochim. Biophys. Acta, doi:10.1016/j.bbalip.2014.12.013
Ferraresi, Titone, Follo, Castiglioni, Chiorino et al., The protein restriction mimetic resveratrol is an autophagy inducer stronger than amino acid starvation in ovarian cancer cells, Mol. Carcinog, doi:10.1002/mc.22711
Ferrari, Bettuzzi, Naponelli, The potential of epigallocatechin Gallate (EGCG) in targeting autophagy for cancer treatment: a narrative review, Int. J. Mol. Sci, doi:10.3390/ijms23116075
Finn, Dice, Ketone bodies stimulate chaperone-mediated autophagy, J. Biol. Chem, doi:10.1074/jbc.M502456200
Forbes-Hernández, Giampieri, Gasparrini, Mazzoni, Quiles et al., The effects of bioactive compounds from plant foods on mitochondrial function: a focus on apoptotic mechanisms, Food Chem. Toxicol, doi:10.1016/j.fct.2014.03.017
Forsyth, Zhang, Bhushan, Swanson, Zhang et al., The SARS-CoV-2 S1 spike protein promotes MAPK and NF-kB activation in human lung cells and inflammatory cytokine production in human lung and intestinal epithelial cells, Microorganisms, doi:10.3390/microorganisms10101996
Frallicciardi, Gabba, Poolman, Determining small-molecule permeation through lipid membranes, Nat. Protoc, doi:10.1038/s41596-022-00734-2
Fuhrman, Sarter, Glaser, Acocella, Changing perceptions of hunger on a high nutrient density diet, Nutr. J, doi:10.1186/1475-2891-9-51
Fujita, Nomura, Hong, Ito, Asada et al., Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan, Biochem. Biophys. Res. Commun, doi:10.1006/bbrc.1993.2624
Furmli, Elmasry, Ramos, Fung, Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin, Case Reports, doi:10.1136/bcr-2017-221854
Galan-Acosta, Xia, Yuan, Vakifahmetoglu-Norberg, Activation of chaperone-mediated autophagy as a potential anticancer therapy, Autophagy, doi:10.1080/15548627.2015.1106666
Georgakopoulos, Wells, Campanella, The pharmacological regulation of cellular mitophagy, Nat. Chem. Biol, doi:10.1038/nchembio.2287
Giannos, Prokopidis, Gut dysbiosis and long COVID-19: feeling gutted, J. Med. Virol, doi:10.1002/jmv.27684
Gill, Tashjian, Duncanson, Autopsy histopathologic cardiac findings in 2 adolescents following the second COVID-19 vaccine dose, Arch. Pathol. Lab. Med, doi:10.5858/arpa.2021-0435-SA
Gillman, CNS toxicity involving methylene blue: the exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity, J. Psychopharmacol, doi:10.1177/0269881109359098
Godar, Ma, Liu, Murphy, Weinheimer et al., Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury, Autophagy, doi:10.1080/15548627.2015.1063768
Gomaa, Abdel-Wadood, Gomaa, Glycyrrhizin and boswellic acids, the golden nutraceuticals: multitargeting for treatment of mild-moderate COVID-19 and prevention of post-COVID cognitive impairment, Inflammopharmacol, doi:10.1007/s10787-022-01062-3
Gomaa, Mohamed, Abd-Ellatief, Gomaa, Hammam, Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial, Inflammopharmacol, doi:10.1007/s10787-022-00939-7
Gomes, Rigolon, Souza, Da, Alvarez-Leite et al., Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: a systematic review, Nutrition, doi:10.1016/j.nut.2019.05.005
Grobbelaar, Venter, Vlok, Ngoepe, Laubscher et al., SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19, Biosci. Rep, doi:10.1042/BSR20210611
Grundler, Mesnage, Cerrada, Wilhelmi De Toledo, Improvements during long-term fasting in patients with long COVIDa case series and literature review, Front. Nutr, doi:10.3389/fnut.2023.1195270
Grune, Jung, Merker, Davies, Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and 'aggresomes' during oxidative stress, aging, and disease, Int. J. Biochem. Cell Biol, doi:10.1016/j.biocel.2004.04.020
Guerrero-Romero, Mercado, Rodriguez-Moran, Ramírez-Renteria, Martínez-Aguilar et al., Magnesium-tocalcium ratio and mortality from COVID-19, Nutrients, doi:10.3390/nu14091686
Guntur, Nemkov, De Boer, Mohning, Baraghoshi et al., Signatures of mitochondrial dysfunction and impaired fatty acid metabolism in plasma of patients with postacute sequelae of COVID-19 (PASC), Metabolites, doi:10.3390/metabo12111026
Guo, Luo, Ye, Yin, Fan et al., Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients, J. Clin. Endocrinol. Metab, doi:10.1210/clinem/dgaa644
Ha, Kim, Park, Jung, Choo et al., Structural modification of (-)-epigallocatechin gallate (EGCG) shows significant enhancement in mitochondrial biogenesis, J. Agric. Food Chem, doi:10.1021/acs.jafc.8b00364
Halajian, Leblanc, Gee, Colpitts, Activation of TLR4 by viral glycoproteins: a double-edged sword? Front, Microbiol
Halma, Plothe, Marik, Lawrie, Strategies for the management of spike protein-related pathology, Microorganisms, doi:10.3390/microorganisms11051308
Halma, Rose, Lawrie, The novelty of mRNA viral vaccines and potential harms: a scoping review, J 2023a, doi:10.3390/j6020017
Hamidi-Alamdari, Hafizi-Lotfabadi, Bagheri-Moghaddam, Safari, Mozdourian et al., Methylene blue for treatment of hospitalized COVID-19 patients: a randomized, controlled, open-label clinical trial, phase 2, Rev. Investig. Clin, doi:10.24875/ric.21000028
Hamidie, Yamada, Ishizawa, Saito, Masuda, Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels, Metab. Clin. Exp, doi:10.1016/j.metabol.2015.07.010
Hao, Shen, Yu, Jia, Li et al., Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2009.03.012
Hartl, Bracher, Hayer-Hartl, Molecular chaperones in protein folding and proteostasis, Nature, doi:10.1038/nature10317
Harvie, Pegington, Mattson, Frystyk, Dillon et al., The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women, Int. J. Obes
Harvie, Wright, Pegington, Mcmullan, Mitchell et al., The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women, Br. J. Nutr
Hattori, Saiki, Imai, Regulation by mitophagy, Int. J. Biochem. Cell Biol, doi:10.1016/j.biocel.2014.05.012
Hazell, Khazova, Cohen, Felton, Raj, Post-exposure persistence of nitric oxide upregulation in skin cells irradiated by UV-A, Sci. Rep, doi:10.1038/s41598-022-13399-4
He, Bassik, Moresi, Sun, Wei et al., Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis, Nature, doi:10.1038/nature10758
He, Xu, Li, Yang, Mao, Intermittent fasting and immunomodulatory effects: a systematic review, Frontiers in Nutrition
He-Ling, Hong-Yuan, Zhen-Xiang, Zhi, Yi-Fen et al., Electrical stimulation induces mitochondrial autophagy via activating oxidative stress and Sirt3 signaling pathway, Chin. Med. J, doi:10.1097/CM9.0000000000001165
Hemilä, De Man, Vitamin C and COVID-19, Front. Med
Henagan, Cefalu, Ribnicky, Noland, Dunville et al., In vivo effects of dietary quercetin and quercetin-rich red onion extract on skeletal muscle mitochondria, metabolism, and insulin sensitivity, Genes Nutr, doi:10.1007/s12263-014-0451-1
Henry, Summa, Patrick, Schwartz, A cohort of cancer patients with no reported cases of SARS-CoV-2 infection: the possible preventive role of methylene blue, Substantia, doi:10.13128/Substantia-888
Heo, Ordureau, Paulo, Rinehart, Harper, The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy, Mol. Cell, doi:10.1016/j.molcel.2015.08.016
Hewlings, Kalman, Curcumin: a review of its effects on human health, Foods, doi:10.3390/foods6100092
Hiramitsu, Shimada, Kuroyanagi, Inoue, Katagiri et al., Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis, Sci. Rep, doi:10.1038/srep03708
Ho, Veldhuis, Johnson, Furlanetto, Evans et al., Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man, J. Clin. Invest
Hobday, Cason, The open-air treatment of pandemic influenza, Am. J. Public Health, doi:10.2105/AJPH.2008.134627
Hoffman, Eating and fasting for god in Sufi tradition, J. Am. Acad. Relig
Holick, Chapter 4 -photobiology of vitamin D
Horne, May, Muhlestein, Le, Bair et al., Association of periodic fasting with lower severity of COVID-19 outcomes in the SARS-CoV-2 prevaccine era: an observational cohort from the INSPIRE Registry, BMJ Nutr Prev Health, doi:10.1136/bmjnph-2022-000462
Hsu, Lee, Wang, Lee, Chen, Amyloid-degrading ability of nattokinase from Bacillus subtilis natto, J. Agric. Food Chem, doi:10.1021/jf803072r
Hsu, Wang, Reid, Veerati, Pathinayake et al., SARS-CoV-2 Spike Protein Promotes Hyper-inflammatory Response That Can Be Ameliorated by Spikeantagonistic Peptide and FDA-approved ER Stress and MAP Kinase Inhibitors in, Vitro
Hu, Li, Wang, Luo, Zhang et al., Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin, Oncotarget, doi:10.18632/oncotarget.7861
Hu, Zhang, Peng, Ma, Han, Efficiency of nicotinamidebased supportive therapy in lymphopenia for patients with ordinary or severe COVID-19: a randomized controlled trial, Medicine, doi:10.1097/MD.0000000000031138
Huang, Chen, Xie, Yang, Shen, Nebivolol stimulates mitochondrial biogenesis in 3T3-L1 adipocytes, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2013.07.055
Huang, Xu, Wan, Shou, Qian et al., Deacetylation of nuclear LC3 drives autophagy initiation under starvation, Mol. Cell, doi:10.1016/j.molcel.2014.12.013
Hubert, Weiss, Rees, Kain, Modulating chaperone-mediated autophagy and its clinical applications in cancer, Cells, doi:10.3390/cells11162562
Hurley, Young, Mechanisms of autophagy initiation, Annu. Rev. Biochem, doi:10.1146/annurev-biochem-061516-044820
Huynh, Rethi, Lee, Higa, Kao et al., Spike protein impairs mitochondrial function in human cardiomyocytes: mechanisms underlying cardiac injury in COVID-19, Cells, doi:10.3390/cells12060877
Hwang, Machek, Cardaci, Wilburn, Kim et al., Effects of pyrroloquinoline quinone (PQQ) supplementation on aerobic exercise performance and indices of mitochondrial biogenesis in untrained men, J. Am. Coll. Nutr, doi:10.1080/07315724.2019.1705203
Islam, Yamamoto, Mizoue, Tanaka, Oshiro et al., Association of impaired fasting glucose and diabetes with SARS-CoV-2 spike antibody titers after the BNT162b2 vaccine among health care workers in a tertiary hospital in Japan, Vaccines, doi:10.3390/vaccines10050776
Izzo, Trimarco, Mone, Aloè, Capra Marzani et al., Combining L-arginine with vitamin C improves long-COVID symptoms: the LINCOLN Survey, Pharmacol. Res, doi:10.1016/j.phrs.2022.106360
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-021-00418-x
Jaimes-Gualdrón, Flórez-Álvarez, Zapata-Cardona, Rojano, Rugeles et al., Corozo (Bactris guineensis) fruit extract has antiviral activity in vitro against SARS-CoV-2, Functional Foods in Health and Disease
Jamshed, Beyl, Della Manna, Yang, Ravussin et al., Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans, Nutrients, doi:10.3390/nu11061234
Jang, Kang, Hwang, Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation, J. Biol. Chem, doi:10.1074/jbc.M112.363747
Jang, Kim, Cai, Kim, Kim et al., Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation, Lab Anim Res, doi:10.5625/lar.2013.29.4.221
Jin, Lazarou, Wang, Kane, Narendra et al., Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL, J. Cell Biol, doi:10.1083/jcb.201008084
Jornayvaz, Shulman, Regulation of mitochondrial biogenesis, Essays Biochem, doi:10.1042/bse0470069
Josifovska, Albert, Nagymihály, Lytvynchuk, Moe et al., Resveratrol as inducer of autophagy, pro-survival, and anti-inflammatory stimuli in cultured human RPE cells, Int. J. Mol. Sci, doi:10.3390/ijms21030813
Kane, Lazarou, Fogel, Li, Yamano et al., PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity, J. Cell Biol, doi:10.1083/jcb.201402104
Kang, Hong, Lee, Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis, J. Pineal Res, doi:10.1111/jpi.12319
Kato, Tanaka, Masuda, Ogasawara, Sakurai et al., Melatonin promotes adipogenesis and mitochondrial biogenesis in 3T3-L1 preadipocytes, J. Pineal Res, doi:10.1111/jpi.12259
Kauser, Rubanyi, Potential cellular signaling mechanisms mediating upregulation of endothelial nitric oxide production by estrogen, J. Vasc. Res, doi:10.1159/000159227
Kaushik, Cuervo, The coming of age of chaperone-mediated autophagy, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-018-0001-6
Kazlauskaite, Kondapalli, Gourlay, Campbell, Ritorto et al., Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65, Biochem. J, doi:10.1042/BJ20140334
Kc, Càrcamo, Golde, Vitamin C enters mitochondria via facilitative glucose transporter 1 (Gluti) and confers mitochondrial protection against oxidative injury, FASEB J, doi:10.1096/fj.05-4107com
Kepp, Chen, Carmona-Gutierrez, Madeo, Kroemer, A discovery platform for the identification of caloric restriction mimetics with broad healthimproving effects, Autophagy, doi:10.1080/15548627.2019.1688984
Khalaji, Behnoush, Alilou, Rezaee, Peiman et al., Adjunctive therapy with lipid-lowering agents in COVID-19: a systematic review and meta-analysis of randomized controlled trials, Lipids Health Dis, doi:10.1186/s12944-023-01828-w
Khan, Shafiei, Longoria, Schoggins, Savani et al., SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway, Elife, doi:10.7554/eLife.68563
Kidd, Lancaster, Anderson, Boogert, Fisher et al., Fetal death after exposure to methylene blue dye during midtrimester amniocentesis in twin pregnancy, Nat. Cell Biol, doi:10.1038/s41556-018-0205-1
Kim, Jeon, Kim, Lee, Kim et al., Spike proteins of SARS-CoV-2 induce pathological changes in molecular delivery and metabolic function in the brain endothelial cells, Viruses, doi:10.3390/v13102021
Kim, Park, Park, Jung, Youn et al., De Candolle ethanolic extract inhibits adipogenic regulators in 3T3-L1 cells and induces mitochondrial biogenesis in primary brown preadipocytes, J. Agric. Food Chem, doi:10.1021/acs.jafc.5b01908
Kou, Li, Wu, Liu, Hu et al., Citrus tangeretin improves skeletal muscle mitochondrial biogenesis via activating the AMPK-PGC1-α pathway in vitro and in vivo: a possible mechanism for its beneficial effect on physical performance, J. Agric. Food Chem, doi:10.1021/acs.jafc.8b04124
Koyano, Okatsu, Kosako, Tamura, Go et al., Ubiquitin is phosphorylated by PINK1 to activate Parkin, Nature, doi:10.1038/nature13392
Krakoff, Fasting and ambulatory blood pressure monitoring, Blood Pressure Monitoring, doi:10.1097/MBP.0000000000000271
Kriegenburg, Ellgaard, Hartmann-Petersen, Molecular chaperones in targeting misfolded proteins for ubiquitin-dependent degradation, FEBS J, doi:10.1111/j.1742-4658.2011.08456.x
Kumar, Liu, Hsu, Chacko, Minard et al., Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: results of a pilot clinical trial, Clin. Transl. Med, doi:10.1002/ctm2.372
Kurosawa, Nirengi, Homma, Esaki, Ohta et al., A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles, Sci. Rep, doi:10.1038/srep11601
Lan, Lee, Chao, Chang, Lu et al., Efficacy of melatonin in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials, J. Med. Virol, doi:10.1002/jmv.27595
Larsson, Hellstrand, Hammarström, Nyström, SARS-CoV-2 Spike Amyloid Fibrils Specifically and Selectively Accelerates Amyloid Fibril Formation of Human Prion Protein and the Amyloid β Peptide
Laufs, La Fata, Plutzky, Liao, Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors, Circulation, doi:10.1161/01.CIR.97.12.1129
Leary, Shoubridge, Mitochondrial biogenesis: which part of "NO" do we understand?, BioEssays, doi:10.1002/bies.10298
Lee, Kim, Effects of isorhamnetin on adipocyte mitochondrial biogenesis and AMPK activation, Molecules, doi:10.3390/molecules23081853
Lee, Kim, Suh, Lee, Involvement of ROS in curcumin-induced autophagic cell death, Korean J Physiol Pharmacol, doi:10.4196/kjpp.2011.15.1.1
Lee, Shin, Jung, Kim, Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of dietinduced obese mice, Food Nutr, doi:10.1080/16546628.2017.1325307
Lei, Klionsky, Transcriptional regulation of autophagy and its implications in human disease, Cell Death Differ, doi:10.1038/s41418-023-01162-9
Lei, Zhang, Schiavon, He, Chen et al., SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2, Circ. Res, doi:10.1161/CIRCRESAHA.121.318902
Lescat, Véron, Mourot, Péron, Chenais et al., Chaperone-mediated autophagy in the light of evolution: insight from fish, Mol. Biol. Evol, doi:10.1093/molbev/msaa127
Lettieri-Barbato, Cannata, Casagrande, Ciriolo, Aquilano, Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle, PLoS One, doi:10.1371/journal.pone.0195912
Levine, Klionsky, Autophagy wins the 2016 Nobel prize in physiology or medicine: breakthroughs in baker's yeast fuel advances in biomedical research, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.1619876114
Lewis Luján, Mccarty, Di Nicolantonio, Gálvez Ruiz, Rosas-Burgos et al., Nutraceuticals/ drugs promoting mitophagy and mitochondrial biogenesis may combat the mitochondrial dysfunction driving progression of dry age-related macular degeneration, Nutrients, doi:10.3390/nu14091985
Li, Hou, Ma, Wang, Wang et al., SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy, Cell. Mol. Immunol, doi:10.1038/s41423-021-00807-4
Li, Li, Wang, Yang, Huang et al., SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling, Biochim. Biophys. Acta (BBA) -Mol. Basis Dis, doi:10.1016/j.bbadis.2021.166260
Li, Ou, Wei, Shang, Carnitine and COVID-19 susceptibility and severity: a Mendelian randomization study, Frontiers. Nutrition
Liang, Bao, Yang, Liu, Sun et al., SARS-CoV-2 spike protein induces IL-18-mediated cardiopulmonary inflammation via reduced mitophagy, Sig Transduct Target Ther, doi:10.1038/s41392-023-01368-w
Liao, Wang, Liu, Xu, Hou et al., Dysfunction of chaperone-mediated autophagy in human diseases, Mol. Cell. Biochem, doi:10.1007/s11010-020-04006-z
Lin, Fu, Tsai, Cheng, Weng, Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy, Int. J. Mol. Sci, doi:10.3390/ijms18071412
Lin, More than a key-the pathological roles of SARS-CoV-2 spike protein in COVID-19 related cardiac injury, Sports Medicine and Health Science, doi:10.1016/j.smhs.2023.03.004
Lindqvist, Epstein, Nielsen, Landin-Olsson, Ingvar et al., Avoidance of Sun exposure as a risk factor for major causes of death: a competing risk analysis of the melanoma in southern Sweden cohort, J. Intern. Med, doi:10.1111/joim.12496
Longo, Mattson, Fasting: molecular mechanisms and clinical applications, Cell Metab, doi:10.1016/j.cmet.2013.12.008
Lyamzaev, Tokarchuk, Panteleeva, Mulkidjanian, Skulachev et al., Induction of autophagy by depolarization of mitochondria, Autophagy, doi:10.1080/15548627.2018.1436937
Madeo, Eisenberg, Pietrocola, Kroemer, Spermidine in health and disease, Science, doi:10.1126/science.aan2788
Madeo, Hofer, Pendl, Bauer, Eisenberg et al., Nutritional aspects of spermidine, Annu. Rev. Nutr, doi:10.1146/annurev-nutr-120419-015419
Mahale, Godavarthy, Marreddy, Gokhale, Funde et al., Intravenous methylene blue as a rescue therapy in the management of refractory hypoxia in COVID-19 ARDS patients: a case series, Indian J Crit Care Med, doi:10.5005/jp-journals-10071-23905
Mao, Jin, Wang, Hu, Chen et al., Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China, JAMA Neurol, doi:10.1001/jamaneurol.2020.1127
Martin, Mattson, Maudsley, Caloric restriction and intermittent fasting: two potential diets for successful brain aging, Ageing Res. Rev, doi:10.1016/j.arr.2006.04.002
Martin-Rincon, Morales-Alamo, Calbet, Exercise-mediated modulation of autophagy in skeletal muscle, Scand. J. Med. Sci. Sports, doi:10.1111/sms.12945
Martinez-Lopez, Tarabra, Toledo, Garcia-Macia, Sahu et al., Systemwide benefits of intermeal fasting by autophagy, Cell Metab, doi:10.1016/j.cmet.2017.09.020
Mattson, Longo, Harvie, Impact of intermittent fasting on health and disease processes, Ageing Res. Rev, doi:10.1016/j.arr.2016.10.005
Mattson, Wan, Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2004.12.007
Mccreary, Schnell, Rhoda, Randomized double-blind placebocontrolled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19), Sci. Rep, doi:10.1038/s41598-022-13920-9
Meng, Xiao, Muhammed, Deng, Chen et al., Anti-inflammatory action and mechanisms of resveratrol, Molecules, doi:10.3390/molecules26010229
Mikirova, Casciari, Rogers, Taylor, Effect of high-dose intravenous vitamin C on inflammation in cancer patients, J. Transl. Med, doi:10.1186/1479-5876-10-189
Mittra, De Souza, Bhadade, Madke, Shankpal et al., Resveratrol and Copper for Treatment of Severe COVID-19: An Observational Study, RESCU
