Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far?
et al., ACS Omega, doi:10.1021/acsomega.2c07326, Mar 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
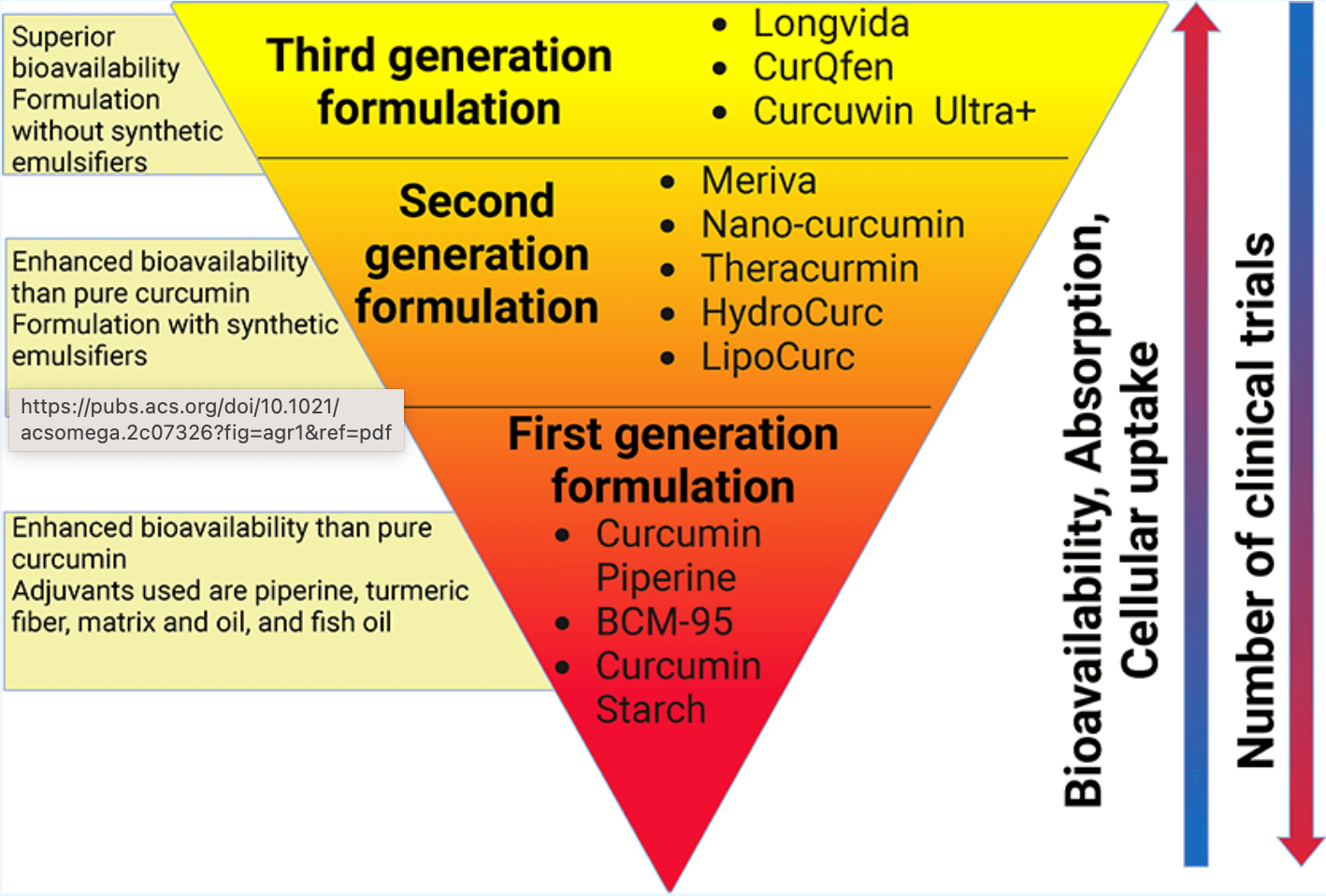
Review of clinical studies on curcumin formulations for improved bioavailability. Authors note that bioavailability has been improved by a factor of >50,000.
1.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
2.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
3.
Duan et al., Bioactive compounds,quercetin, curcumin and β-glucan,regulate innate immunity via the gut-liver-brain axis, Trends in Food Science & Technology, doi:10.1016/j.tifs.2024.104864.
4.
Rajak et al., Antiallergic Implications of Curcumin During COVID-19: Current Status and Perspectives, Biotechnology of Medicinal Plants with Antiallergy Properties, doi:10.1007/978-981-97-1467-4_4.
5.
Kali et al., Curcumin as a Promising Therapy for COVID-19: A Review, Global Journal of Medical, Pharmaceutical, and Biomedical Update, doi:10.25259/GJMPBU_78_2023.
6.
Vajdi et al., Effect of polyphenols against complications of COVID-19: current evidence and potential efficacy, Pharmacological Reports, doi:10.1007/s43440-024-00585-6.
7.
Yong et al., Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases, International Journal of Nanomedicine, doi:10.2147/ijn.s451206.
8.
Halma et al., Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology, Endocrine and Metabolic Science, doi:10.1016/j.endmts.2024.100163.
9.
Arab et al., Immunoregulatory effects of nanocurcumin in inflammatory milieu: Focus on COVID-19, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2024.116131.
10.
Daskou et al., The Role of the NRF2 Pathway in the Pathogenesis of Viral Respiratory Infections, Pathogens, doi:10.3390/pathogens13010039.
11.
Law et al., Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review, Pharmaceuticals, doi:10.3390/ph17010034.
12.
Donzelli, A., Neglected Effective Early Therapies against COVID-19: Focus on Functional Foods and Related Active Substances. A Review, MDPI AG, doi:10.20944/preprints202312.1178.v1.
13.
Hulscher et al., Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination, Cureus, doi:10.7759/cureus.49204.
14.
Hegde et al., Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far?, ACS Omega, doi:10.1021/acsomega.2c07326.
Hegde et al., 13 Mar 2023, peer-reviewed, 5 authors.
Contact: ajai78@gmail.com.
Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far?
ACS Omega, doi:10.1021/acsomega.2c07326
Curcumin has been credited with a wide spectrum of pharmacological properties for the prevention and treatment of several chronic diseases such as arthritis, autoimmune diseases, cancer, cardiovascular diseases, diabetes, hemoglobinopathies, hypertension, infectious diseases, inflammation, metabolic syndrome, neurological diseases, obesity, and skin diseases. However, due to its weak solubility and bioavailability, it has limited potential as an oral medication. Numerous factors including low water solubility, poor intestinal permeability, instability at alkaline pH, and fast metabolism contribute to curcumin's limited oral bioavailability. In order to improve its oral bioavailability, different formulation techniques such as coadministration with piperine, incorporation into micelles, micro/nanoemulsions, nanoparticles, liposomes, solid dispersions, spray drying, and noncovalent complex formation with galactomannosides have been investigated with in vitro cell culture models, in vivo animal models, and humans. In the current study, we extensively reviewed clinical trials on various generations of curcumin formulations and their safety and efficacy in the treatment of many diseases. We also summarized the dose, duration, and mechanism of action of these formulations. We have also critically reviewed the advantages and limitations of each of these formulations compared to various placebo and/or available standard care therapies for these ailments. The highlighted integrative concept embodied in the development of next-generation formulations helps to minimize bioavailability and safety issues with least or no adverse side effects and the provisional new dimensions presented in this direction may add value in the prevention and cure of complex chronic diseases.
Author Contributions M.H. contributed to the initial drafting of the manuscript, review of literature, table preparation, visualization, and overall editing; S.G. contributed to the initial drafting of the manuscript and overall editing; B.B. and R.V. provided critical overall manuscript editing and revision; A.B.K. contributed to conceptualization, funding, overall supervision, and supported review development and overall editing.
Notes The authors declare no competing financial interest.
■ ACKNOWLEDGMENTS Mangala Hegde acknowledges Science and Engineering Research Board (SERB)-National Post-Doctoral Fellowship (NPDF) (PDF/2021/004053). All the figures were created using BioRender.com.
References
Abbasian, Soltani-Zangbar, Khabbazi, Farzaneh, Malek Mahdavi et al., Nanocurcumin supplementation ameliorates Behcet's disease by modulating regulatory T cells: A randomized, double-blind, placebo-controlled trial, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.108237
Abdolahi, Jafarieh, Sarraf, Sedighiyan, Yousefi et al., The Neuromodulatory Effects of omega-3 Fatty Acids and Nano-Curcumin on the COX-2/ iNOS Network in Migraines: A Clinical Trial Study from Gene Expression to Clinical Symptoms, Endocr Metab Immune Disord Drug Targets, doi:10.2174/1871530319666190212170140
Abdolahi, Karimi, Sarraf, Tafakhori, Siri et al., The omega-3 and Nano-curcumin effects on vascular cell adhesion molecule (VCAM) in episodic migraine patients: a randomized clinical trial, BMC Res. Notes, doi:10.1186/s13104-021-05700-x
Abdolahi, Sarraf, Javanbakht, Honarvar, Hatami et al., A Novel Combination of omega-3 Fatty Acids and Nano-Curcumin Modulates Interleukin-6 Gene Expression and High Sensitivity C-reactive Protein Serum Levels in Patients with Migraine: A Randomized Clinical Trial Study, CNS Neurol Disord Drug Targets, doi:10.2174/1871527317666180625101643
Abdolahi, Tafakhori, Togha, Okhovat, Siassi et al., The synergistic effects of omega-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-alpha gene expression and serum level in migraine patients, Immunogenetics, doi:10.1007/s00251-017-0992-8