Mogalli, Matsukawa, Shimomura, Isoda, Ohkohchi, Cyanidin-3glucoside enhances mitochondrial function and biogenesis in a human hepatocyte cell line, Cytotechnology, doi:10.1007/s10616-018-0242-4
Montella-Manuel, Pujol-Carrion, Mechoud, De La Torre-Ruiz, Bulk autophagy induction and life extension is achieved when iron is the only limited nutrient in Saccharomyces cerevisiae, Biochem. J, doi:10.1042/BCJ20200849
Moser, Salvador, Deniz, Swanson, Tuszynski et al., The mechanisms of action of tumor treating fields, Cancer Res, doi:10.1158/0008-5472.CAN-22-0887
Mörz, A case report: multifocal necrotizing encephalitis and myocarditis after BNT162b2 mRNA vaccination against COVID-19, Vaccines, doi:10.3390/vaccines10101651
Nair, Mohanty, Knowledge, Attitude and practice of therapeutic fasting among naturopathy physicians: across sectional national survey, Journal of Nutrition, Fasting and Health, doi:10.22038/jfh.2015.6075
Narendra, Tanaka, Suen, Youle, Parkin is recruited selectively to impaired mitochondria and promotes their autophagy, J. Cell Biol, doi:10.1083/jcb.200809125
Nazir, Asghar, Rathore, Shahzad, Shahid et al., Menstrual abnormalities after COVID-19 vaccines: a systematic review, Vacunas (English Edition), doi:10.1016/j.vacune.2022.10.019
Negro, Turco, Povero, Nebivolol: an effective option against longlasting dyspnoea following COVID-19 pneumonia -a pivotal double-blind, cross-over controlled study, Multidisciplinary Respiratory Medicine, doi:10.4081/mrm.2022.886
Nejatifard, Asefi, Jamali, Hamblin, Fekrazad, Probable positive effects of the photobiomodulation as an adjunctive treatment in COVID-19: a systematic review, Cytokine, doi:10.1016/j.cyto.2020.155312
Nguyen, Malamo, Larkin-Kaiser, Borsa, Adhihetty, Effect of near-infrared light exposure on mitochondrial signaling in C2C12 muscle cells, Mitochondrion, doi:10.1016/j.mito.2013.11.001
Nguyen, Zhang, Gao, Cao, Tian et al., The spike protein of SARS-CoV-2 impairs lipid metabolism and increases susceptibility to lipotoxicity: implication for a role of Nrf2, Cells, doi:10.3390/cells11121916
Nicassio, Fracasso, Sirago, Musicco, Picca et al., Dietary supplementation with acetyll-carnitine counteracts age-related alterations of mitochondrial biogenesis, dynamics and antioxidant defenses in brain of old rats, Exp. Gerontol, doi:10.1016/j.exger.2017.08.017
Nisoli, Clementi, Paolucci, Cozzi, Tonello et al., Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide, Science, doi:10.1126/science.1079368
Niu, Zhou, Nie, Shin, Cui, Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos, J. Pineal Res, doi:10.1111/jpi.12627
Nopp, Moik, Klok, Gattinger, Petrovic et al., Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life, Respiration, doi:10.1159/000522118
Nouri-Majd, Ebrahimzadeh, Mousavi, Zargarzadeh, Eslami et al., Higher intake of dietary magnesium is inversely associated with COVID-19 severity and symptoms in hospitalized patients: a cross-sectional study, Frontiers. Nutrition
Nunez-Castilla, Stebliankin, Baral, Balbin, Sobhan et al., Potential autoimmunity resulting from molecular mimicry between SARS-CoV-2 spike and human proteins, Viruses, doi:10.3390/v14071415
Nunn, Guy, Brysch, Bell, Understanding long COVID; mitochondrial health and adaptation-old pathways. new problems, Biomedicines, doi:10.3390/biomedicines10123113
Nyström, Hammarström, Amyloidogenesis of SARS-CoV-2 spike protein, J. Am. Chem. Soc, doi:10.1021/jacs.2c03925
Oba, Rongduo, Saito, Okabayashi, Yokota et al., Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2021.07.034
Oliveira, Boulé, Berg, Sharma, Elliott et al., Consumption of a high-protein meal replacement leads to higher fat oxidation, suppression of hunger, and improved metabolic profile after an exercise session, Nutrients, doi:10.3390/nu13010155
Otten, Bourgonje, Peters, Alizadeh, Dijkstra et al., Vitamin C supplementation in healthy individuals leads to shifts of bacterial populations in the gut-a pilot study, Antioxidants (Basel), doi:10.3390/antiox10081278
Ozono, Zhang, Ode, Sano, Tan et al., SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity, Nat. Commun, doi:10.1038/s41467-021-21118-2
Pais, Alexy, Holsworth, Meiselman, Effects of nattokinase, a profibrinolytic enzyme, on red blood cell aggregation and whole blood viscosity, Clin. Hemorheol. Microcirc
Paraskevas, Kantanis, Karalis, Michailides, Karamouzos et al., N-acetylcysteine efficacy in patients hospitalized with COVID-19 pneumonia: a systematic review and meta-analysis, Rom. J. Intern. Med, doi:10.2478/rjim-2023-0001
Park, Jeong, Lee, Koh, Kwon et al., Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition, Sci. Rep
Park, Kang, Lee, Autophagy in neurodegenerative diseases: a hunter for aggregates, Int. J. Mol. Sci, doi:10.3390/ijms21093369
Parry, Lefringhausen, Turni, Neil, Cosford et al., Spikeopathy': COVID-19 spike protein is pathogenic, from both virus and vaccine mRNA, Biomedicines, doi:10.3390/biomedicines11082287
Pasquereau, Nehme, Haidar Ahmad, Daouad, Van Assche et al., Resveratrol inhibits HCoV-229E and SARS-CoV-2 coronavirus replication in vitro, Viruses, doi:10.3390/v13020354
Patidar, Sharma, Bhoraskar, Tripathi, Dhaneriya, The role of nebulized methylene blue [NMB] in the management of COVID-19 cases: an observational study, International Journal of Medical Arts, doi:10.21608/ijma.2022.112347.1416
Patikorn, Roubal, Veettil, Chandran, Pham et al., Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.39558
Patterson, Laughlin, Sears, Lacroix, Marinac et al., Intermittent fasting and human metabolic health, J. Acad. Nutr. Diet, doi:10.1016/j.jand.2015.02.018
Patterson, Sears, Metabolic effects of intermittent fasting, Annu. Rev. Nutr, doi:10.1146/annurev-nutr-071816-064634
Pattnaik, Bhatraju, Kashyap, Verma, Madan et al., Effect of a nutraceutical drug in COPD condition: a pilot study of in vitro, in vivo and clinical trial, Eur. Respir. J, doi:10.1183/13993003.congress-2022.1661
Pawar, Mastud, Pawar, Pawar, Bhoite et al., Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial, Front. Pharmacol
Pesce, Fracasso, Cassano, Lezza, Cantatore et al., Acetyl-L-carnitine supplementation to old rats partially reverts the age-related mitochondrial decay of soleus muscle by activating peroxisome proliferatoractivated receptor gamma coactivator-1alpha-dependent mitochondrial biogenesis, Rejuvenation Res, doi:10.1089/rej.2009.0955
Pesce, Nicassio, Fracasso, Musicco, Cantatore et al., Acetyl-L-carnitine activates the peroxisome proliferator-activated receptor-γ coactivators PGC-1α/PGC-1β-dependent signaling cascade of mitochondrial biogenesis and decreases the oxidized peroxiredoxins content in old rat liver, Rejuvenation Res, doi:10.1089/rej.2011.1255
Petrlova, Samsudin, Bond, Schmidtchen, SARS-CoV-2 spike protein aggregation is triggered by bacterial lipopolysaccharide, FEBS Lett, doi:10.1002/1873-3468.14490
Petrovszki, Walter, Vigh, Kocsis, Valkai et al., Penetration of the SARS-CoV-2 spike protein across the blood-brain barrier, as revealed by a combination of a human cell culture model system and optical biosensing, Biomedicines, doi:10.3390/biomedicines10010188
Pickrell, Youle, The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson's disease, Neuron, doi:10.1016/j.neuron.2014.12.007
Pietrocola, Lachkar, Enot, Niso-Santano, Pedro et al., Spermidine induces autophagy by inhibiting the acetyltransferase EP300, Cell Death Differ, doi:10.1038/cdd.2014.215
Pietrocola, Malik, Mariño, Vacchelli, Senovilla et al., Coffee induces autophagy in vivo, Cell Cycle, doi:10.4161/cc.28929
Pilchova, Klacanova, Tatarkova, Kaplan, Racay, The involvement of Mg 2+ in regulation of cellular and mitochondrial functions, Oxidative Med. Cell. Longev, doi:10.1155/2017/6797460
Poteet, Winters, Yan, Shufelt, Green et al., Neuroprotective actions of methylene blue and its derivatives, PLoS One, doi:10.1371/journal.pone.0048279
Prasada Kabekkodu, Chakrabarty, Jayaram, Mallya, Thangaraj et al., Severe acute respiratory syndrome coronaviruses contributing to mitochondrial dysfunction: implications for post-COVID complications, Mitochondrion, doi:10.1016/j.mito.2023.01.005
Pujhari, Paul, Ahluwalia, Rasgon, Clotting disorder in severe acute respiratory syndrome coronavirus 2, Rev. Med. Virol, doi:10.1002/rmv.2177
Pulido Perez, Póndigo De Los Angeles, Perez Peralta, Ramirez Mojica, Torres Rasgado et al., Reduction in serum magnesium levels and renal function are associated with increased mortality in obese COVID-19 patients, Nutrients, doi:10.3390/nu14194054
Puyaubert, Baudouin, New clues for a cold case: nitric oxide response to low temperature, Plant Cell Environ, doi:10.1111/pce.12329
Racay, Effect of magnesium on calcium-induced depolarisation of mitochondrial transmembrane potential, Cell Biol. Int, doi:10.1016/j.cellbi.2007.08.024
Rai, Russell, Lightowlers, Turnbull, Potential compounds for the treatment of mitochondrial disease, Br. Med. Bull, doi:10.1093/bmb/ldv046
Rajpal, Ismail-Beigi, Intermittent fasting and 'metabolic switch': effects on metabolic syndrome, prediabetes and type 2 diabetes, Diabetes Obes. Metab, doi:10.1111/dom.14080
Rangaraju, Verrier, Madorsky, Nicks, Dunn et al., Rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice, J. Neurosci, doi:10.1523/JNEUROSCI.1356-10.2010
Rasbach, Schnellmann, Isoflavones promote mitochondrial biogenesis, J. Pharmacol. Exp. Ther, doi:10.1124/jpet.107.134882
Rashedinia, Saberzadeh, Khosravi Bakhtiari, Hozhabri, Arabsolghar, Glycyrrhizic acid ameliorates mitochondrial function and biogenesis against aluminum toxicity in PC12 cells, Neurotox. Res, doi:10.1007/s12640-018-9967-2
Rawat, Roy, Maitra, Gulati, Khanna et al., Vitamin C and COVID-19 treatment: a systematic review and meta-analysis of randomized controlled trials, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2021.102324
Ray, Caffeine is a potent stimulator of autophagy to reduce hepatic lipid content-a coffee for NAFLD?, Nat. Rev. Gastroenterol. Hepatol, doi:10.1038/nrgastro.2013.170
Real-Hohn, Navegantes, Ramos, Ramos-Filho, Cahuê et al., The synergism of high-intensity intermittent exercise and everyother-day intermittent fasting regimen on energy metabolism adaptations includes hexokinase activity and mitochondrial efficiency, PLoS One, doi:10.1371/journal.pone.0202784
Regmi, Heilbronn, Time-restricted eating: benefits, mechanisms, and challenges in translation, iScience, doi:10.1016/j.isci.2020.101161
Rehman, Krishnasamy, Haque, Thurman, Lemasters et al., Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporin A treatment in rats, PLoS One, doi:10.1371/journal.pone.0065029
Rhea, Logsdon, Hansen, Williams, Reed et al., The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice, Nat. Neurosci, doi:10.1038/s41593-020-00771-8
Roberts, Barnard, Jasman, Balon, Acute exercise increases nitric oxide synthase activity in skeletal muscle, American Journal of Physiology-Endocrinology and Metabolism, doi:10.1152/ajpendo.1999.277.2.E390
Rojas-Morales, León-Contreras, Granados-Pineda, Hernández-Pando, Gonzaga et al., Protection against renal ischemia and reperfusion injury by shortterm time-restricted feeding involves the mitochondrial unfolded protein response, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2020.04.025
Romanet, Wormser, Fels, Lucas, Prudat et al., Effectiveness of exercise training on the dyspnoea of individuals with long COVID: a randomised controlled multicentre trial, Ann. Phys. Rehabil. Med, doi:10.1016/j.rehab.2023.101765
Rubinsztein, Nixon, Rapamycin induces autophagic flux in neurons, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1014633107
Rushworth, Megson, Existing and potential therapeutic uses for Nacetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits, Pharmacol. Ther, doi:10.1016/j.pharmthera.2013.09.006
Sanchetee, Sanchetee, Garg, Effect of Jain fasting on anthropometric, clinical and biochemical parameters, Indian J Endocrinol Metab, doi:10.4103/ijem.IJEM_601_19
Sanz-Biset, Cañigueral, Plant use in the medicinal practices known as "strict diets" in Chazuta Valley (Peruvian Amazon), J. Ethnopharmacol, doi:10.1016/j.jep.2011.05.021
Sarkar, Ravikumar, Floto, Rubinsztein, Rapamycin and mTORindependent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies, Cell Death Differ, doi:10.1038/cdd.2008.110
Schiavi, Maglioni, Palikaras, Shaik, Strappazzon et al., Ironstarvation-induced mitophagy mediates lifespan extension upon mitochondrial stress in C. elegans, Curr. Biol, doi:10.1016/j.cub.2015.05.059
Schneider, Suh, Cuervo, Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation, Cell Metab, doi:10.1016/j.cmet.2014.06.009
Schwab, Domke, Hartmann, Stenzinger, Longerich et al., Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination, Clin. Res. Cardiol, doi:10.1007/s00392-022-02129-5
Schwarz, Benson, Horn, Wurdack, Grittner et al., Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline: a randomized clinical trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2022.13875
Schwarz, Stekovic, Wirth, Benson, Royer et al., Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline, Aging, doi:10.18632/aging.101354
Segev, Sagir, Matetzky, Segev, Atar et al., Admission serum magnesium levels is associated with short and long-term clinical outcomes in COVID-19 patients, Nutrients, doi:10.3390/nu15092016
Semmarath, Mapoung, Umsumarng, Arjsri, Srisawad et al., Cyanidin-3-O-glucoside and peonidin-3-Oglucoside-rich fraction of black rice germ and bran suppresses inflammatory responses from SARS-CoV-2 spike glycoprotein S1-induction in vitro in A549 lung cells and THP-1 macrophages via inhibition of the NLRP3 inflammasome pathway, Nutrients, doi:10.3390/nu14132738
Senekowitsch, Wietkamp, Grimm, Schmelter, Schick et al., High-dose spermidine supplementation does not increase spermidine levels in blood plasma and saliva of healthy adults: a randomized placebo-controlled pharmacokinetic and metabolomic study, Nutrients, doi:10.3390/nu15081852
Sessa, Salerno, Esposito, Di Nunno, Zamboni et al., Autopsy findings and causality relationship between death and COVID-19 vaccination: a systematic review, J. Clin. Med, doi:10.3390/jcm10245876
Shakeri, Cicero, Panahi, Mohajeri, Sahebkar, Curcumin: a naturally occurring autophagy modulator, J. Cell. Physiol, doi:10.1002/jcp.27404
Shamloo, Defensor, Ciari, Ogawa, Vidano et al., The anti-inflammatory effects of photobiomodulation are mediated by cytokines: evidence from a mouse model of inflammation, Front. Neurosci
Shang, Liu, Zhu, Lu, Ge et al., SARS-CoV-2 causes mitochondrial dysfunction and mitophagy impairment, Front. Microbiol, doi:10.3389/fmicb.2021.780768
Shen, Hao, Feng, Tian, Chen et al., Lipoamide or lipoic acid stimulates mitochondrial biogenesis in 3T3-L1 adipocytes via the endothelial NO synthase-cGMP-protein kinase G signalling pathway: lipoamide, lipoic acid and mitochondrial biogenesis, Br. J. Pharmacol, doi:10.1111/j.1476-5381.2010.01134.x
Shen, Liu, Tian, Yang, Li et al., R-alpha-lipoic acid and acetyl-L-carnitine complementarily promote mitochondrial biogenesis in murine 3T3-L1 adipocytes, Diabetologia, doi:10.1007/s00125-007-0852-4
Shen, Zhang, Zhao, Wolin, Sessa et al., Nitric oxide production and NO synthase gene expression contribute to vascular regulation during exercise, Med. Sci. Sports Exerc
Shiba-Fukushima, Arano, Matsumoto, Inoshita, Yoshida et al., Phosphorylation of mitochondrial polyubiquitin by PINK1 promotes Parkin mitochondrial tethering, PLoS Genet, doi:10.1371/journal.pgen.1004861
Shirato, Kizaki, SARS-CoV-2 spike protein S1 subunit induces proinflammatory responses via Toll-like receptor 4 signaling in murine and human macrophages, Heliyon, doi:10.1016/j.heliyon.2021.e06187
Shoba, Joy, Joseph, Majeed, Rajendran et al., Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers, Planta Med, doi:10.1055/s-2006-957450
Shripathi Adiga, Adiga, Concept and canons of fasting in Ayurveda, Journal of Nutrition, Fasting and Health, doi:10.22038/jfh.2013.419
Singh, Bharara Singh, S2 subunit of SARS-nCoV-2 interacts with tumor suppressor protein P53 and BRCA: an in silico study, Transl. Oncol, doi:10.1016/j.tranon.2020.100814
Singh, Mohapatra, Tripathi, Targeting mitochondrial biogenesis: a potential approach for preventing and controlling diabetes, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-021-00360-x
Soda, Kano, Sakuragi, Takao, Lefor et al., Long-term oral polyamine intake increases blood polyamine concentrations, J. Nutr. Sci. Vitaminol, doi:10.3177/jnsv.55.361
Soda, Uemura, Sanayama, Igarashi, Fukui, Polyamine-rich diet elevates blood Spermine levels and inhibits pro-inflammatory status: an interventional study, Med Sci, doi:10.3390/medsci9020022
Solaini, Baracca, Lenaz, Sgarbi, Hypoxia and mitochondrial oxidative metabolism, Biochimica et Biophysica Acta (BBA) -Bioenergetics, doi:10.1016/j.bbabio.2010.02.011
Solis, Beccari, Iaconis, Talarico, Ruiz-Bedoya et al., The SARS-CoV-2 spike protein binds and modulates estrogen receptors, Sci. Adv, doi:10.1126/sciadv.add4150
Sotthibundhu, Mcdonagh, Von Kriegsheim, Garcia-Munoz, Klawiter et al., Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells, Stem Cell Res Ther, doi:10.1186/s13287-016-0425-x
Stein, Ramelli, Grazioli, Chung, Singh et al., SARS-CoV-2 infection and persistence in the human body and brain at autopsy, Nature, doi:10.1038/s41586-022-05542-y
Su, Yu, Chen, Huang, Chen et al., Rutin, a flavonoid and principal component of Saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model, Int. J. Med. Sci, doi:10.7150/ijms.8220
Su, Zhang, Gomez, Kellum, Peng, Mitochondria ROS and mitophagy in acute kidney injury, Autophagy, doi:10.1080/15548627.2022.2084862
Sumi, Hamada, Tsushima, Mihara, Muraki, A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese natto; a typical and popular soybean food in the Japanese diet, Experientia, doi:10.1007/BF01956052
Summers, Valentine, Zhang, Wu, SARS-CoV-2 targets the lysosome to mediate airway inflammatory cell death, Front. Physiol, doi:10.1080/15548627.2021.2021496
Sutton, Beyl, Early, Cefalu, Ravussin et al., Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes, Cell Metab, doi:10.1016/j.cmet.2018.04.010
Suzuki, Onodera, Ohsumi, Starvation induced cell death in autophagydefective yeast mutants is caused by mitochondria dysfunction, PLoS One, doi:10.1371/journal.pone.0017412
Swank, Senussi, Manickas-Hill, Yu, Li et al., Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae, Clin. Infect. Dis, doi:10.1093/cid/ciac722
Sánchez-Vicente, Lorenzo, Nitric oxide regulation of temperature acclimation: a molecular genetic perspective, J. Exp. Bot, doi:10.1093/jxb/erab049
Takeshige, Baba, Tsuboi, Noda, Ohsumi, Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction, J. Cell Biol, doi:10.1083/jcb.119.2.301
Tan, Ho, Kalimuddin, Cherng, Teh et al., Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19), Nutrition, doi:10.1016/j.nut.2020.111017
Tanaka, The PINK1-Parkin axis: an overview, Neurosci. Res, doi:10.1016/j.neures.2020.01.006
Tanikawa, Kiba, Yu, Hsu, Chen et al., Degradative effect of nattokinase on spike protein of SARS-CoV-2, Molecules, doi:10.3390/molecules27175405
Temkin, The Falling Sickness: A History of Epilepsy From the Greeks to the Beginnings of Modern Neurology
Tenório, Graciliano, Moura, Oliveira, De et al., N-acetylcysteine (NAC): impacts on human health, Antioxidants, doi:10.3390/antiox10060967
Theoharides, Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome?, Mol. Neurobiol, doi:10.1007/s12035-021-02696-0
Thissen, Ketelslegers, Underwood, Nutritional regulation of the insulin-like growth factors, Endocr. Rev
Tian, Tang, Liu, Li, Chen et al., Populations in lowmagnesium areas were associated with higher risk of infection in COVID-19's Early transmission: a nationwide retrospective cohort study in the United States, Nutrients, doi:10.3390/nu14040909
Tillman, Chen, Bondarenko, Coleman, Xu et al., SARS-CoV-2 spike protein downregulates cell surface α7nAChR through a helical motif in the spike neck, ACS Chem. Neurosci, doi:10.1021/acschemneuro.2c00610
Tosato, Calvani, Picca, Ciciarello, Galluzzo et al., Effects of L-arginine plus vitamin C supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: a single-blind randomized controlled trial, Nutrients, doi:10.3390/nu14234984
Tsai, Hamblin, Biological effects and medical applications of infrared radiation, J. Photochem. Photobiol. B, doi:10.1016/j.jphotobiol.2017.04.014
Tsukada, Ohsumi, Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae, FEBS Lett, doi:10.1016/0014-5793(93)80398-E
Tsutsumi, Yoshida, Nii, Okahisa, Iwata et al., Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle, Nutrition & Metabolism, doi:10.1186/1743-7075-11-32
Tucker, Lu, Zhang, From mitochondrial function to neuroprotection-an emerging role for methylene blue, Mol. Neurobiol, doi:10.1007/s12035-017-0712-2
Vahedian-Azimi, Abbasifard, Rahimi-Bashar, Guest, Majeed et al., Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials, Nutrients, doi:10.3390/nu14020256
Vainshtein, Hood, The regulation of autophagy during exercise in skeletal muscle, J. Appl. Physiol, doi:10.1152/japplphysiol.00550.2015
Valenti, De Rasmo, Signorile, Rossi, De Bari et al., Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down's syndrome, Biochim. Biophys. Acta (BBA) -Mol. Basis Dis, doi:10.1016/j.bbadis.2012.12.011
Vargas, Hamasaki, Kawabata, Youle, Yoshimori, The mechanisms and roles of selective autophagy in mammals, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-022-00542-2
Venegas-Borsellino, Sonikpreet, Martindale, From religion to secularism: the benefits of fasting, Curr Nutr Rep, doi:10.1007/s13668-018-0233-2
Vetrici, Mokmeli, Bohm, Monici, Sigman, Evaluation of adjunctive photobiomodulation (PBMT) for COVID-19 pneumonia via clinical status and pulmonary severity indices in a preliminary trial, J. Inflamm. Res, doi:10.2147/JIR.S301625
Vineetha, Mathews, Nair, Chapter 28 -ascorbic acid and the mitochondria
Visioli, Mucignat-Caretta, Anile, Panaite, Traditional and medical applications of fasting, Nutrients, doi:10.3390/nu14030433
Vollbracht, Kraft, Feasibility of vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV vitamin C on fatigue, Nutrients, doi:10.3390/nu13041154
Vásquez-Reyes, Velázquez-Villegas, Vargas-Castillo, Noriega, Torres et al., Dietary bioactive compounds as modulators of mitochondrial function, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2021.108768
Walski, Dąbrowska, Drohomirecka, Jędruchniewicz, Trochanowska-Pauk et al., The effect of red-to-near-infrared (R/NIR) irradiation on inflammatory processes, Int. J. Radiat. Biol, doi:10.1080/09553002.2019.1625464
Wang, Chen, The effects of sunlight exposure therapy on the improvement of depression and quality of life in post-stroke patients: a RCT study, Heliyon, doi:10.1016/j.heliyon.2020.e04379
Wang, Chen, Zhang, Deng, Lian et al., CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells, Sig Transduct Target Ther, doi:10.1038/s41392-020-00426-x
Wang, Ming, Wang, Cao, Wang et al., Long-term extreme fasting following a traditional Chinese "Bigu" regimen: a preliminary retrospective and prospective cohort study, SSRN Journal, doi:10.2139/ssrn.3237012
Winchester, John, Jabbar, John, Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection, Journal of Infection, doi:10.1016/j.jinf.2021.05.009
Wirth, Benson, Schwarz, Köbe, Grittner et al., The effect of spermidine on memory performance in older adults at risk for dementia: a randomized controlled trial, Cortex, doi:10.1016/j.cortex.2018.09.014
Wissler Gerdes, Vanichkachorn, Verdoorn, Hanson, Joshi et al., Role of senescence in the chronic health consequences of COVID-19, Transl. Res, doi:10.1016/j.trsl.2021.10.003
Yamamoto, Morino, Mengistu, Ishibashi, Kiriyama et al., Amla enhances mitochondrial spare respiratory capacity by increasing mitochondrial biogenesis and antioxidant systems in a murine skeletal muscle cell line, Oxidative Med. Cell. Longev, doi:10.1155/2016/1735841
Yamamoto, Zhang, Mizushima, Autophagy genes in biology and disease, Nat. Rev. Genet, doi:10.1038/s41576-022-00562-w
Yamano, Youle, PINK1 is degraded through the N-end rule pathway, Autophagy, doi:10.4161/auto.24633
Yanagisawa, Chatake, Chiba-Kamoshida, Naito, Ohsugi et al., Purification, crystallization and preliminary X-ray diffraction experiment of nattokinase from Bacillus subtilis natto, Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun, doi:10.1107/S1744309110043137
Yang, Petitjean, Koehler, Zhang, Dumitru et al., Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor, Nat. Commun, doi:10.1038/s41467-020-18319-6
Yang, Zhang, Nakahira, Gu, Mitochondrial DNA mutation, diseases, and nutrient-regulated mitophagy, Annu. Rev. Nutr, doi:10.1146/annurev-nutr-082018-124643
Yella, Yella, Sasanka, Thangaraju, Does methylene blue satisfy an option in COVID-19 ARDS, Infectious Disorders -Drug TargetsDisorders, doi:10.2174/1871526522666220317155947
You, Liang, Han, Guo, Ren et al., Mulberry anthocyanins, cyanidin 3-glucoside and cyanidin 3-rutinoside, increase the quantity of mitochondria during brown adipogenesis, J. Funct. Foods, doi:10.1016/j.jff.2017.07.007
You, Yuan, Lee, Huang, Jin et al., Mulberry and mulberry wine extract increase the number of mitochondria during brown adipogenesis, Food Funct, doi:10.1039/C4FO00719K
Yu, Mcphee, Zheng, Mardones, Rong et al., Termination of autophagy and reformation of lysosomes regulated by mTOR, Nature, doi:10.1038/nature09076
Zauner, Schneeweiss, Kranz, Madl, Ratheiser et al., Resting energy expenditure in short-term starvation is increased as a result of an increase in serum norepinephrine, Am. J. Clin. Nutr
Zhang, Si, Lu, Zhang, Zheng et al., SARS-CoV-2 omicron variant clearance delayed in breakthrough cases with elevated fasting blood glucose, Virol. J, doi:10.1186/s12985-022-01877-0
Zhao, Jaber, Lukiw, SARS-CoV-2, long COVID. prion disease and neurodegeneration, Front Neurosci, doi:10.3389/fnins.2022.1002770
Zhao, Kuang, Li, Zhu, Jia et al., SARS-CoV-2 spike protein interacts with and activates TLR41, Cell Res, doi:10.1038/s41422-021-00495-9
Zhen, Lu, Wang, Vaziri, Zhou, Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species, Am. J. Hypertens, doi:10.1038/ajh.2007.14
Zhong, Sun, Xiao, Yao, Sang et al., A randomized, single-blind, group sequential, activecontrolled study to evaluate the clinical efficacy and safety of α-lipoic acid for critically ill patients with coronavirus disease 2019, doi:10.1038/ajh.2007.14
Zhou, Farah, Sinha, Wu, Singh et al., Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance, PLoS One, doi:10.1371/journal.pone.0087161
Zong, Wei, Ren, Zhang, Zhou, The intersection of COVID-19 and cancer: signaling pathways and treatment implications, Mol. Cancer, doi:10.1186/s12943-021-01363-1
DOI record:
{
"DOI": "10.1016/j.endmts.2024.100163",
"ISSN": [
"2666-3961"
],
"URL": "http://dx.doi.org/10.1016/j.endmts.2024.100163",
"alternative-id": [
"S2666396124000074"
],
"article-number": "100163",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Endocrine and Metabolic Science"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.endmts.2024.100163"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Halma",
"given": "Matthew T.J.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Marik",
"given": "Paul E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saleeby",
"given": "Yusuf M.",
"sequence": "additional"
}
],
"container-title": "Endocrine and Metabolic Science",
"container-title-short": "Endocrine and Metabolic Science",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
30
]
],
"date-time": "2024-01-30T18:03:51Z",
"timestamp": 1706637831000
},
"deposited": {
"date-parts": [
[
2024,
2,
8
]
],
"date-time": "2024-02-08T08:05:14Z",
"timestamp": 1707379514000
},
"indexed": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T00:32:04Z",
"timestamp": 1707438724960
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
25
]
],
"date-time": "2024-01-25T00:00:00Z",
"timestamp": 1706140800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2666396124000074?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2666396124000074?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100163",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
3
]
]
},
"published-print": {
"date-parts": [
[
2024,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"author": "Abramczyk",
"key": "10.1016/j.endmts.2024.100163_bb0005",
"series-title": "Decoding COVID-19 mRNA Vaccine Immunometabolism in Central Nervous System: Human Brain Normal Glial and Glioma Cells by Raman Imaging",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-24386-0",
"article-title": "Effects of hypoxia and reoxygenation on mitochondrial functions and transcriptional profiles of isolated brain and muscle porcine cells",
"author": "Adzigbli",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.endmts.2024.100163_bb0010",
"volume": "12",
"year": "2022"
},
{
"article-title": "Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19",
"author": "Ajaz",
"first-page": "C57",
"journal-title": "Am. J. Phys. Cell Phys.",
"key": "10.1016/j.endmts.2024.100163_bb0015",
"volume": "320",
"year": "2021"
},
{
"DOI": "10.1016/j.ejphar.2020.173494",
"article-title": "Application of methylene blue -vitamin C–N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial",
"author": "Alamdari",
"doi-asserted-by": "crossref",
"journal-title": "Eur. J. Pharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb0020",
"volume": "885",
"year": "2020"
},
{
"DOI": "10.1186/s40842-020-00116-1",
"article-title": "Intermittent fasting: is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians",
"author": "Albosta",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Clinical Diabetes and Endocrinology",
"key": "10.1016/j.endmts.2024.100163_bb0025",
"volume": "7",
"year": "2021"
},
{
"article-title": "Polyamines in foods: development of a food database",
"author": "Ali",
"first-page": "5572",
"journal-title": "Food Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb0030",
"volume": "55",
"year": "2011"
},
{
"DOI": "10.4161/auto.6.6.12376",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb0035",
"unstructured": "Alirezaei, M.; Kemball, C.C.; Flynn, C.T.; Wood, M.R.; Whitton, J.L.; Kiosses, W.B. Short-term fasting induces profound neuronal autophagy. Autophagy 2010, 6, 702–710, doi:https://doi.org/10.4161/auto.6.6.12376."
},
{
"DOI": "10.1007/s00277-022-04907-7",
"article-title": "Hemolytic anemia in COVID-19",
"author": "Al-kuraishy",
"doi-asserted-by": "crossref",
"first-page": "1887",
"journal-title": "Ann. Hematol.",
"key": "10.1016/j.endmts.2024.100163_bb0040",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1002/advs.202101222",
"article-title": "Combined metabolic activators accelerates recovery in mild-to-moderate COVID-19",
"author": "Altay",
"doi-asserted-by": "crossref",
"journal-title": "Adv. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0045",
"volume": "8",
"year": "2021"
},
{
"article-title": "Association between the expression of toll-like receptors, cytokines, and homeostatic chemokines in SARS-CoV-2 infection and COVID-19 severity",
"author": "Alturaiki",
"journal-title": "Heliyon",
"key": "10.1016/j.endmts.2024.100163_bb0050",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1021/mp700113r",
"article-title": "Bioavailability of curcumin: problems and promises",
"author": "Anand",
"doi-asserted-by": "crossref",
"first-page": "807",
"journal-title": "Mol. Pharm.",
"key": "10.1016/j.endmts.2024.100163_bb0055",
"volume": "4",
"year": "2007"
},
{
"DOI": "10.3389/fimmu.2023.1080043",
"article-title": "Gut and airway microbiota dysbiosis and their role in COVID-19 and long-COVID",
"author": "Ancona",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.endmts.2024.100163_bb0060",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1002/oby.22065",
"article-title": "Flipping the metabolic switch: understanding and applying the health benefits of fasting",
"author": "Anton",
"doi-asserted-by": "crossref",
"first-page": "254",
"journal-title": "Obesity (Silver Spring)",
"key": "10.1016/j.endmts.2024.100163_bb0065",
"volume": "26",
"year": "2018"
},
{
"DOI": "10.1016/j.freeradbiomed.2018.11.005",
"article-title": "Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid",
"author": "Aparicio-Trejo",
"doi-asserted-by": "crossref",
"first-page": "379",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0070",
"volume": "130",
"year": "2019"
},
{
"DOI": "10.1007/s00018-007-7188-6",
"article-title": "To be, or not to be — molecular chaperones in protein degradation",
"author": "Arndt",
"doi-asserted-by": "crossref",
"first-page": "2525",
"journal-title": "Cell. Mol. Life Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0075",
"volume": "64",
"year": "2007"
},
{
"DOI": "10.1080/23744235.2021.1945675",
"article-title": "N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study",
"author": "Assimakopoulos",
"doi-asserted-by": "crossref",
"first-page": "847",
"journal-title": "Infect. Dis. Ther.",
"key": "10.1016/j.endmts.2024.100163_bb0080",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1016/j.coph.2007.04.005",
"article-title": "N-acetylcysteine—a safe antidote for cysteine/glutathione deficiency",
"author": "Atkuri",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Curr. Opin. Pharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb0085",
"volume": "7",
"year": "2007"
},
{
"DOI": "10.3390/nu13051570",
"article-title": "Fasting: how to guide",
"author": "Attinà",
"doi-asserted-by": "crossref",
"first-page": "1570",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb0090",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1042/CS20210735",
"article-title": "The SARS-CoV-2 spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease",
"author": "Avolio",
"doi-asserted-by": "crossref",
"first-page": "2667",
"journal-title": "Clin. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0095",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.1016/j.molstruc.2022.134642",
"article-title": "Glycyrrhizin as a promising kryptonite against SARS-CoV-2: clinical, experimental, and theoretical evidences",
"author": "Banerjee",
"doi-asserted-by": "crossref",
"journal-title": "J. Mol. Struct.",
"key": "10.1016/j.endmts.2024.100163_bb0100",
"volume": "1275",
"year": "2023"
},
{
"DOI": "10.3390/nu13020463",
"article-title": "Magnesium in aging, health and diseases",
"author": "Barbagallo",
"doi-asserted-by": "crossref",
"first-page": "463",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb0105",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1093/eurjpc/zwac019",
"article-title": "Effects of exercise rehabilitation in patients with long coronavirus disease 2019",
"author": "Barbara",
"doi-asserted-by": "crossref",
"first-page": "e258",
"journal-title": "Eur. J. Prev. Cardiol.",
"key": "10.1016/j.endmts.2024.100163_bb0110",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1016/j.tins.2022.02.006",
"article-title": "The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2",
"author": "Bauer",
"doi-asserted-by": "crossref",
"first-page": "358",
"journal-title": "Trends Neurosci.",
"key": "10.1016/j.endmts.2024.100163_bb0115",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1098/rsbl.2015.0073",
"article-title": "Near-infrared light increases ATP, extends lifespan and improves mobility in aged drosophila melanogaster",
"author": "Begum",
"doi-asserted-by": "crossref",
"journal-title": "Biol. Lett.",
"key": "10.1016/j.endmts.2024.100163_bb0120",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.3390/biomedicines11020451",
"article-title": "Immune response and molecular mechanisms of cardiovascular adverse effects of spike proteins from SARS-CoV-2 and mRNA vaccines",
"author": "Bellavite",
"doi-asserted-by": "crossref",
"first-page": "451",
"journal-title": "Biomedicines",
"key": "10.1016/j.endmts.2024.100163_bb0125",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1146/annurev-biochem-060815-014556",
"article-title": "Mammalian autophagy: how does it work?",
"author": "Bento",
"doi-asserted-by": "crossref",
"first-page": "685",
"journal-title": "Annu. Rev. Biochem.",
"key": "10.1016/j.endmts.2024.100163_bb0130",
"volume": "85",
"year": "2016"
},
{
"DOI": "10.1016/j.nbd.2015.10.011",
"article-title": "Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity",
"author": "Bertholet",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Neurobiol. Dis.",
"key": "10.1016/j.endmts.2024.100163_bb0135",
"volume": "90",
"year": "2016"
},
{
"DOI": "10.3390/covid1010002",
"article-title": "Efficacy of a polyphenolic, standardized green tea extract for the treatment of COVID-19 syndrome: a proof-of-principle study",
"author": "Bettuzzi",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "COVID",
"key": "10.1016/j.endmts.2024.100163_bb0140",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.3390/ijms231810436",
"article-title": "SARS-CoV-2 spike proteins and cell-cell communication inhibits TFPI and induces thrombogenic factors in human lung microvascular endothelial cells and neutrophils: implications for COVID-19 coagulopathy pathogenesis",
"author": "Bhargavan",
"doi-asserted-by": "crossref",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0145",
"volume": "23",
"year": "2022"
},
{
"article-title": "Rationale for nicotinamide adenine dinucleotide (NAD+) metabolome disruption as a pathogenic mechanism of post-acute COVID-19 syndrome",
"author": "Block",
"journal-title": "Clin Med Insights Pathol",
"key": "10.1016/j.endmts.2024.100163_bb0150",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/bs.pmbts.2015.07.016",
"article-title": "Chapter six - endurance exercise and the regulation of skeletal muscle metabolism",
"author": "Booth",
"doi-asserted-by": "crossref",
"first-page": "129",
"key": "10.1016/j.endmts.2024.100163_bb0155",
"series-title": "Progress in Molecular Biology and Translational Science",
"volume": "vol. 135",
"year": "2015"
},
{
"DOI": "10.1016/j.ijheh.2005.03.003",
"article-title": "Daily time spent indoors in German homes – baseline data for the assessment of indoor exposure of German occupants",
"author": "Brasche",
"doi-asserted-by": "crossref",
"first-page": "247",
"journal-title": "Int. J. Hyg. Environ. Health",
"key": "10.1016/j.endmts.2024.100163_bb0160",
"volume": "208",
"year": "2005"
},
{
"DOI": "10.1038/s41581-020-00381-4",
"article-title": "The COVID-19 pandemic: consequences for nephrology",
"author": "Bruchfeld",
"doi-asserted-by": "crossref",
"first-page": "81",
"journal-title": "Nat. Rev. Nephrol.",
"key": "10.1016/j.endmts.2024.100163_bb0165",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/j.nbd.2020.105131",
"article-title": "The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier",
"author": "Buzhdygan",
"doi-asserted-by": "crossref",
"journal-title": "Neurobiol. Dis.",
"key": "10.1016/j.endmts.2024.100163_bb0170",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1126/science.abe4832",
"article-title": "Senolytics reduce coronavirus-related mortality in old mice",
"author": "Camell",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "10.1016/j.endmts.2024.100163_bb0175",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.3390/biom12111646",
"article-title": "Possible application of melatonin in long COVID",
"author": "Cardinali",
"doi-asserted-by": "crossref",
"first-page": "1646",
"journal-title": "Biomolecules",
"key": "10.1016/j.endmts.2024.100163_bb0180",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/nu9111211",
"article-title": "Vitamin C and immune function",
"author": "Carr",
"doi-asserted-by": "crossref",
"first-page": "1211",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb0185",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1002/oby.21581",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb0190",
"unstructured": "Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883."