Ablon, Kogan, Six, -Month, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of a Nutraceutical Supplement for Promoting Hair Growth in Women With Self-Perceived Thinning Hair, J. Drugs Dermatol
Adibian, Hodaei, Nikpayam, Sohrab, Hekmatdoost et al., The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, doubleblind, placebo-controlled trial, Phytother Res, doi:10.1002/ptr.6328
Ahmadi, Agah, Nafissi, Jaafari, Harirchian et al., Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients With Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial, Neurotherapeutics, doi:10.1007/s13311-018-0606-7
Ahmadi, Salari, Sharifi, Reihani, Rostamiani et al., Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial, Food Sci. Nutr, doi:10.1002/fsn3.2226
Al-Askar, Almubarak, Alqutub, Mokeem, Javed et al., Analgesic Efficacy of Curcuma longa (Curcumin) after Surgical Periodontal Therapy, Oral Health Prev Dent, doi:10.3290/j.ohpd.b2572979
Alizadeh, Javadi, Karami, Gholaminejad, Kavianpour et al., Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial, Phytother Res, doi:10.1002/ptr.5998
Amalraj, Divya, Gopi, The Effects of Bioavailable Curcumin (Cureit) on Delayed Onset Muscle Soreness Induced By Eccentric Continuous Exercise: A Randomized, Placebo-Controlled, Double-Blind Clinical Study, J. Med. Food, doi:10.1089/jmf.2019.4533
Amalraj, Varma, Jacob, Divya, Kunnumakkara et al., A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study, J. Med. Food, doi:10.1089/jmf.2017.3930
Amalraj, Varma, Jacob, Kuttappan, Efficacy and safety of a gut health product (Actbiome) prepared by incorporation of asafoetida-curcumin complex onto the turmeric dietary fiber in the management of gut health and intestinal microflora in healthy subjects: A randomized, double-blind, placebo controlled study, Bioactive Carbohydrates and Dietary Fibre, doi:10.1016/j.bcdf.2021.100280
Antiga, Bonciolini, Volpi, Del Bianco, Caproni, Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris, Biomed Res. Int, doi:10.1155/2015/283634
Antony, Merina, Iyer, Judy, Lennertz et al., A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin, Indian J. Pharm. Sci, doi:10.4103/0250-474X.44591
Anusha, Chaly, Junaid, Nijesh, Shivashankar et al., Efficacy of a mouthwash containing essential oils and curcumin as an adjunct to nonsurgical periodontal therapy among rheumatoid arthritis patients with chronic periodontitis: A randomized controlled trial, Indian J. Dent Res, doi:10.4103/ijdr.IJDR_662_17
Appendino, Belcaro, Cornelli, Luzzi, Togni et al., Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study, Panminerva Med
Arabnezhad, Mohammadifard, Rahmani, Majidi, Ferns et al., Effects of curcumin supplementation on vitamin D levels in women with premenstrual syndrome and dysmenorrhea: a randomized controlled study, BMC Complement Med. Ther, doi:10.1186/s12906-022-03515-2
Arun, Nalini, Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats, Plant Foods Hum Nutr, doi:10.1023/A:1013106527829
Asada, Ohara, Muroyama, Yamamoto, Murosaki, Effects of hot water extract of Curcuma longa on human epidermal keratinocytes in vitro and skin conditions in healthy participants: A randomized, double-blind, placebo-controlled trial, J. Cosmet Dermatol, doi:10.1111/jocd.12890
Asadi, Gholami, Siassi, Qorbani, Khamoshian et al., Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial, Complement Ther Med, doi:10.1016/j.ctim.2019.02.014
Asadi, Gholami, Siassi, Qorbani, Sotoudeh, Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: A randomized, double-blind, placebo-controlled clinical trial, Phytother Res, doi:10.1002/ptr.6571
Asadirad, Nashibi, Khodadadi, Ghadiri, Sadeghi et al., Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebocontrolled clinical trial, Phytother Res, doi:10.1002/ptr.7375
Asawanonda, Klahan, Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: a preliminary randomized controlled study, Photomed Laser Surg, doi:10.1089/pho.2009.2637
Asher, Xie, Moaddel, Sanghvi, Dossou et al., Randomized Pharmacokinetic Crossover Study Comparing 2 Curcumin Preparations in Plasma and Rectal Tissue of Healthy Human Volunteers, J. Clin Pharmacol, doi:10.1002/jcph.806
Askari, Sahebkar, Soleimani, Mahdavi, Rafiee et al., The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial, Trials, doi:10.1186/s13063-022-06375-w
Aslanabadi, Entezari-Maleki, Rezaee, Jafarzadeh, Vahedpour, Curcumin for the prevention of myocardial injury following elective percutaneous coronary intervention; a pilot randomized clinical trial, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2019.172471
Atabaki, Shariati-Sarabi, Tavakkol-Afshari, Mohammadi, Significant immunomodulatory properties of curcumin in patients with osteoarthritis; a successful clinical trial in Iran, Int. Immunopharmacol, doi:10.1016/j.intimp.2020.106607
Atabaki, Shariati-Sarabi, Tavakkol-Afshari, Taghipour, Jafari et al., Curcumin as an effective suppressor of miRNA expression in patients with knee osteoarthritis, Avicenna J. Phytomed, doi:10.22038/AJP.2021.19380
Bahrami, Zarban, Rezapour, Agha Amini Fashami, Ferns, Effects of curcumin on menstrual pattern, premenstrual syndrome, and dysmenorrhea: A triple-blind, placebocontrolled clinical trial, Phytother