},
{
"DOI": "10.3390/jcm11082228",
"article-title": "Exercise training in post-COVID-19 patients: the need for a multifactorial protocol for a multifactorial pathophysiology",
"author": "Cattadori",
"doi-asserted-by": "crossref",
"first-page": "2228",
"journal-title": "J. Clin. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0195",
"volume": "11",
"year": "2022"
},
{
"article-title": "A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications",
"author": "Changeux",
"first-page": "33",
"journal-title": "C. R. Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0200",
"volume": "343",
"year": "2020"
},
{
"DOI": "10.1007/s11130-022-00954-7",
"article-title": "Resveratrol induces the fasting state and alters circadian metabolism in hepatocytes",
"author": "Chatam",
"doi-asserted-by": "crossref",
"first-page": "128",
"journal-title": "Plant Foods Hum. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb0205",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1002/rmv.2427",
"article-title": "Quercetin for the treatment of COVID-19 patients: a systematic review and meta-analysis",
"author": "Cheema",
"doi-asserted-by": "crossref",
"journal-title": "Rev. Med. Virol.",
"key": "10.1016/j.endmts.2024.100163_bb0210",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1177/1177271918785130",
"article-title": "Nattokinase: a promising alternative in prevention and treatment of cardiovascular diseases",
"author": "Chen",
"doi-asserted-by": "crossref",
"journal-title": "Biomark. Insights",
"key": "10.1016/j.endmts.2024.100163_bb0215",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1080/09637486.2020.1754351",
"article-title": "Effect of resveratrol on intestinal tight junction proteins and the gut microbiome in high-fat diet-fed insulin resistant mice",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "965",
"journal-title": "Int. J. Food Sci. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb0220",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1111/jpi.12774",
"article-title": "Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer’s disease",
"author": "Chen",
"doi-asserted-by": "crossref",
"journal-title": "J. Pineal Res.",
"key": "10.1016/j.endmts.2024.100163_bb0225",
"volume": "71",
"year": "2021"
},
{
"DOI": "10.1016/j.stem.2014.04.014",
"article-title": "Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "810",
"journal-title": "Cell Stem Cell",
"key": "10.1016/j.endmts.2024.100163_bb0230",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1016/j.kint.2020.03.005",
"article-title": "Kidney disease is associated with in-hospital death of patients with COVID-19",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "829",
"journal-title": "Kidney Int.",
"key": "10.1016/j.endmts.2024.100163_bb0235",
"volume": "97",
"year": "2020"
},
{
"article-title": "Natural phytochemicals that affect autophagy in the treatment of oral diseases and infections: a review",
"author": "Cheng",
"first-page": "13",
"journal-title": "Front. Pharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb0240",
"year": "2022"
},
{
"DOI": "10.1155/2021/4946711",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb0245",
"unstructured": "Chodari, L.; Dilsiz Aytemir, M.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting mitochondrial biogenesis with polyphenol compounds. Oxidative Med. Cell. Longev. 2021, 2021, e4946711, doi:https://doi.org/10.1155/2021/4946711."
},
{
"DOI": "10.3346/jkms.2021.36.e286",
"article-title": "Myocarditis-induced sudden death after BNT162b2 mRNA COVID-19 vaccination in Korea: case report focusing on histopathological findings",
"author": "Choi",
"doi-asserted-by": "crossref",
"journal-title": "J. Korean Med. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0250",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1016/j.bbrc.2022.07.113",
"article-title": "SARS-CoV-2 spike S1 subunit protein-mediated increase of beta-secretase 1 (BACE1) impairs human brain vessel cells",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "66",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.endmts.2024.100163_bb0255",
"volume": "626",
"year": "2022"
},
{
"DOI": "10.1186/s40170-015-0130-8",
"author": "Chourasia",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Mitophagy and cancer. Cancer & Metabolism",
"key": "10.1016/j.endmts.2024.100163_bb0260",
"volume": "3",
"year": "2015"
},
{
"DOI": "10.1038/ijo.2015.154",
"article-title": "Effect of extended morning fasting upon ad libitum lunch intake and associated metabolic and hormonal responses in obese adults",
"author": "Chowdhury",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "Int. J. Obes.",
"key": "10.1016/j.endmts.2024.100163_bb0265",
"volume": "40",
"year": "2016"
},
{
"DOI": "10.3390/ph15050621",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb0270",
"unstructured": "Chuang, S.-T.; Papp, H.; Kuczmog, A.; Eells, R.; Condor Capcha, J.M.; Shehadeh, L.A.; Jakab, F.; Buchwald, P. Methylene blue is a nonspecific protein–protein interaction inhibitor with potential for repurposing as an antiviral for COVID-19. Pharmaceuticals 2022, 15, 621, doi:https://doi.org/10.3390/ph15050621."
},
{
"DOI": "10.20463/jenb.2017.0020",
"article-title": "The effects of exercise and cold exposure on mitochondrial biogenesis in skeletal muscle and white adipose tissue",
"author": "Chung",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "J. Exerc. Nutr. Biochem.",
"key": "10.1016/j.endmts.2024.100163_bb0275",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.3322/caac.21694",
"article-title": "Intermittent fasting in the prevention and treatment of cancer",
"author": "Clifton",
"doi-asserted-by": "crossref",
"first-page": "527",
"journal-title": "CA Cancer J. Clin.",
"key": "10.1016/j.endmts.2024.100163_bb0280",
"volume": "71",
"year": "2021"
},
{
"DOI": "10.1007/s11481-021-10015-6",
"article-title": "Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: implications for neuro-COVID",
"author": "Clough",
"doi-asserted-by": "crossref",
"first-page": "770",
"journal-title": "J. NeuroImmune Pharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb0285",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/S1382-6689(01)00077-1",
"article-title": "The interplay of glutathione-related processes in antioxidant defense",
"author": "Cnubben",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Environ. Toxicol. Pharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb0290",
"volume": "10",
"year": "2001"
},
{
"article-title": "Native American medicine",
"author": "Cohen",
"first-page": "45",
"journal-title": "Altern. Ther. Health Med.",
"key": "10.1016/j.endmts.2024.100163_bb0295",
"volume": "4",
"year": "1998"
},
{
"DOI": "10.3390/ijms231810881",
"article-title": "Understanding the pharmacology of COVID-19 mRNA vaccines: playing dice with the spike?",
"author": "Cosentino",
"doi-asserted-by": "crossref",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0300",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.mce.2012.04.009",
"article-title": "Role of melatonin in the regulation of autophagy and mitophagy: a review",
"author": "Coto-Montes",
"doi-asserted-by": "crossref",
"first-page": "12",
"journal-title": "Mol. Cell. Endocrinol.",
"key": "10.1016/j.endmts.2024.100163_bb0305",
"volume": "361",
"year": "2012"
},
{
"DOI": "10.1126/science.368.6490.455",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb0310",
"unstructured": "Couzin-Frankel, J. The mystery of the pandemic's ‘happy hypoxia.’ Science 2020, 368, 455–456, doi:https://doi.org/10.1126/science.368.6490.455."
},
{
"DOI": "10.1016/S1440-2440(99)80172-8",
"article-title": "Physiological determinants of endurance exercise performance",
"author": "Coyle",
"doi-asserted-by": "crossref",
"first-page": "181",
"journal-title": "J. Sci. Med. Sport",
"key": "10.1016/j.endmts.2024.100163_bb0315",
"volume": "2",
"year": "1999"
},
{
"article-title": "Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation",
"author": "Cuervo",
"first-page": "C1200",
"journal-title": "Am. J. Phys. Cell Phys.",
"key": "10.1016/j.endmts.2024.100163_bb0320",
"volume": "269",
"year": "1995"
},
{
"DOI": "10.1016/j.biopha.2021.112023",
"article-title": "Repurposing methylene blue in the management of COVID-19: mechanistic aspects and clinical investigations",
"author": "Dabholkar",
"doi-asserted-by": "crossref",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.endmts.2024.100163_bb0325",
"volume": "142",
"year": "2021"
},
{
"DOI": "10.1515/hsz-2014-0164",
"article-title": "Inflammatory outcomes of apoptosis",
"author": "Davidovich",
"doi-asserted-by": "crossref",
"first-page": "1163",
"journal-title": "necrosis and necroptosis. Biol Chem",
"key": "10.1016/j.endmts.2024.100163_bb0330",
"volume": "395",
"year": "2014"
},
{
"DOI": "10.1111/acel.13727",
"article-title": "COVID-19 and neurodegeneration: the mitochondrial connection",
"author": "Denaro",
"doi-asserted-by": "crossref",
"journal-title": "Aging Cell",
"key": "10.1016/j.endmts.2024.100163_bb0335",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1007/s11481-021-10029-0",
"article-title": "SARS-CoV-2 spike protein disrupts blood–brain barrier integrity via RhoA activation",
"author": "DeOre",
"doi-asserted-by": "crossref",
"first-page": "722",
"journal-title": "J. NeuroImmune Pharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb0340",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.3390/v15051045",
"article-title": "Molecular mimicry of the viral spike in the SARS-CoV-2 vaccine possibly triggers transient dysregulation of ACE2, leading to vascular and coagulation dysfunction similar to SARS-CoV-2 infection",
"author": "Devaux",
"doi-asserted-by": "crossref",
"first-page": "1045",
"journal-title": "Viruses",
"key": "10.1016/j.endmts.2024.100163_bb0345",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.2119/molmed.2015.00038",
"article-title": "Methylene blue reduces acute cerebral ischemic injury via the induction of mitophagy",
"author": "Di",
"doi-asserted-by": "crossref",
"first-page": "420",
"journal-title": "Mol. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0350",
"volume": "21",
"year": "2015"
},
{
"DOI": "10.1002/JLB.3MA0322-279RRR",
"article-title": "Loss of mitochondrial membrane potential (ΔΨm) in leucocytes as post-COVID-19 sequelae",
"author": "Díaz-Resendiz",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "J. Leukoc. Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0355",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.3390/md20020099",
"article-title": "Effect of fucoidan on the mitochondrial membrane potential (ΔΨm) of leukocytes from patients with active COVID-19 and subjects that recovered from SARS-CoV-2 infection",
"author": "Díaz-Resendiz",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Mar. Drugs",
"key": "10.1016/j.endmts.2024.100163_bb0360",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.4161/auto.4144",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb0365",
"unstructured": "Dice, J.F. Chaperone-mediated autophagy. Autophagy 2007, 3, 295–299, doi:https://doi.org/10.4161/auto.4144."
},
{
"DOI": "10.1055/a-1899-8862",
"article-title": "Serum calcium, magnesium, and phosphorus levels in patients with COVID-19: relationships with poor outcome and mortality",
"author": "Díez",
"doi-asserted-by": "crossref",
"first-page": "31",
"journal-title": "Horm. Metab. Res.",
"key": "10.1016/j.endmts.2024.100163_bb0370",
"volume": "55",
"year": "2023"
},
{
"DOI": "10.3390/molecules27082376",
"article-title": "The importance of nutraceuticals in COVID-19: what’s the role of resveratrol?",
"author": "Domi",
"doi-asserted-by": "crossref",
"first-page": "2376",
"journal-title": "Molecules",
"key": "10.1016/j.endmts.2024.100163_bb0375",
"volume": "27",
"year": "2022"
},
{
"article-title": "Time-activity-patterns of some selected small groups as a basis for exposure estimation: a methodological study",
"author": "Dörre",
"first-page": "471",
"journal-title": "J. Expo. Anal. Environ. Epidemiol.",
"key": "10.1016/j.endmts.2024.100163_bb0380",
"volume": "7",
"year": "1997"
},
{
"DOI": "10.1038/s12276-019-0245-z",
"article-title": "Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis",
"author": "Dusabimana",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Exp. Mol. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0385",
"volume": "51",
"year": "2019"
},
{
"DOI": "10.1007/s13304-013-0242-z",
"article-title": "Preoperative fasting: current practice and areas for improvement",
"author": "Falconer",
"doi-asserted-by": "crossref",
"first-page": "31",
"journal-title": "Updat. Surg.",
"key": "10.1016/j.endmts.2024.100163_bb0390",
"volume": "66",
"year": "2014"
},
{
"DOI": "10.1177/014107689809100505",
"article-title": "Medical implications of controlled fasting",
"author": "Fazel",
"doi-asserted-by": "crossref",
"first-page": "260",
"journal-title": "J. R. Soc. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0395",
"volume": "91",
"year": "1998"
},
{
"article-title": "The multifaceted role of nutrient sensing and mTORC1 signaling in physiology and aging",
"author": "Fernandes",
"first-page": "2",
"journal-title": "Frontiers in Aging",
"key": "10.1016/j.endmts.2024.100163_bb0400",
"year": "2021"
},
{
"DOI": "10.1016/j.bbalip.2014.12.013",
"article-title": "α-Lipoic acid treatment increases mitochondrial biogenesis and promotes beige adipose features in subcutaneous adipocytes from overweight/obese subjects",
"author": "Fernández-Galilea",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "Biochim. Biophys. Acta",
"key": "10.1016/j.endmts.2024.100163_bb0405",
"volume": "1851",
"year": "2015"
},
{
"DOI": "10.1002/mc.22711",
"article-title": "The protein restriction mimetic resveratrol is an autophagy inducer stronger than amino acid starvation in ovarian cancer cells",
"author": "Ferraresi",
"doi-asserted-by": "crossref",
"first-page": "2681",
"journal-title": "Mol. Carcinog.",
"key": "10.1016/j.endmts.2024.100163_bb0410",
"volume": "56",
"year": "2017"
},
{
"DOI": "10.3390/ijms23116075",
"article-title": "The potential of epigallocatechin Gallate (EGCG) in targeting autophagy for cancer treatment: a narrative review",
"author": "Ferrari",
"doi-asserted-by": "crossref",
"first-page": "6075",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0415",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1074/jbc.M502456200",
"article-title": "Ketone bodies stimulate chaperone-mediated autophagy",
"author": "Finn",
"doi-asserted-by": "crossref",
"first-page": "25864",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.endmts.2024.100163_bb0420",
"volume": "280",
"year": "2005"
},
{
"DOI": "10.1016/j.fct.2014.03.017",
"article-title": "The effects of bioactive compounds from plant foods on mitochondrial function: a focus on apoptotic mechanisms",
"author": "Forbes-Hernández",
"doi-asserted-by": "crossref",
"first-page": "154",
"journal-title": "Food Chem. Toxicol.",
"key": "10.1016/j.endmts.2024.100163_bb0425",
"volume": "68",
"year": "2014"
},
{
"article-title": "The SARS-CoV-2 S1 spike protein promotes MAPK and NF-kB activation in human lung cells and inflammatory cytokine production in human lung and intestinal epithelial cells",
"author": "Forsyth",
"first-page": "10",
"journal-title": "Microorganisms",
"key": "10.1016/j.endmts.2024.100163_bb0430",
"volume": "2022",
"year": "1996"
},
{
"DOI": "10.1038/s41596-022-00734-2",
"article-title": "Determining small-molecule permeation through lipid membranes",
"author": "Frallicciardi",
"doi-asserted-by": "crossref",
"first-page": "2620",
"journal-title": "Nat. Protoc.",
"key": "10.1016/j.endmts.2024.100163_bb0435",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1186/1475-2891-9-51",
"article-title": "Changing perceptions of hunger on a high nutrient density diet",
"author": "Fuhrman",
"doi-asserted-by": "crossref",
"first-page": "51",
"journal-title": "Nutr. J.",
"key": "10.1016/j.endmts.2024.100163_bb0440",
"volume": "9",
"year": "2010"
},
{
"DOI": "10.1006/bbrc.1993.2624",
"article-title": "Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan",
"author": "Fujita",
"doi-asserted-by": "crossref",
"first-page": "1340",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.endmts.2024.100163_bb0445",
"volume": "197",
"year": "1993"
},
{
"article-title": "Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin",
"author": "Furmli",
"journal-title": "Case Reports",
"key": "10.1016/j.endmts.2024.100163_bb0450",
"volume": "2018",
"year": "2018"
},
{
"DOI": "10.1080/15548627.2015.1106666",
"article-title": "Activation of chaperone-mediated autophagy as a potential anticancer therapy",
"author": "Galan-Acosta",
"doi-asserted-by": "crossref",
"first-page": "2370",
"journal-title": "Autophagy",
"key": "10.1016/j.endmts.2024.100163_bb0455",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1038/nchembio.2287",
"article-title": "The pharmacological regulation of cellular mitophagy",
"author": "Georgakopoulos",
"doi-asserted-by": "crossref",
"first-page": "136",
"journal-title": "Nat. Chem. Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0460",
"volume": "13",
"year": "2017"
},
{
"DOI": "10.1002/jmv.27684",
"article-title": "Gut dysbiosis and long COVID-19: feeling gutted",
"author": "Giannos",
"doi-asserted-by": "crossref",
"first-page": "2917",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.endmts.2024.100163_bb0465",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.5858/arpa.2021-0435-SA",
"article-title": "Autopsy histopathologic cardiac findings in 2 adolescents following the second COVID-19 vaccine dose",
"author": "Gill",
"doi-asserted-by": "crossref",
"first-page": "925",
"journal-title": "Arch. Pathol. Lab. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0470",
"volume": "146",
"year": "2022"
},
{
"DOI": "10.1177/0269881109359098",
"article-title": "CNS toxicity involving methylene blue: the exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity",
"author": "Gillman",
"doi-asserted-by": "crossref",
"first-page": "429",
"journal-title": "J. Psychopharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb0475",
"volume": "25",
"year": "2011"
},
{
"DOI": "10.1080/15548627.2015.1063768",
"article-title": "Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury",
"author": "Godar",
"doi-asserted-by": "crossref",
"first-page": "1537",
"journal-title": "Autophagy",
"key": "10.1016/j.endmts.2024.100163_bb0480",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1007/s10787-022-00939-7",
"article-title": "Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial",
"author": "Gomaa",
"doi-asserted-by": "crossref",
"first-page": "477",
"journal-title": "Inflammopharmacol",
"key": "10.1016/j.endmts.2024.100163_bb0485",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1007/s10787-022-01062-3",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb0490",
"unstructured": "Gomaa, A.A.; Abdel-Wadood, Y.A.; Gomaa, M.A. Glycyrrhizin and boswellic acids, the golden nutraceuticals: multitargeting for treatment of mild–moderate COVID-19 and prevention of post-COVID cognitive impairment. Inflammopharmacol 2022b, 30, 1977–1992, doi:https://doi.org/10.1007/s10787-022-01062-3."
},
{
"DOI": "10.1016/j.nut.2019.05.005",
"article-title": "Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: a systematic review",
"author": "Gomes",
"doi-asserted-by": "crossref",
"first-page": "192",
"journal-title": "Nutrition",
"key": "10.1016/j.endmts.2024.100163_bb0495",
"volume": "66",
"year": "2019"
},
{
"DOI": "10.1042/BSR20210611",
"article-title": "SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19",
"author": "Grobbelaar",
"doi-asserted-by": "crossref",
"journal-title": "Biosci. Rep.",
"key": "10.1016/j.endmts.2024.100163_bb0500",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.3389/fnut.2023.1195270",
"article-title": "Improvements during long-term fasting in patients with long COVID – a case series and literature review",
"author": "Grundler",
"doi-asserted-by": "crossref",
"journal-title": "Front. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb0505",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1016/j.biocel.2004.04.020",
"article-title": "Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease",
"author": "Grune",
"doi-asserted-by": "crossref",
"first-page": "2519",
"journal-title": "Int. J. Biochem. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0510",
"volume": "36",
"year": "2004"
},
{
"DOI": "10.3390/nu14091686",
"article-title": "Magnesium-to-calcium ratio and mortality from COVID-19",
"author": "Guerrero-Romero",
"doi-asserted-by": "crossref",
"first-page": "1686",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb0515",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/metabo12111026",
"article-title": "Signatures of mitochondrial dysfunction and impaired fatty acid metabolism in plasma of patients with post-acute sequelae of COVID-19 (PASC)",
"author": "Guntur",
"doi-asserted-by": "crossref",
"first-page": "1026",
"journal-title": "Metabolites",
"key": "10.1016/j.endmts.2024.100163_bb0520",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1210/clinem/dgaa644",
"article-title": "Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "64",
"journal-title": "J. Clin. Endocrinol. Metab.",
"key": "10.1016/j.endmts.2024.100163_bb0525",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1021/acs.jafc.8b00364",
"article-title": "Structural modification of (−)-epigallocatechin gallate (EGCG) shows significant enhancement in mitochondrial biogenesis",
"author": "Ha",
"doi-asserted-by": "crossref",
"first-page": "3850",
"journal-title": "J. Agric. Food Chem.",
"key": "10.1016/j.endmts.2024.100163_bb0530",
"volume": "66",
"year": "2018"
},
{
"DOI": "10.3389/fmicb.2022.1007081",
"article-title": "Activation of TLR4 by viral glycoproteins: a double-edged sword?",
"author": "Halajian",
"doi-asserted-by": "crossref",
"journal-title": "Front. Microbiol.",
"key": "10.1016/j.endmts.2024.100163_bb0535",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/j6020017",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb0540",
"unstructured": "Halma, M.T.J.; Rose, J.; Lawrie, T. The novelty of mRNA viral vaccines and potential harms: a scoping review. J 2023a, 6, 220–235, doi:https://doi.org/10.3390/j6020017."
},
{
"DOI": "10.3390/microorganisms11051308",
"article-title": "Strategies for the management of spike protein-related pathology",
"author": "Halma",
"doi-asserted-by": "crossref",
"first-page": "1308",
"journal-title": "Microorganisms",
"key": "10.1016/j.endmts.2024.100163_bb0545",
"volume": "11",
"year": "2023"
},
{
"article-title": "Methylene blue for treatment of hospitalized COVID-19 patients: a randomized, controlled, open-label clinical trial, phase 2",
"author": "Hamidi-Alamdari",
"first-page": "190",
"journal-title": "Rev. Investig. Clin.",
"key": "10.1016/j.endmts.2024.100163_bb0550",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.metabol.2015.07.010",
"article-title": "Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels",
"author": "Hamidie",
"doi-asserted-by": "crossref",
"first-page": "1334",
"journal-title": "Metab. Clin. Exp.",
"key": "10.1016/j.endmts.2024.100163_bb0555",
"volume": "64",
"year": "2015"
},
{
"DOI": "10.1016/j.jnutbio.2009.03.012",
"article-title": "Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes",
"author": "Hao",
"doi-asserted-by": "crossref",
"first-page": "634",
"journal-title": "J. Nutr. Biochem.",
"key": "10.1016/j.endmts.2024.100163_bb0560",
"volume": "21",
"year": "2010"
},
{
"DOI": "10.1038/nature10317",
"article-title": "Molecular chaperones in protein folding and proteostasis",
"author": "Hartl",
"doi-asserted-by": "crossref",
"first-page": "324",
"journal-title": "Nature",
"key": "10.1016/j.endmts.2024.100163_bb0565",
"volume": "475",
"year": "2011"
},
{
"DOI": "10.1038/ijo.2010.171",
"article-title": "The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women",
"author": "Harvie",
"doi-asserted-by": "crossref",
"first-page": "714",
"journal-title": "Int. J. Obes.",
"key": "10.1016/j.endmts.2024.100163_bb0570",
"volume": "35",
"year": "2011"
},
{
"DOI": "10.1017/S0007114513000792",
"article-title": "The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women",
"author": "Harvie",
"doi-asserted-by": "crossref",
"first-page": "1534",
"journal-title": "Br. J. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb0575",
"volume": "110",
"year": "2013"
},
{
"DOI": "10.1016/j.biocel.2014.05.012",
"article-title": "Regulation by mitophagy",
"author": "Hattori",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "Int. J. Biochem. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0580",
"volume": "53",
"year": "2014"
},
{
"DOI": "10.1038/s41598-022-13399-4",
"article-title": "Post-exposure persistence of nitric oxide upregulation in skin cells irradiated by UV-A",
"author": "Hazell",
"doi-asserted-by": "crossref",
"first-page": "9465",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.endmts.2024.100163_bb0585",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1038/nature10758",
"article-title": "Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "511",
"journal-title": "Nature",
"key": "10.1016/j.endmts.2024.100163_bb0590",
"volume": "481",
"year": "2012"
},
{
"DOI": "10.3389/fnut.2023.1048230",
"article-title": "Intermittent fasting and immunomodulatory effects: a systematic review",
"author": "He",
"doi-asserted-by": "crossref",
"journal-title": "Frontiers in Nutrition",
"key": "10.1016/j.endmts.2024.100163_bb0595",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1097/CM9.0000000000001165",
"article-title": "Electrical stimulation induces mitochondrial autophagy via activating oxidative stress and Sirt3 signaling pathway",
"author": "He-Ling",
"doi-asserted-by": "crossref",
"first-page": "628",
"journal-title": "Chin. Med. J.",
"key": "10.1016/j.endmts.2024.100163_bb0600",
"volume": "134",
"year": "2021"
},
{
"article-title": "Vitamin C and COVID-19",
"author": "Hemilä",
"first-page": "7",
"journal-title": "Front. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0605",
"year": "2021"
},
{
"DOI": "10.1007/s12263-014-0451-1",
"article-title": "In vivo effects of dietary quercetin and quercetin-rich red onion extract on skeletal muscle mitochondria, metabolism, and insulin sensitivity",
"author": "Henagan",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Genes Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb0610",
"volume": "10",
"year": "2014"
},
{
"article-title": "A cohort of cancer patients with no reported cases of SARS-CoV-2 infection: the possible preventive role of methylene blue",
"author": "Henry",
"first-page": "888",
"journal-title": "Substantia",
"key": "10.1016/j.endmts.2024.100163_bb0615",
"year": "2020"
},
{
"DOI": "10.1016/j.molcel.2015.08.016",
"article-title": "The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy",
"author": "Heo",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Mol. Cell",
"key": "10.1016/j.endmts.2024.100163_bb0620",
"volume": "60",
"year": "2015"
},
{
"DOI": "10.3390/foods6100092",
"article-title": "Curcumin: a review of its effects on human health",
"author": "Hewlings",
"doi-asserted-by": "crossref",
"first-page": "92",
"journal-title": "Foods",
"key": "10.1016/j.endmts.2024.100163_bb0625",
"volume": "6",
"year": "2017"
},
{
"DOI": "10.1038/srep03708",
"article-title": "Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis",
"author": "Hiramitsu",
"doi-asserted-by": "crossref",
"first-page": "3708",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.endmts.2024.100163_bb0630",
"volume": "4",
"year": "2014"
},
{
"DOI": "10.1172/JCI113450",
"article-title": "Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man",
"author": "Ho",
"doi-asserted-by": "crossref",
"first-page": "968",
"journal-title": "J. Clin. Invest.",
"key": "10.1016/j.endmts.2024.100163_bb0635",
"volume": "81",
"year": "1988"
},
{
"DOI": "10.2105/AJPH.2008.134627",
"article-title": "The open-air treatment of pandemic influenza",
"author": "Hobday",
"doi-asserted-by": "crossref",
"first-page": "S236",
"journal-title": "Am. J. Public Health",
"key": "10.1016/j.endmts.2024.100163_bb0640",
"volume": "99",
"year": "2009"
},
{
"DOI": "10.1093/jaarel/LXIII.3.465",
"article-title": "Eating and fasting for god in Sufi tradition",
"author": "Hoffman",
"doi-asserted-by": "crossref",
"first-page": "465",
"journal-title": "J. Am. Acad. Relig.",
"key": "10.1016/j.endmts.2024.100163_bb0645",
"volume": "63",
"year": "1995"
},
{
"DOI": "10.1016/B978-0-12-809965-0.00004-5",
"ISBN": "http://id.crossref.org/isbn/9780128099650",
"article-title": "Chapter 4 - photobiology of vitamin D",
"author": "Holick",
"doi-asserted-by": "crossref",
"first-page": "45",
"isbn-type": "print",
"key": "10.1016/j.endmts.2024.100163_bb0650",
"series-title": "Vitamin D",
"year": "2018"
},
{
"DOI": "10.1136/bmjnph-2022-000462",
"article-title": "Association of periodic fasting with lower severity of COVID-19 outcomes in the SARS-CoV-2 prevaccine era: an observational cohort from the INSPIRE Registry",
"author": "Horne",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "BMJ Nutr Prev Health",
"key": "10.1016/j.endmts.2024.100163_bb0655",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1021/jf803072r",
"article-title": "Amyloid-degrading ability of nattokinase from Bacillus subtilis natto",
"author": "Hsu",
"doi-asserted-by": "crossref",
"first-page": "503",
"journal-title": "J. Agric. Food Chem.",
"key": "10.1016/j.endmts.2024.100163_bb0660",
"volume": "57",
"year": "2009"
},
{
"author": "Hsu",
"key": "10.1016/j.endmts.2024.100163_bb0665",
"series-title": "SARS-CoV-2 Spike Protein Promotes Hyper-inflammatory Response That Can Be Ameliorated by Spike-antagonistic Peptide and FDA-approved ER Stress and MAP Kinase Inhibitors in Vitro",
"year": "2020"
},
{
"DOI": "10.18632/oncotarget.7861",
"article-title": "Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "17356",
"journal-title": "Oncotarget",
"key": "10.1016/j.endmts.2024.100163_bb0670",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1097/MD.0000000000031138",
"article-title": "Efficiency of nicotinamide-based supportive therapy in lymphopenia for patients with ordinary or severe COVID-19: a randomized controlled trial",
"author": "Hu",
"doi-asserted-by": "crossref",
"journal-title": "Medicine (Baltimore)",
"key": "10.1016/j.endmts.2024.100163_bb0675",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1016/j.bbrc.2013.07.055",
"article-title": "Nebivolol stimulates mitochondrial biogenesis in 3T3-L1 adipocytes",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "211",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.endmts.2024.100163_bb0680",
"volume": "438",
"year": "2013"
},
{
"DOI": "10.1016/j.molcel.2014.12.013",
"article-title": "Deacetylation of nuclear LC3 drives autophagy initiation under starvation",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "456",
"journal-title": "Mol. Cell",
"key": "10.1016/j.endmts.2024.100163_bb0685",
"volume": "57",
"year": "2015"
},
{
"DOI": "10.3390/cells11162562",
"article-title": "Modulating chaperone-mediated autophagy and its clinical applications in cancer",
"author": "Hubert",
"doi-asserted-by": "crossref",
"first-page": "2562",
"journal-title": "Cells",
"key": "10.1016/j.endmts.2024.100163_bb0690",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1146/annurev-biochem-061516-044820",
"article-title": "Mechanisms of autophagy initiation",
"author": "Hurley",
"doi-asserted-by": "crossref",
"first-page": "225",
"journal-title": "Annu. Rev. Biochem.",
"key": "10.1016/j.endmts.2024.100163_bb0695",
"volume": "86",
"year": "2017"
},
{
"DOI": "10.3390/cells12060877",
"article-title": "Spike protein impairs mitochondrial function in human cardiomyocytes: mechanisms underlying cardiac injury in COVID-19",
"author": "Huynh",
"doi-asserted-by": "crossref",
"first-page": "877",
"journal-title": "Cells",
"key": "10.1016/j.endmts.2024.100163_bb0700",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1080/07315724.2019.1705203",
"article-title": "Effects of pyrroloquinoline quinone (PQQ) supplementation on aerobic exercise performance and indices of mitochondrial biogenesis in untrained men",
"author": "Hwang",
"doi-asserted-by": "crossref",
"first-page": "547",
"journal-title": "J. Am. Coll. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb0705",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.3390/vaccines10050776",
"article-title": "Association of impaired fasting glucose and diabetes with SARS-CoV-2 spike antibody titers after the BNT162b2 vaccine among health care workers in a tertiary hospital in Japan",
"author": "Islam",
"doi-asserted-by": "crossref",
"first-page": "776",
"journal-title": "Vaccines",
"key": "10.1016/j.endmts.2024.100163_bb0710",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.phrs.2022.106360",
"article-title": "Combining L-arginine with vitamin C improves long-COVID symptoms: the LINCOLN Survey",
"author": "Izzo",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol. Res.",
"key": "10.1016/j.endmts.2024.100163_bb0715",
"volume": "183",
"year": "2022"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 entry into cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0720",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.31989/ffhd.v12i9.918",
"article-title": "Corozo (Bactris guineensis) fruit extract has antiviral activity in vitro against SARS-CoV-2",
"author": "Jaimes-Gualdrón",
"doi-asserted-by": "crossref",
"first-page": "534",
"journal-title": "Functional Foods in Health and Disease",
"key": "10.1016/j.endmts.2024.100163_bb0725",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/nu11061234",
"article-title": "Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans",
"author": "Jamshed",
"doi-asserted-by": "crossref",
"first-page": "1234",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb0730",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1074/jbc.M112.363747",
"article-title": "Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "19304",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.endmts.2024.100163_bb0735",
"volume": "287",
"year": "2012"
},
{
"DOI": "10.5625/lar.2013.29.4.221",
"article-title": "Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Lab Anim Res",
"key": "10.1016/j.endmts.2024.100163_bb0740",
"volume": "29",
"year": "2013"
},
{
"DOI": "10.1083/jcb.201008084",
"article-title": "Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "933",
"journal-title": "J. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0745",
"volume": "191",
"year": "2010"
},
{
"DOI": "10.1042/bse0470069",
"article-title": "Regulation of mitochondrial biogenesis",
"author": "Jornayvaz",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Essays Biochem.",
"key": "10.1016/j.endmts.2024.100163_bb0750",
"volume": "47",
"year": "2010"
},
{
"DOI": "10.3390/ijms21030813",
"article-title": "Resveratrol as inducer of autophagy, pro-survival, and anti-inflammatory stimuli in cultured human RPE cells",
"author": "Josifovska",
"doi-asserted-by": "crossref",
"first-page": "813",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0755",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1083/jcb.201402104",
"article-title": "PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity",
"author": "Kane",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "J. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0760",
"volume": "205",
"year": "2014"
},
{
"DOI": "10.1111/jpi.12319",
"article-title": "Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis",
"author": "Kang",
"doi-asserted-by": "crossref",
"first-page": "383",
"journal-title": "J. Pineal Res.",
"key": "10.1016/j.endmts.2024.100163_bb0765",
"volume": "60",
"year": "2016"
},
{
"DOI": "10.1111/jpi.12259",
"article-title": "Melatonin promotes adipogenesis and mitochondrial biogenesis in 3T3-L1 preadipocytes",
"author": "Kato",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "J. Pineal Res.",
"key": "10.1016/j.endmts.2024.100163_bb0770",
"volume": "59",
"year": "2015"
},
{
"DOI": "10.1159/000159227",
"article-title": "Potential cellular signaling mechanisms mediating upregulation of endothelial nitric oxide production by estrogen",
"author": "Kauser",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "J. Vasc. Res.",
"key": "10.1016/j.endmts.2024.100163_bb0775",
"volume": "34",
"year": "2008"
},
{
"DOI": "10.1038/s41580-018-0001-6",
"article-title": "The coming of age of chaperone-mediated autophagy",
"author": "Kaushik",
"doi-asserted-by": "crossref",
"first-page": "365",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0780",
"volume": "19",
"year": "2018"
},
{
"DOI": "10.1042/BJ20140334",
"article-title": "Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65",
"author": "Kazlauskaite",
"doi-asserted-by": "crossref",
"first-page": "127",
"journal-title": "Biochem. J.",
"key": "10.1016/j.endmts.2024.100163_bb0785",
"volume": "460",
"year": "2014"
},
{
"DOI": "10.1096/fj.05-4107com",
"article-title": "Vitamin C enters mitochondria via facilitative glucose transporter 1 (Gluti) and confers mitochondrial protection against oxidative injury",
"author": "Kc",
"doi-asserted-by": "crossref",
"first-page": "1657",
"journal-title": "FASEB J.",
"key": "10.1016/j.endmts.2024.100163_bb0790",
"volume": "19",
"year": "2005"
},
{
"DOI": "10.1080/15548627.2019.1688984",
"article-title": "A discovery platform for the identification of caloric restriction mimetics with broad health-improving effects",
"author": "Kepp",
"doi-asserted-by": "crossref",
"first-page": "188",
"journal-title": "Autophagy",
"key": "10.1016/j.endmts.2024.100163_bb0795",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1186/s12944-023-01828-w",
"article-title": "Adjunctive therapy with lipid-lowering agents in COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"author": "Khalaji",
"doi-asserted-by": "crossref",
"first-page": "61",
"journal-title": "Lipids Health Dis.",
"key": "10.1016/j.endmts.2024.100163_bb0800",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.7554/eLife.68563",
"article-title": "SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway",
"author": "Khan",
"doi-asserted-by": "crossref",
"journal-title": "Elife",
"key": "10.1016/j.endmts.2024.100163_bb0805",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1002/(SICI)1097-0223(199601)16:1<39::AID-PD789>3.0.CO;2-P",
"article-title": "Fetal death after exposure to methylene blue dye during mid-trimester amniocentesis in twin pregnancy",
"author": "Kidd",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Prenat. Diagn.",
"key": "10.1016/j.endmts.2024.100163_bb0810",
"volume": "16",
"year": "1996"
},
{
"DOI": "10.1038/s41556-018-0205-1",
"article-title": "mTOR as a central hub of nutrient signalling and cell growth",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "63",
"journal-title": "Nat. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb0815",
"volume": "21",
"year": "2019"
},
{
"DOI": "10.1021/acs.jafc.5b01908",
"article-title": "De Candolle ethanolic extract inhibits adipogenic regulators in 3T3-L1 cells and induces mitochondrial biogenesis in primary brown preadipocytes",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "7721",
"journal-title": "J. Agric. Food Chem.",
"key": "10.1016/j.endmts.2024.100163_bb0820",
"volume": "63",
"year": "2015"
},
{
"article-title": "Spike proteins of SARS-CoV-2 induce pathological changes in molecular delivery and metabolic function in the brain endothelial cells",
"author": "Kim",
"first-page": "13",
"journal-title": "Viruses",
"key": "10.1016/j.endmts.2024.100163_bb0825",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1021/acs.jafc.8b04124",
"article-title": "Citrus tangeretin improves skeletal muscle mitochondrial biogenesis via activating the AMPK-PGC1-α pathway in vitro and in vivo: a possible mechanism for its beneficial effect on physical performance",
"author": "Kou",
"doi-asserted-by": "crossref",
"first-page": "11917",
"journal-title": "J. Agric. Food Chem.",
"key": "10.1016/j.endmts.2024.100163_bb0830",
"volume": "66",
"year": "2018"
},
{
"DOI": "10.1038/nature13392",
"article-title": "Ubiquitin is phosphorylated by PINK1 to activate Parkin",
"author": "Koyano",
"doi-asserted-by": "crossref",
"first-page": "162",
"journal-title": "Nature",
"key": "10.1016/j.endmts.2024.100163_bb0835",
"volume": "510",
"year": "2014"
},
{
"DOI": "10.1097/MBP.0000000000000271",
"article-title": "Fasting and ambulatory blood pressure monitoring",
"author": "Krakoff",
"doi-asserted-by": "crossref",
"first-page": "258",
"journal-title": "Blood Pressure Monitoring",
"key": "10.1016/j.endmts.2024.100163_bb0840",
"volume": "22",
"year": "2017"
},
{
"DOI": "10.1111/j.1742-4658.2011.08456.x",
"article-title": "Molecular chaperones in targeting misfolded proteins for ubiquitin-dependent degradation",
"author": "Kriegenburg",
"doi-asserted-by": "crossref",
"first-page": "532",
"journal-title": "FEBS J.",
"key": "10.1016/j.endmts.2024.100163_bb0845",
"volume": "279",
"year": "2012"
},
{
"DOI": "10.1002/ctm2.372",
"author": "Kumar",
"doi-asserted-by": "crossref",
"journal-title": "Clin. Transl. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0850",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/srep11601",
"article-title": "A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles",
"author": "Kurosawa",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.endmts.2024.100163_bb0855",
"volume": "5",
"year": "2015"
},
{
"DOI": "10.3390/nu15040830",
"article-title": "Prognostic value of magnesium in COVID-19: findings from the COMEPA study",
"author": "La Carrubba",
"doi-asserted-by": "crossref",
"first-page": "830",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb0860",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1002/jmv.27595",
"article-title": "Efficacy of melatonin in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "2102",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.endmts.2024.100163_bb0865",
"volume": "94",
"year": "2022"
},
{
"author": "Larsson",
"key": "10.1016/j.endmts.2024.100163_bb0870",
"series-title": "SARS-CoV-2 Spike Amyloid Fibrils Specifically and Selectively Accelerates Amyloid Fibril Formation of Human Prion Protein and the Amyloid β Peptide",
"year": "2023"
},
{
"DOI": "10.1161/01.CIR.97.12.1129",
"article-title": "Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors",
"author": "Laufs",
"doi-asserted-by": "crossref",
"first-page": "1129",
"journal-title": "Circulation",
"key": "10.1016/j.endmts.2024.100163_bb0875",
"volume": "97",
"year": "1998"
},
{
"DOI": "10.1002/bies.10298",
"article-title": "Mitochondrial biogenesis: which part of “NO” do we understand?",
"author": "Leary",
"doi-asserted-by": "crossref",
"first-page": "538",
"journal-title": "BioEssays",
"key": "10.1016/j.endmts.2024.100163_bb0880",
"volume": "25",
"year": "2003"
},
{
"article-title": "Effects of isorhamnetin on adipocyte mitochondrial biogenesis and AMPK activation",
"author": "Lee",
"first-page": "23",
"journal-title": "Molecules",
"key": "10.1016/j.endmts.2024.100163_bb0885",
"volume": "2018",
"year": "1853"
},
{
"DOI": "10.4196/kjpp.2011.15.1.1",
"article-title": "Involvement of ROS in curcumin-induced autophagic cell death",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Korean J Physiol Pharmacol",
"key": "10.1016/j.endmts.2024.100163_bb0890",
"volume": "15",
"year": "2011"
},
{
"article-title": "Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice",
"author": "Lee",
"journal-title": "Food Nutr",
"key": "10.1016/j.endmts.2024.100163_bb0895",
"volume": "61",
"year": "2017"
},
{
"article-title": "Transcriptional regulation of autophagy and its implications in human disease",
"author": "Lei",
"first-page": "1",
"journal-title": "Cell Death Differ.",
"key": "10.1016/j.endmts.2024.100163_bb0900",
"year": "2023"
},
{
"DOI": "10.1161/CIRCRESAHA.121.318902",
"article-title": "SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2",
"author": "Lei",
"doi-asserted-by": "crossref",
"first-page": "1323",
"journal-title": "Circ. Res.",
"key": "10.1016/j.endmts.2024.100163_bb0905",
"volume": "128",
"year": "2021"
},
{
"DOI": "10.1093/molbev/msaa127",
"article-title": "Chaperone-mediated autophagy in the light of evolution: insight from fish",
"author": "Lescat",
"doi-asserted-by": "crossref",
"first-page": "2887",
"journal-title": "Mol. Biol. Evol.",
"key": "10.1016/j.endmts.2024.100163_bb0910",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0195912",
"article-title": "Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle",
"author": "Lettieri-Barbato",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.endmts.2024.100163_bb0915",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1073/pnas.1619876114",
"article-title": "Autophagy wins the 2016 Nobel prize in physiology or medicine: breakthroughs in baker’s yeast fuel advances in biomedical research",
"author": "Levine",
"doi-asserted-by": "crossref",
"first-page": "201",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "10.1016/j.endmts.2024.100163_bb0920",
"volume": "114",
"year": "2017"
},
{
"article-title": "Nutraceuticals/drugs promoting mitophagy and mitochondrial biogenesis may combat the mitochondrial dysfunction driving progression of dry age-related macular degeneration",
"author": "Lewis Luján",
"first-page": "14",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb0925",
"volume": "2022",
"year": "1985"
},
{
"article-title": "SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling",
"author": "Li",
"journal-title": "Biochim. Biophys. Acta (BBA) - Mol. Basis Dis.",
"key": "10.1016/j.endmts.2024.100163_bb0930",
"volume": "2021",
"year": "1867"
},
{
"article-title": "Carnitine and COVID-19 susceptibility and severity: a Mendelian randomization study. Frontiers",
"author": "Li",
"first-page": "8",
"journal-title": "Nutrition",
"key": "10.1016/j.endmts.2024.100163_bb0935",
"year": "2021"
},
{
"DOI": "10.1038/s41423-021-00807-4",
"article-title": "SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "Cell. Mol. Immunol.",
"key": "10.1016/j.endmts.2024.100163_bb0940",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1038/s41392-023-01368-w",
"article-title": "SARS-CoV-2 spike protein induces IL-18-mediated cardiopulmonary inflammation via reduced mitophagy",
"author": "Liang",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Sig Transduct Target Ther",
"key": "10.1016/j.endmts.2024.100163_bb0945",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1007/s11010-020-04006-z",
"article-title": "Dysfunction of chaperone-mediated autophagy in human diseases",
"author": "Liao",
"doi-asserted-by": "crossref",
"first-page": "1439",
"journal-title": "Mol. Cell. Biochem.",
"key": "10.1016/j.endmts.2024.100163_bb0950",
"volume": "476",
"year": "2021"
},
{
"DOI": "10.1016/j.smhs.2023.03.004",
"article-title": "More than a key—the pathological roles of SARS-CoV-2 spike protein in COVID-19 related cardiac injury",
"author": "Lin",
"doi-asserted-by": "crossref",
"journal-title": "Sports Medicine and Health Science",
"key": "10.1016/j.endmts.2024.100163_bb0955",
"year": "2023"
},
{
"DOI": "10.3390/ijms18071412",
"article-title": "Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "1412",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb0960",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.1111/joim.12496",
"article-title": "Avoidance of Sun exposure as a risk factor for major causes of death: a competing risk analysis of the melanoma in southern Sweden cohort",
"author": "Lindqvist",
"doi-asserted-by": "crossref",
"first-page": "375",
"journal-title": "J. Intern. Med.",
"key": "10.1016/j.endmts.2024.100163_bb0965",
"volume": "280",
"year": "2016"
},
{
"DOI": "10.1016/j.cmet.2013.12.008",
"article-title": "Fasting: molecular mechanisms and clinical applications",
"author": "Longo",
"doi-asserted-by": "crossref",
"first-page": "181",
"journal-title": "Cell Metab.",
"key": "10.1016/j.endmts.2024.100163_bb0970",
"volume": "19",
"year": "2014"
},
{
"DOI": "10.1080/15548627.2018.1436937",
"article-title": "Induction of autophagy by depolarization of mitochondria",
"author": "Lyamzaev",
"doi-asserted-by": "crossref",
"first-page": "921",
"journal-title": "Autophagy",
"key": "10.1016/j.endmts.2024.100163_bb0975",
"volume": "14",
"year": "2018"
},
{
"DOI": "10.1126/science.aan2788",
"article-title": "Spermidine in health and disease",
"author": "Madeo",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "10.1016/j.endmts.2024.100163_bb0980",
"volume": "359",
"year": "2018"
},
{
"DOI": "10.1146/annurev-nutr-120419-015419",
"article-title": "Nutritional aspects of spermidine",
"author": "Madeo",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "Annu. Rev. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb0985",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.5005/jp-journals-10071-23905",
"article-title": "Intravenous methylene blue as a rescue therapy in the management of refractory hypoxia in COVID-19 ARDS patients: a case series",
"author": "Mahale",
"doi-asserted-by": "crossref",
"first-page": "934",
"journal-title": "Indian J Crit Care Med",
"key": "10.1016/j.endmts.2024.100163_bb0990",
"volume": "25",
"year": "2021"
},
{
"article-title": "Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan",
"author": "Mao",
"first-page": "1",
"journal-title": "China. JAMA Neurol",
"key": "10.1016/j.endmts.2024.100163_bb0995",
"volume": "77",
"year": "2020"
},
{
"DOI": "10.1016/j.arr.2006.04.002",
"article-title": "Caloric restriction and intermittent fasting: two potential diets for successful brain aging",
"author": "Martin",
"doi-asserted-by": "crossref",
"first-page": "332",
"journal-title": "Ageing Res. Rev.",
"key": "10.1016/j.endmts.2024.100163_bb1000",
"volume": "5",
"year": "2006"
},
{
"DOI": "10.1016/j.cmet.2017.09.020",
"article-title": "System-wide benefits of intermeal fasting by autophagy",
"author": "Martinez-Lopez",
"doi-asserted-by": "crossref",
"first-page": "856",
"journal-title": "Cell Metab.",
"key": "10.1016/j.endmts.2024.100163_bb1005",
"volume": "26",
"year": "2017"
},
{
"DOI": "10.1111/sms.12945",
"article-title": "Exercise-mediated modulation of autophagy in skeletal muscle",
"author": "Martin-Rincon",
"doi-asserted-by": "crossref",
"first-page": "772",
"journal-title": "Scand. J. Med. Sci. Sports",
"key": "10.1016/j.endmts.2024.100163_bb1010",
"volume": "28",
"year": "2018"
},
{
"DOI": "10.1016/j.jnutbio.2004.12.007",
"article-title": "Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems",
"author": "Mattson",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "J. Nutr. Biochem.",
"key": "10.1016/j.endmts.2024.100163_bb1015",
"volume": "16",
"year": "2005"
},
{
"DOI": "10.1016/j.arr.2016.10.005",
"article-title": "Impact of intermittent fasting on health and disease processes",
"author": "Mattson",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Ageing Res. Rev.",
"key": "10.1016/j.endmts.2024.100163_bb1020",
"volume": "39",
"year": "2017"
},
{
"DOI": "10.1038/s41598-022-13920-9",
"article-title": "Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19)",
"author": "McCreary",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.endmts.2024.100163_bb1025",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/molecules26010229",
"article-title": "Anti-inflammatory action and mechanisms of resveratrol",
"author": "Meng",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "Molecules",
"key": "10.1016/j.endmts.2024.100163_bb1030",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1186/1479-5876-10-189",
"article-title": "Effect of high-dose intravenous vitamin C on inflammation in cancer patients",
"author": "Mikirova",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "J. Transl. Med.",
"key": "10.1016/j.endmts.2024.100163_bb1035",
"volume": "10",
"year": "2012"
},
{
"author": "Mittra",
"key": "10.1016/j.endmts.2024.100163_bb1040",
"series-title": "Resveratrol and Copper for Treatment of Severe COVID-19: An Observational Study (RESCU 002)",
"year": "2020"
},
{
"DOI": "10.1007/s10616-018-0242-4",
"article-title": "Cyanidin-3-glucoside enhances mitochondrial function and biogenesis in a human hepatocyte cell line",
"author": "Mogalli",
"doi-asserted-by": "crossref",
"first-page": "1519",
"journal-title": "Cytotechnology",
"key": "10.1016/j.endmts.2024.100163_bb1045",
"volume": "70",
"year": "2018"
},
{
"DOI": "10.1042/BCJ20200849",
"article-title": "Bulk autophagy induction and life extension is achieved when iron is the only limited nutrient in Saccharomyces cerevisiae",
"author": "Montella-Manuel",
"doi-asserted-by": "crossref",
"first-page": "811",
"journal-title": "Biochem. J.",
"key": "10.1016/j.endmts.2024.100163_bb1050",
"volume": "478",
"year": "2021"
},
{
"DOI": "10.3390/vaccines10101651",
"article-title": "A case report: multifocal necrotizing encephalitis and myocarditis after BNT162b2 mRNA vaccination against COVID-19",
"author": "Mörz",
"doi-asserted-by": "crossref",
"first-page": "1651",
"journal-title": "Vaccines",
"key": "10.1016/j.endmts.2024.100163_bb1055",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1158/0008-5472.CAN-22-0887",
"article-title": "The mechanisms of action of tumor treating fields",
"author": "Moser",
"doi-asserted-by": "crossref",
"first-page": "3650",
"journal-title": "Cancer Res.",
"key": "10.1016/j.endmts.2024.100163_bb1060",
"volume": "82",
"year": "2022"
},
{
"article-title": "Attitude and practice of therapeutic fasting among naturopathy physicians: across sectional national survey",
"author": "Nair",
"first-page": "177",
"journal-title": "Journal of Nutrition, Fasting and Health",
"key": "10.1016/j.endmts.2024.100163_bb1065",
"volume": "3",
"year": "2015"
},
{
"DOI": "10.1083/jcb.200809125",
"article-title": "Parkin is recruited selectively to impaired mitochondria and promotes their autophagy",
"author": "Narendra",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "J. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb1070",
"volume": "183",
"year": "2008"
},
{
"DOI": "10.1016/j.vacune.2022.10.019",
"article-title": "Menstrual abnormalities after COVID-19 vaccines: a systematic review",
"author": "Nazir",
"doi-asserted-by": "crossref",
"first-page": "S77",
"journal-title": "Vacunas (English Edition)",
"key": "10.1016/j.endmts.2024.100163_bb1075",
"volume": "23",
"year": "2022"
},
{
"article-title": "Nebivolol: an effective option against long-lasting dyspnoea following COVID-19 pneumonia - a pivotal double-blind, cross-over controlled study",
"author": "Negro",
"journal-title": "Multidisciplinary Respiratory Medicine",
"key": "10.1016/j.endmts.2024.100163_bb1080",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.cyto.2020.155312",
"article-title": "Probable positive effects of the photobiomodulation as an adjunctive treatment in COVID-19: a systematic review",
"author": "Nejatifard",
"doi-asserted-by": "crossref",
"journal-title": "Cytokine",
"key": "10.1016/j.endmts.2024.100163_bb1085",
"volume": "137",
"year": "2021"
},
{
"article-title": "The spike protein of SARS-CoV-2 impairs lipid metabolism and increases susceptibility to lipotoxicity: implication for a role of Nrf2",
"author": "Nguyen",
"first-page": "11",
"journal-title": "Cells",
"key": "10.1016/j.endmts.2024.100163_bb1090",
"volume": "2022",
"year": "1916"
},
{
"DOI": "10.1016/j.mito.2013.11.001",
"article-title": "Effect of near-infrared light exposure on mitochondrial signaling in C2C12 muscle cells",
"author": "Nguyen",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "Mitochondrion",
"key": "10.1016/j.endmts.2024.100163_bb1095",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1016/j.exger.2017.08.017",
"article-title": "Dietary supplementation with acetyl-l-carnitine counteracts age-related alterations of mitochondrial biogenesis, dynamics and antioxidant defenses in brain of old rats",
"author": "Nicassio",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Exp. Gerontol.",
"key": "10.1016/j.endmts.2024.100163_bb1100",
"volume": "98",
"year": "2017"
},
{
"DOI": "10.1126/science.1079368",
"article-title": "Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide",
"author": "Nisoli",
"doi-asserted-by": "crossref",
"first-page": "896",
"journal-title": "Science",
"key": "10.1016/j.endmts.2024.100163_bb1105",
"volume": "299",
"year": "2003"
},
{
"DOI": "10.1111/jpi.12627",
"article-title": "Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos",
"author": "Niu",
"doi-asserted-by": "crossref",
"journal-title": "J. Pineal Res.",
"key": "10.1016/j.endmts.2024.100163_bb1110",
"volume": "68",
"year": "2020"
},
{
"DOI": "10.1159/000522118",
"article-title": "Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life",
"author": "Nopp",
"doi-asserted-by": "crossref",
"first-page": "593",
"journal-title": "Respiration",
"key": "10.1016/j.endmts.2024.100163_bb1115",
"volume": "101",
"year": "2022"
},
{
"article-title": "Higher intake of dietary magnesium is inversely associated with COVID-19 severity and symptoms in hospitalized patients: a cross-sectional study. Frontiers",
"author": "Nouri-Majd",
"first-page": "9",
"journal-title": "Nutrition",
"key": "10.1016/j.endmts.2024.100163_bb1120",
"year": "2022"
},
{
"DOI": "10.3390/v14071415",
"article-title": "Potential autoimmunity resulting from molecular mimicry between SARS-CoV-2 spike and human proteins",
"author": "Nunez-Castilla",
"doi-asserted-by": "crossref",
"first-page": "1415",
"journal-title": "Viruses",
"key": "10.1016/j.endmts.2024.100163_bb1125",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/biomedicines10123113",
"article-title": "Understanding long COVID; mitochondrial health and adaptation—old pathways",
"author": "Nunn",
"doi-asserted-by": "crossref",
"first-page": "3113",
"journal-title": "new problems. Biomedicines",
"key": "10.1016/j.endmts.2024.100163_bb1130",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1021/jacs.2c03925",
"article-title": "Amyloidogenesis of SARS-CoV-2 spike protein",
"author": "Nyström",
"doi-asserted-by": "crossref",
"first-page": "8945",
"journal-title": "J. Am. Chem. Soc.",
"key": "10.1016/j.endmts.2024.100163_bb1135",
"volume": "144",
"year": "2022"
},
{
"DOI": "10.1016/j.bbrc.2021.07.034",
"article-title": "Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro",
"author": "Oba",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.endmts.2024.100163_bb1140",
"volume": "570",
"year": "2021"
},
{
"DOI": "10.3390/nu13010155",
"article-title": "Consumption of a high-protein meal replacement leads to higher fat oxidation, suppression of hunger, and improved metabolic profile after an exercise session",
"author": "Oliveira",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1145",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/antiox10081278",
"article-title": "Vitamin C supplementation in healthy individuals leads to shifts of bacterial populations in the gut-a pilot study",
"author": "Otten",
"doi-asserted-by": "crossref",
"first-page": "1278",
"journal-title": "Antioxidants (Basel)",
"key": "10.1016/j.endmts.2024.100163_bb1150",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-21118-2",
"article-title": "SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity",
"author": "Ozono",
"doi-asserted-by": "crossref",
"first-page": "848",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.endmts.2024.100163_bb1155",
"volume": "12",
"year": "2021"
},
{
"article-title": "Effects of nattokinase, a pro-fibrinolytic enzyme, on red blood cell aggregation and whole blood viscosity",
"author": "Pais",
"first-page": "139",
"journal-title": "Clin. Hemorheol. Microcirc.",
"key": "10.1016/j.endmts.2024.100163_bb1160",
"volume": "35",
"year": "2006"
},
{
"article-title": "N-acetylcysteine efficacy in patients hospitalized with COVID-19 pneumonia: a systematic review and meta-analysis",
"author": "Paraskevas",
"first-page": "41",
"journal-title": "Rom. J. Intern. Med.",
"key": "10.1016/j.endmts.2024.100163_bb1165",
"volume": "61",
"year": "2023"
},
{
"article-title": "Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition",
"author": "Park",
"first-page": "1",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.endmts.2024.100163_bb1170",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.3390/ijms21093369",
"article-title": "Autophagy in neurodegenerative diseases: a hunter for aggregates",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "3369",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb1175",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.3390/biomedicines11082287",
"article-title": "‘Spikeopathy’: COVID-19 spike protein is pathogenic, from both virus and vaccine mRNA",
"author": "Parry",
"doi-asserted-by": "crossref",
"first-page": "2287",
"journal-title": "Biomedicines",
"key": "10.1016/j.endmts.2024.100163_bb1180",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.3390/v13020354",
"article-title": "Resveratrol inhibits HCoV-229E and SARS-CoV-2 coronavirus replication in vitro",
"author": "Pasquereau",
"doi-asserted-by": "crossref",
"first-page": "354",
"journal-title": "Viruses",
"key": "10.1016/j.endmts.2024.100163_bb1185",
"volume": "13",
"year": "2021"
},
{
"article-title": "The role of nebulized methylene blue [NMB] in the management of COVID-19 cases: an observational study",
"author": "Patidar",
"first-page": "2129",
"journal-title": "International Journal of Medical Arts",
"key": "10.1016/j.endmts.2024.100163_bb1190",
"volume": "4",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.39558",
"article-title": "Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials",
"author": "Patikorn",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.endmts.2024.100163_bb1195",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1146/annurev-nutr-071816-064634",
"article-title": "Metabolic effects of intermittent fasting",
"author": "Patterson",
"doi-asserted-by": "crossref",
"first-page": "371",
"journal-title": "Annu. Rev. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb1200",
"volume": "37",
"year": "2017"
},
{
"DOI": "10.1016/j.jand.2015.02.018",
"article-title": "Intermittent fasting and human metabolic health",
"author": "Patterson",
"doi-asserted-by": "crossref",
"first-page": "1203",
"journal-title": "J. Acad. Nutr. Diet.",
"key": "10.1016/j.endmts.2024.100163_bb1205",
"volume": "115",
"year": "2015"
},
{
"article-title": "Effect of a nutraceutical drug in COPD condition: a pilot study of in vitro, in vivo and clinical trial",
"author": "Pattnaik",
"journal-title": "Eur. Respir. J.",
"key": "10.1016/j.endmts.2024.100163_bb1210",
"volume": "60",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.669362",
"article-title": "Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial",
"author": "Pawar",
"doi-asserted-by": "crossref",
"journal-title": "Front. Pharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb1215",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1089/rej.2009.0955",
"article-title": "Acetyl-L-carnitine supplementation to old rats partially reverts the age-related mitochondrial decay of soleus muscle by activating peroxisome proliferator-activated receptor gamma coactivator-1alpha-dependent mitochondrial biogenesis",
"author": "Pesce",
"doi-asserted-by": "crossref",
"first-page": "148",
"journal-title": "Rejuvenation Res.",
"key": "10.1016/j.endmts.2024.100163_bb1220",
"volume": "13",
"year": "2010"
},
{
"DOI": "10.1089/rej.2011.1255",
"article-title": "Acetyl-L-carnitine activates the peroxisome proliferator-activated receptor-γ coactivators PGC-1α/PGC-1β-dependent signaling cascade of mitochondrial biogenesis and decreases the oxidized peroxiredoxins content in old rat liver",
"author": "Pesce",
"doi-asserted-by": "crossref",
"first-page": "136",
"journal-title": "Rejuvenation Res.",
"key": "10.1016/j.endmts.2024.100163_bb1225",
"volume": "15",
"year": "2012"
},
{
"DOI": "10.1002/1873-3468.14490",
"article-title": "SARS-CoV-2 spike protein aggregation is triggered by bacterial lipopolysaccharide",
"author": "Petrlova",
"doi-asserted-by": "crossref",
"first-page": "2566",
"journal-title": "FEBS Lett.",
"key": "10.1016/j.endmts.2024.100163_bb1230",
"volume": "596",
"year": "2022"
},
{
"DOI": "10.3390/biomedicines10010188",
"article-title": "Penetration of the SARS-CoV-2 spike protein across the blood-brain barrier, as revealed by a combination of a human cell culture model system and optical biosensing",
"author": "Petrovszki",
"doi-asserted-by": "crossref",
"first-page": "188",
"journal-title": "Biomedicines",
"key": "10.1016/j.endmts.2024.100163_bb1235",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.neuron.2014.12.007",
"article-title": "The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson’s disease",
"author": "Pickrell",
"doi-asserted-by": "crossref",
"first-page": "257",
"journal-title": "Neuron",
"key": "10.1016/j.endmts.2024.100163_bb1240",
"volume": "85",
"year": "2015"
},
{
"DOI": "10.4161/cc.28929",
"article-title": "Coffee induces autophagy in vivo",
"author": "Pietrocola",
"doi-asserted-by": "crossref",
"first-page": "1987",
"journal-title": "Cell Cycle",
"key": "10.1016/j.endmts.2024.100163_bb1245",
"volume": "13",
"year": "2014"
},
{
"DOI": "10.1038/cdd.2014.215",
"article-title": "Spermidine induces autophagy by inhibiting the acetyltransferase EP300",
"author": "Pietrocola",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "Cell Death Differ.",
"key": "10.1016/j.endmts.2024.100163_bb1250",
"volume": "22",
"year": "2015"
},
{
"DOI": "10.1155/2017/6797460",
"article-title": "The involvement of Mg2+ in regulation of cellular and mitochondrial functions",
"author": "Pilchova",
"doi-asserted-by": "crossref",
"journal-title": "Oxidative Med. Cell. Longev.",
"key": "10.1016/j.endmts.2024.100163_bb1255",
"volume": "2017",
"year": "2017"
},
{
"DOI": "10.1371/journal.pone.0048279",
"article-title": "Neuroprotective actions of methylene blue and its derivatives",
"author": "Poteet",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.endmts.2024.100163_bb1260",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.1016/j.mito.2023.01.005",
"article-title": "Severe acute respiratory syndrome coronaviruses contributing to mitochondrial dysfunction: implications for post-COVID complications",
"author": "Prasada Kabekkodu",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Mitochondrion",
"key": "10.1016/j.endmts.2024.100163_bb1265",
"volume": "69",
"year": "2023"
},
{
"DOI": "10.1002/rmv.2177",
"article-title": "Clotting disorder in severe acute respiratory syndrome coronavirus 2",
"author": "Pujhari",
"doi-asserted-by": "crossref",
"journal-title": "Rev. Med. Virol.",
"key": "10.1016/j.endmts.2024.100163_bb1270",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.3390/nu14194054",
"article-title": "Reduction in serum magnesium levels and renal function are associated with increased mortality in obese COVID-19 patients",
"author": "Pulido Perez",
"doi-asserted-by": "crossref",
"first-page": "4054",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1275",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1111/pce.12329",
"article-title": "New clues for a cold case: nitric oxide response to low temperature",
"author": "Puyaubert",
"doi-asserted-by": "crossref",
"first-page": "2623",
"journal-title": "Plant Cell Environ.",
"key": "10.1016/j.endmts.2024.100163_bb1280",
"volume": "37",
"year": "2014"
},
{
"DOI": "10.1016/j.cellbi.2007.08.024",
"article-title": "Effect of magnesium on calcium-induced depolarisation of mitochondrial transmembrane potential",
"author": "Racay",
"doi-asserted-by": "crossref",
"first-page": "136",
"journal-title": "Cell Biol. Int.",
"key": "10.1016/j.endmts.2024.100163_bb1285",
"volume": "32",
"year": "2008"
},
{
"article-title": "Potential compounds for the treatment of mitochondrial disease",
"author": "Rai",
"first-page": "5",
"journal-title": "Br. Med. Bull.",
"key": "10.1016/j.endmts.2024.100163_bb1290",
"volume": "116",
"year": "2015"
},
{
"DOI": "10.1111/dom.14080",
"article-title": "Intermittent fasting and ‘metabolic switch’: effects on metabolic syndrome, prediabetes and type 2 diabetes",
"author": "Rajpal",
"doi-asserted-by": "crossref",
"first-page": "1496",
"journal-title": "Diabetes Obes. Metab.",
"key": "10.1016/j.endmts.2024.100163_bb1295",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1523/JNEUROSCI.1356-10.2010",
"article-title": "Rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice",
"author": "Rangaraju",
"doi-asserted-by": "crossref",
"first-page": "11388",
"journal-title": "J. Neurosci.",
"key": "10.1016/j.endmts.2024.100163_bb1300",
"volume": "30",
"year": "2010"
},
{
"DOI": "10.1124/jpet.107.134882",
"article-title": "Isoflavones promote mitochondrial biogenesis",
"author": "Rasbach",
"doi-asserted-by": "crossref",
"first-page": "536",
"journal-title": "J. Pharmacol. Exp. Ther.",
"key": "10.1016/j.endmts.2024.100163_bb1305",
"volume": "325",
"year": "2008"
},
{
"DOI": "10.1007/s12640-018-9967-2",
"article-title": "Glycyrrhizic acid ameliorates mitochondrial function and biogenesis against aluminum toxicity in PC12 cells",
"author": "Rashedinia",
"doi-asserted-by": "crossref",
"first-page": "584",
"journal-title": "Neurotox. Res.",
"key": "10.1016/j.endmts.2024.100163_bb1310",
"volume": "35",
"year": "2019"
},
{
"article-title": "Vitamin C and COVID-19 treatment: a systematic review and meta-analysis of randomized controlled trials",
"author": "Rawat",
"journal-title": "Diabetes Metab. Syndr. Clin. Res. Rev.",
"key": "10.1016/j.endmts.2024.100163_bb1315",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1038/nrgastro.2013.170",
"article-title": "Caffeine is a potent stimulator of autophagy to reduce hepatic lipid content—a coffee for NAFLD?",
"author": "Ray",
"doi-asserted-by": "crossref",
"first-page": "563",
"journal-title": "Nat. Rev. Gastroenterol. Hepatol.",
"key": "10.1016/j.endmts.2024.100163_bb1320",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1371/journal.pone.0202784",
"article-title": "The synergism of high-intensity intermittent exercise and every-other-day intermittent fasting regimen on energy metabolism adaptations includes hexokinase activity and mitochondrial efficiency",
"author": "Real-Hohn",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.endmts.2024.100163_bb1325",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1016/j.isci.2020.101161",
"article-title": "Time-restricted eating: benefits, mechanisms, and challenges in translation",
"author": "Regmi",
"doi-asserted-by": "crossref",
"journal-title": "iScience",
"key": "10.1016/j.endmts.2024.100163_bb1330",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0065029",
"article-title": "Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporin A treatment in rats",
"author": "Rehman",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.endmts.2024.100163_bb1335",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1038/s41593-020-00771-8",
"doi-asserted-by": "crossref",
"key": "10.1016/j.endmts.2024.100163_bb1340",
"unstructured": "Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378, doi:https://doi.org/10.1038/s41593-020-00771-8."
},
{
"DOI": "10.1152/ajpendo.1999.277.2.E390",
"article-title": "Acute exercise increases nitric oxide synthase activity in skeletal muscle",
"author": "Roberts",
"doi-asserted-by": "crossref",
"first-page": "E390",
"journal-title": "American Journal of Physiology-Endocrinology and Metabolism",
"key": "10.1016/j.endmts.2024.100163_bb1345",
"volume": "277",
"year": "1999"
},
{
"DOI": "10.1016/j.freeradbiomed.2020.04.025",
"article-title": "Protection against renal ischemia and reperfusion injury by short-term time-restricted feeding involves the mitochondrial unfolded protein response",
"author": "Rojas-Morales",
"doi-asserted-by": "crossref",
"first-page": "75",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.endmts.2024.100163_bb1350",
"volume": "154",
"year": "2020"
},
{
"DOI": "10.1016/j.rehab.2023.101765",
"article-title": "Effectiveness of exercise training on the dyspnoea of individuals with long COVID: a randomised controlled multicentre trial",
"author": "Romanet",
"doi-asserted-by": "crossref",
"journal-title": "Ann. Phys. Rehabil. Med.",
"key": "10.1016/j.endmts.2024.100163_bb1355",
"volume": "66",
"year": "2023"
},
{
"DOI": "10.1073/pnas.1014633107",
"article-title": "Rapamycin induces autophagic flux in neurons",
"author": "Rubinsztein",
"doi-asserted-by": "crossref",
"first-page": "E181",
"journal-title": "Proc. Natl. Acad. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb1360",
"volume": "107",
"year": "2010"
},
{
"DOI": "10.1016/j.pharmthera.2013.09.006",
"article-title": "Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits",
"author": "Rushworth",
"doi-asserted-by": "crossref",
"first-page": "150",
"journal-title": "Pharmacol. Ther.",
"key": "10.1016/j.endmts.2024.100163_bb1365",
"volume": "141",
"year": "2014"
},
{
"DOI": "10.4103/ijem.IJEM_601_19",
"article-title": "Effect of Jain fasting on anthropometric, clinical and biochemical parameters",
"author": "Sanchetee",
"doi-asserted-by": "crossref",
"first-page": "187",
"journal-title": "Indian J Endocrinol Metab",
"key": "10.1016/j.endmts.2024.100163_bb1370",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1093/jxb/erab049",
"article-title": "Nitric oxide regulation of temperature acclimation: a molecular genetic perspective",
"author": "Sánchez-Vicente",
"doi-asserted-by": "crossref",
"first-page": "5789",
"journal-title": "J. Exp. Bot.",
"key": "10.1016/j.endmts.2024.100163_bb1375",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1016/j.jep.2011.05.021",
"article-title": "Plant use in the medicinal practices known as “strict diets” in Chazuta Valley (Peruvian Amazon)",
"author": "Sanz-Biset",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb1380",
"volume": "137",
"year": "2011"
},
{
"DOI": "10.1038/cdd.2008.110",
"article-title": "Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies",
"author": "Sarkar",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Cell Death Differ.",
"key": "10.1016/j.endmts.2024.100163_bb1385",
"volume": "16",
"year": "2009"
},
{
"DOI": "10.1016/j.cub.2015.05.059",
"article-title": "Iron-starvation-induced mitophagy mediates lifespan extension upon mitochondrial stress in C. elegans",
"author": "Schiavi",
"doi-asserted-by": "crossref",
"first-page": "1810",
"journal-title": "Curr. Biol.",
"key": "10.1016/j.endmts.2024.100163_bb1390",
"volume": "25",
"year": "2015"
},
{
"DOI": "10.1016/j.cmet.2014.06.009",
"article-title": "Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation",
"author": "Schneider",
"doi-asserted-by": "crossref",
"first-page": "417",
"journal-title": "Cell Metab.",
"key": "10.1016/j.endmts.2024.100163_bb1395",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1007/s00392-022-02129-5",
"article-title": "Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination",
"author": "Schwab",
"doi-asserted-by": "crossref",
"first-page": "431",
"journal-title": "Clin. Res. Cardiol.",
"key": "10.1016/j.endmts.2024.100163_bb1400",
"volume": "112",
"year": "2023"
},
{
"DOI": "10.18632/aging.101354",
"article-title": "Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline",
"author": "Schwarz",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "Aging",
"key": "10.1016/j.endmts.2024.100163_bb1405",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1001/jamanetworkopen.2022.13875",
"article-title": "Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline: a randomized clinical trial",
"author": "Schwarz",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.endmts.2024.100163_bb1410",
"volume": "5",
"year": "2022"
},
{
"article-title": "Admission serum magnesium levels is associated with short and long-term clinical outcomes in COVID-19 patients",
"author": "Segev",
"first-page": "15",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1415",
"volume": "2023",
"year": "2016"
},
{
"DOI": "10.3390/nu14132738",
"author": "Semmarath",
"doi-asserted-by": "crossref",
"first-page": "2738",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1420",
"volume": "14",
"year": "2022"
},
{
"article-title": "High-dose spermidine supplementation does not increase spermidine levels in blood plasma and saliva of healthy adults: a randomized placebo-controlled pharmacokinetic and metabolomic study",
"author": "Senekowitsch",
"first-page": "15",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1425",
"volume": "2023",
"year": "1852"
},
{
"DOI": "10.3390/jcm10245876",
"article-title": "Autopsy findings and causality relationship between death and COVID-19 vaccination: a systematic review",
"author": "Sessa",
"doi-asserted-by": "crossref",
"first-page": "5876",
"journal-title": "J. Clin. Med.",
"key": "10.1016/j.endmts.2024.100163_bb1430",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1002/jcp.27404",
"article-title": "Curcumin: a naturally occurring autophagy modulator",
"author": "Shakeri",
"doi-asserted-by": "crossref",
"first-page": "5643",
"journal-title": "J. Cell. Physiol.",
"key": "10.1016/j.endmts.2024.100163_bb1435",
"volume": "234",
"year": "2019"
},
{
"DOI": "10.3389/fnins.2023.1150156",
"article-title": "The anti-inflammatory effects of photobiomodulation are mediated by cytokines: evidence from a mouse model of inflammation",
"author": "Shamloo",
"doi-asserted-by": "crossref",
"journal-title": "Front. Neurosci.",
"key": "10.1016/j.endmts.2024.100163_bb1440",
"volume": "17",
"year": "2023"
},
{
"article-title": "SARS-CoV-2 causes mitochondrial dysfunction and mitophagy impairment",
"author": "Shang",
"journal-title": "Front. Microbiol.",
"key": "10.1016/j.endmts.2024.100163_bb1445",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1249/00005768-199508000-00005",
"article-title": "Nitric oxide production and NO synthase gene expression contribute to vascular regulation during exercise",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "1125",
"journal-title": "Med. Sci. Sports Exerc.",
"key": "10.1016/j.endmts.2024.100163_bb1450",
"volume": "27",
"year": "1995"
},
{
"DOI": "10.1007/s00125-007-0852-4",
"article-title": "R-alpha-lipoic acid and acetyl-L-carnitine complementarily promote mitochondrial biogenesis in murine 3T3-L1 adipocytes",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "165",
"journal-title": "Diabetologia",
"key": "10.1016/j.endmts.2024.100163_bb1455",
"volume": "51",
"year": "2008"
},
{
"DOI": "10.1111/j.1476-5381.2010.01134.x",
"article-title": "Lipoamide or lipoic acid stimulates mitochondrial biogenesis in 3T3-L1 adipocytes via the endothelial NO synthase-cGMP-protein kinase G signalling pathway: lipoamide, lipoic acid and mitochondrial biogenesis",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "1213",
"journal-title": "Br. J. Pharmacol.",
"key": "10.1016/j.endmts.2024.100163_bb1460",
"volume": "162",
"year": "2011"
},
{
"DOI": "10.1371/journal.pgen.1004861",
"article-title": "Phosphorylation of mitochondrial polyubiquitin by PINK1 promotes Parkin mitochondrial tethering",
"author": "Shiba-Fukushima",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Genet.",
"key": "10.1016/j.endmts.2024.100163_bb1465",
"volume": "10",
"year": "2014"
},
{
"DOI": "10.1016/j.heliyon.2021.e06187",
"article-title": "SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via Toll-like receptor 4 signaling in murine and human macrophages",
"author": "Shirato",
"doi-asserted-by": "crossref",
"journal-title": "Heliyon",
"key": "10.1016/j.endmts.2024.100163_bb1470",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1055/s-2006-957450",
"article-title": "Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers",
"author": "Shoba",
"doi-asserted-by": "crossref",
"first-page": "353",
"journal-title": "Planta Med.",
"key": "10.1016/j.endmts.2024.100163_bb1475",
"volume": "64",
"year": "1998"
},
{
"article-title": "Concept and canons of fasting in Ayurveda",
"author": "Shripathi Adiga",
"first-page": "37",
"journal-title": "Journal of Nutrition, Fasting and Health",
"key": "10.1016/j.endmts.2024.100163_bb1480",
"volume": "1",
"year": "2013"
},
{
"DOI": "10.1016/j.tranon.2020.100814",
"article-title": "S2 subunit of SARS-nCoV-2 interacts with tumor suppressor protein P53 and BRCA: an in silico study",
"author": "Singh",
"doi-asserted-by": "crossref",
"journal-title": "Transl. Oncol.",
"key": "10.1016/j.endmts.2024.100163_bb1485",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1186/s43094-021-00360-x",
"article-title": "Targeting mitochondrial biogenesis: a potential approach for preventing and controlling diabetes",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "212",
"journal-title": "Future Journal of Pharmaceutical Sciences",
"key": "10.1016/j.endmts.2024.100163_bb1490",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.3177/jnsv.55.361",
"article-title": "Long-term oral polyamine intake increases blood polyamine concentrations",
"author": "Soda",
"doi-asserted-by": "crossref",
"first-page": "361",
"journal-title": "J. Nutr. Sci. Vitaminol. (Tokyo)",
"key": "10.1016/j.endmts.2024.100163_bb1495",
"volume": "55",
"year": "2009"
},
{
"article-title": "Polyamine-rich diet elevates blood Spermine levels and inhibits pro-inflammatory status: an interventional study",
"author": "Soda",
"first-page": "22",
"journal-title": "Med Sci (Basel)",
"key": "10.1016/j.endmts.2024.100163_bb1500",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.bbabio.2010.02.011",
"article-title": "Hypoxia and mitochondrial oxidative metabolism",
"author": "Solaini",
"doi-asserted-by": "crossref",
"first-page": "1171",
"journal-title": "Biochimica et Biophysica Acta (BBA) - Bioenergetics",
"key": "10.1016/j.endmts.2024.100163_bb1505",
"volume": "1797",
"year": "2010"
},
{
"DOI": "10.1126/sciadv.add4150",
"article-title": "The SARS-CoV-2 spike protein binds and modulates estrogen receptors",
"author": "Solis",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Adv.",
"key": "10.1016/j.endmts.2024.100163_bb1510",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1186/s13287-016-0425-x",
"article-title": "Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells",
"author": "Sotthibundhu",
"doi-asserted-by": "crossref",
"first-page": "166",
"journal-title": "Stem Cell Res Ther",
"key": "10.1016/j.endmts.2024.100163_bb1515",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1038/s41586-022-05542-y",
"article-title": "SARS-CoV-2 infection and persistence in the human body and brain at autopsy",
"author": "Stein",
"doi-asserted-by": "crossref",
"first-page": "758",
"journal-title": "Nature",
"key": "10.1016/j.endmts.2024.100163_bb1520",
"volume": "612",
"year": "2022"
},
{
"DOI": "10.7150/ijms.8220",
"article-title": "Rutin, a flavonoid and principal component of Saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "528",
"journal-title": "Int. J. Med. Sci.",
"key": "10.1016/j.endmts.2024.100163_bb1525",
"volume": "11",
"year": "2014"
},
{
"DOI": "10.1080/15548627.2022.2084862",
"article-title": "Mitochondria ROS and mitophagy in acute kidney injury",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "401",
"journal-title": "Autophagy",
"key": "10.1016/j.endmts.2024.100163_bb1530",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1007/BF01956052",
"article-title": "A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese natto; a typical and popular soybean food in the Japanese diet",
"author": "Sumi",
"doi-asserted-by": "crossref",
"first-page": "1110",
"journal-title": "Experientia",
"key": "10.1016/j.endmts.2024.100163_bb1535",
"volume": "43",
"year": "1987"
},
{
"DOI": "10.3389/fphys.2019.01521",
"article-title": "Acute heat exposure alters autophagy signaling in C2C12 myotubes",
"author": "Summers",
"doi-asserted-by": "crossref",
"journal-title": "Front. Physiol.",
"key": "10.1016/j.endmts.2024.100163_bb1540",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1080/15548627.2021.2021496",
"article-title": "SARS-CoV-2 targets the lysosome to mediate airway inflammatory cell death",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "2246",
"journal-title": "Autophagy",
"key": "10.1016/j.endmts.2024.100163_bb1545",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1016/j.cmet.2018.04.010",
"article-title": "Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes",
"author": "Sutton",
"doi-asserted-by": "crossref",
"first-page": "1212",
"journal-title": "Cell Metab.",
"key": "10.1016/j.endmts.2024.100163_bb1550",
"volume": "27",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0017412",
"article-title": "Starvation induced cell death in autophagy-defective yeast mutants is caused by mitochondria dysfunction",
"author": "Suzuki",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.endmts.2024.100163_bb1555",
"volume": "6",
"year": "2011"
},
{
"DOI": "10.1093/cid/ciac722",
"article-title": "Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae",
"author": "Swank",
"doi-asserted-by": "crossref",
"first-page": "e487",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.endmts.2024.100163_bb1560",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1083/jcb.119.2.301",
"article-title": "Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction",
"author": "Takeshige",
"doi-asserted-by": "crossref",
"first-page": "301",
"journal-title": "J. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb1565",
"volume": "119",
"year": "1992"
},
{
"article-title": "Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19)",
"author": "Tan",
"journal-title": "Nutrition",
"key": "10.1016/j.endmts.2024.100163_bb1570",
"volume": "79–80",
"year": "2020"
},
{
"DOI": "10.1016/j.neures.2020.01.006",
"article-title": "The PINK1–Parkin axis: an overview",
"author": "Tanaka",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Neurosci. Res.",
"key": "10.1016/j.endmts.2024.100163_bb1575",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.3390/molecules27175405",
"article-title": "Degradative effect of nattokinase on spike protein of SARS-CoV-2",
"author": "Tanikawa",
"doi-asserted-by": "crossref",
"first-page": "5405",
"journal-title": "Molecules",
"key": "10.1016/j.endmts.2024.100163_bb1580",
"volume": "27",
"year": "2022"
},
{
"ISBN": "http://id.crossref.org/isbn/0801848490",
"author": "Temkin",
"isbn-type": "print",
"key": "10.1016/j.endmts.2024.100163_bb1585",
"series-title": "The Falling Sickness: A History of Epilepsy From the Greeks to the Beginnings of Modern Neurology",
"volume": "vol. 4",
"year": "1994"
},
{
"DOI": "10.3390/antiox10060967",
"article-title": "N-acetylcysteine (NAC): impacts on human health",
"author": "Tenório",
"doi-asserted-by": "crossref",
"first-page": "967",
"journal-title": "Antioxidants",
"key": "10.1016/j.endmts.2024.100163_bb1590",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1007/s12035-021-02696-0",
"article-title": "Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome?",
"author": "Theoharides",
"doi-asserted-by": "crossref",
"first-page": "1850",
"journal-title": "Mol. Neurobiol.",
"key": "10.1016/j.endmts.2024.100163_bb1595",
"volume": "59",
"year": "2022"
},
{
"article-title": "Nutritional regulation of the insulin-like growth factors",
"author": "Thissen",
"first-page": "80",
"journal-title": "Endocr. Rev.",
"key": "10.1016/j.endmts.2024.100163_bb1600",
"volume": "15",
"year": "1994"
},
{
"DOI": "10.3390/nu14040909",
"article-title": "Populations in low-magnesium areas were associated with higher risk of infection in COVID-19’s Early transmission: a nationwide retrospective cohort study in the United States",
"author": "Tian",
"doi-asserted-by": "crossref",
"first-page": "909",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1605",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1021/acschemneuro.2c00610",
"article-title": "SARS-CoV-2 spike protein downregulates cell surface α7nAChR through a helical motif in the spike neck",
"author": "Tillman",
"doi-asserted-by": "crossref",
"first-page": "689",
"journal-title": "ACS Chem. Neurosci.",
"key": "10.1016/j.endmts.2024.100163_bb1610",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3390/nu14234984",
"article-title": "Effects of L-arginine plus vitamin C supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: a single-blind randomized controlled trial",
"author": "Tosato",
"doi-asserted-by": "crossref",
"first-page": "4984",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1615",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.jphotobiol.2017.04.014",
"article-title": "Biological effects and medical applications of infrared radiation",
"author": "Tsai",
"doi-asserted-by": "crossref",
"first-page": "197",
"journal-title": "J. Photochem. Photobiol. B",
"key": "10.1016/j.endmts.2024.100163_bb1620",
"volume": "170",
"year": "2017"
},
{
"DOI": "10.1016/0014-5793(93)80398-E",
"article-title": "Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae",
"author": "Tsukada",
"doi-asserted-by": "crossref",
"first-page": "169",
"journal-title": "FEBS Lett.",
"key": "10.1016/j.endmts.2024.100163_bb1625",
"volume": "333",
"year": "1993"
},
{
"DOI": "10.1186/1743-7075-11-32",
"article-title": "Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle",
"author": "Tsutsumi",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "Nutrition & Metabolism",
"key": "10.1016/j.endmts.2024.100163_bb1630",
"volume": "11",
"year": "2014"
},
{
"DOI": "10.1007/s12035-017-0712-2",
"article-title": "From mitochondrial function to neuroprotection—an emerging role for methylene blue",
"author": "Tucker",
"doi-asserted-by": "crossref",
"first-page": "5137",
"journal-title": "Mol. Neurobiol.",
"key": "10.1016/j.endmts.2024.100163_bb1635",
"volume": "55",
"year": "2018"
},
{
"DOI": "10.3390/nu14020256",
"article-title": "Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials",
"author": "Vahedian-Azimi",
"doi-asserted-by": "crossref",
"first-page": "256",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1640",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1152/japplphysiol.00550.2015",
"article-title": "The regulation of autophagy during exercise in skeletal muscle",
"author": "Vainshtein",
"doi-asserted-by": "crossref",
"first-page": "664",
"journal-title": "J. Appl. Physiol.",
"key": "10.1016/j.endmts.2024.100163_bb1645",
"volume": "120",
"year": "2016"
},
{
"DOI": "10.1016/j.bbadis.2012.12.011",
"article-title": "Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome",
"author": "Valenti",
"doi-asserted-by": "crossref",
"first-page": "542",
"journal-title": "Biochim. Biophys. Acta (BBA) - Mol. Basis Dis.",
"key": "10.1016/j.endmts.2024.100163_bb1650",
"volume": "1832",
"year": "2013"
},
{
"DOI": "10.1038/s41580-022-00542-2",
"article-title": "The mechanisms and roles of selective autophagy in mammals",
"author": "Vargas",
"doi-asserted-by": "crossref",
"first-page": "167",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "10.1016/j.endmts.2024.100163_bb1655",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/j.jnutbio.2021.108768",
"article-title": "Dietary bioactive compounds as modulators of mitochondrial function",
"author": "Vásquez-Reyes",
"doi-asserted-by": "crossref",
"journal-title": "J. Nutr. Biochem.",
"key": "10.1016/j.endmts.2024.100163_bb1660",
"volume": "96",
"year": "2021"
},
{
"DOI": "10.1007/s13668-018-0233-2",
"article-title": "From religion to secularism: the benefits of fasting",
"author": "Venegas-Borsellino",
"doi-asserted-by": "crossref",
"first-page": "131",
"journal-title": "Curr Nutr Rep",
"key": "10.1016/j.endmts.2024.100163_bb1665",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.2147/JIR.S301625",
"article-title": "Evaluation of adjunctive photobiomodulation (PBMT) for COVID-19 pneumonia via clinical status and pulmonary severity indices in a preliminary trial",
"author": "Vetrici",
"doi-asserted-by": "crossref",
"first-page": "965",
"journal-title": "J. Inflamm. Res.",
"key": "10.1016/j.endmts.2024.100163_bb1670",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1016/B978-0-12-821562-3.00034-4",
"ISBN": "http://id.crossref.org/isbn/9780128215623",
"article-title": "Chapter 28 - ascorbic acid and the mitochondria",
"author": "Vineetha",
"doi-asserted-by": "crossref",
"first-page": "613",
"isbn-type": "print",
"key": "10.1016/j.endmts.2024.100163_bb1675",
"series-title": "Mitochondrial Physiology and Vegetal Molecules",
"year": "2021"
},
{
"DOI": "10.3390/nu14030433",
"article-title": "Traditional and medical applications of fasting",
"author": "Visioli",
"doi-asserted-by": "crossref",
"first-page": "433",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1680",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/nu13041154",
"article-title": "Feasibility of vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV vitamin C on fatigue",
"author": "Vollbracht",
"doi-asserted-by": "crossref",
"first-page": "1154",
"journal-title": "Nutrients",
"key": "10.1016/j.endmts.2024.100163_bb1685",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1080/09553002.2019.1625464",
"article-title": "The effect of red-to-near-infrared (R/NIR) irradiation on inflammatory processes",
"author": "Walski",
"doi-asserted-by": "crossref",
"first-page": "1326",
"journal-title": "Int. J. Radiat. Biol.",
"key": "10.1016/j.endmts.2024.100163_bb1690",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1016/j.heliyon.2020.e04379",
"article-title": "The effects of sunlight exposure therapy on the improvement of depression and quality of life in post-stroke patients: a RCT study",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "Heliyon",
"key": "10.1016/j.endmts.2024.100163_bb1695",
"volume": "6",
"year": "2020"
},
{
"article-title": "Long-term extreme fasting following a traditional Chinese “Bigu” regimen: a preliminary retrospective and prospective cohort study",
"author": "Wang",
"journal-title": "SSRN Journal",
"key": "10.1016/j.endmts.2024.100163_bb1700",
"year": "2018"
},
{
"DOI": "10.1038/s41392-020-00426-x",
"article-title": "CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Sig Transduct Target Ther",
"key": "10.1016/j.endmts.2024.100163_bb1705",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2021.05.009",
"article-title": "Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection",
"author": "Winchester",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "Journal of Infection",
"key": "10.1016/j.endmts.2024.100163_bb1710",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.1016/j.cortex.2018.09.014",
"article-title": "The effect of spermidine on memory performance in older adults at risk for dementia: a randomized controlled trial",
"author": "Wirth",
"doi-asserted-by": "crossref",
"first-page": "181",
"journal-title": "Cortex",
"key": "10.1016/j.endmts.2024.100163_bb1715",
"volume": "109",
"year": "2018"
},
{
"DOI": "10.1016/j.trsl.2021.10.003",
"article-title": "Role of senescence in the chronic health consequences of COVID-19",
"author": "Wissler Gerdes",
"doi-asserted-by": "crossref",
"first-page": "96",
"journal-title": "Transl. Res.",
"key": "10.1016/j.endmts.2024.100163_bb1720",
"volume": "241",
"year": "2022"
},
{
"DOI": "10.1155/2016/1735841",
"article-title": "Amla enhances mitochondrial spare respiratory capacity by increasing mitochondrial biogenesis and antioxidant systems in a murine skeletal muscle cell line",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"journal-title": "Oxidative Med. Cell. Longev.",
"key": "10.1016/j.endmts.2024.100163_bb1725",
"volume": "2016",
"year": "2016"
},
{
"DOI": "10.1038/s41576-022-00562-w",
"article-title": "Autophagy genes in biology and disease",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"first-page": "382",
"journal-title": "Nat. Rev. Genet.",
"key": "10.1016/j.endmts.2024.100163_bb1730",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.4161/auto.24633",
"article-title": "PINK1 is degraded through the N-end rule pathway",
"author": "Yamano",
"doi-asserted-by": "crossref",
"first-page": "1758",
"journal-title": "Autophagy",
"key": "10.1016/j.endmts.2024.100163_bb1735",
"volume": "9",
"year": "2013"
},
{
"DOI": "10.1107/S1744309110043137",
"article-title": "Purification, crystallization and preliminary X-ray diffraction experiment of nattokinase from Bacillus subtilis natto",
"author": "Yanagisawa",
"doi-asserted-by": "crossref",
"first-page": "1670",
"journal-title": "Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun.",
"key": "10.1016/j.endmts.2024.100163_bb1740",
"volume": "66",
"year": "2010"
},
{
"DOI": "10.1146/annurev-nutr-082018-124643",
"article-title": "Mitochondrial DNA mutation, diseases, and nutrient-regulated mitophagy",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "201",
"journal-title": "Annu. Rev. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb1745",
"volume": "39",
"year": "2019"
},
{
"DOI": "10.1038/s41467-020-18319-6",
"article-title": "Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "4541",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.endmts.2024.100163_bb1750",
"volume": "11",
"year": "2020"
},
{
"article-title": "Does methylene blue satisfy an option in COVID-19 ARDS",
"author": "Yella",
"first-page": "62",
"journal-title": "Infectious Disorders - Drug TargetsDisorders",
"key": "10.1016/j.endmts.2024.100163_bb1755",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1039/C4FO00719K",
"article-title": "Mulberry and mulberry wine extract increase the number of mitochondria during brown adipogenesis",
"author": "You",
"doi-asserted-by": "crossref",
"first-page": "401",
"journal-title": "Food Funct.",
"key": "10.1016/j.endmts.2024.100163_bb1760",
"volume": "6",
"year": "2015"
},
{
"DOI": "10.1016/j.jff.2017.07.007",
"article-title": "Mulberry anthocyanins, cyanidin 3-glucoside and cyanidin 3-rutinoside, increase the quantity of mitochondria during brown adipogenesis",
"author": "You",
"doi-asserted-by": "crossref",
"first-page": "348",
"journal-title": "J. Funct. Foods",
"key": "10.1016/j.endmts.2024.100163_bb1765",
"volume": "36",
"year": "2017"
},
{
"DOI": "10.1038/nature09076",
"article-title": "Termination of autophagy and reformation of lysosomes regulated by mTOR",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "942",
"journal-title": "Nature",
"key": "10.1016/j.endmts.2024.100163_bb1770",
"volume": "465",
"year": "2010"
},
{
"DOI": "10.1093/ajcn/71.6.1511",
"article-title": "Resting energy expenditure in short-term starvation is increased as a result of an increase in serum norepinephrine",
"author": "Zauner",
"doi-asserted-by": "crossref",
"first-page": "1511",
"journal-title": "Am. J. Clin. Nutr.",
"key": "10.1016/j.endmts.2024.100163_bb1775",
"volume": "71",
"year": "2000"
},
{
"DOI": "10.1186/s12985-022-01877-0",
"article-title": "SARS-CoV-2 omicron variant clearance delayed in breakthrough cases with elevated fasting blood glucose",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "148",
"journal-title": "Virol. J.",
"key": "10.1016/j.endmts.2024.100163_bb1780",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1038/s41422-021-00495-9",
"article-title": "SARS-CoV-2 spike protein interacts with and activates TLR41",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "818",
"journal-title": "Cell Res.",
"key": "10.1016/j.endmts.2024.100163_bb1785",
"volume": "31",
"year": "2021"
},
{
"article-title": "SARS-CoV-2, long COVID",
"author": "Zhao",
"journal-title": "prion disease and neurodegeneration. Front Neurosci",
"key": "10.1016/j.endmts.2024.100163_bb1790",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1038/ajh.2007.14",
"article-title": "Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species",
"author": "Zhen",
"doi-asserted-by": "crossref",
"first-page": "28",
"journal-title": "Am. J. Hypertens.",
"key": "10.1016/j.endmts.2024.100163_bb1795",
"volume": "21",
"year": "2008"
},
{
"DOI": "10.3389/fmed.2021.566609",
"article-title": "A randomized, single-blind, group sequential, active-controlled study to evaluate the clinical efficacy and safety of α-lipoic acid for critically ill patients with coronavirus disease 2019 (COVID-19)",
"author": "Zhong",
"doi-asserted-by": "crossref",
"journal-title": "Front. Med.",
"key": "10.1016/j.endmts.2024.100163_bb1800",
"volume": "8",
"year": "2022"
},
{
"article-title": "Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance",
"author": "Zhou",
"journal-title": "PLoS One",
"key": "10.1016/j.endmts.2024.100163_bb1805",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1186/s12943-021-01363-1",
"article-title": "The intersection of COVID-19 and cancer: signaling pathways and treatment implications",
"author": "Zong",
"doi-asserted-by": "crossref",
"first-page": "76",
"journal-title": "Mol. Cancer",
"key": "10.1016/j.endmts.2024.100163_bb1810",
"volume": "20",
"year": "2021"
}
],
"reference-count": 362,
"references-count": 362,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2666396124000074"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Endocrinology",
"Endocrinology, Diabetes and Metabolism"
],
"subtitle": [],
"title": "Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "14"
}
halma
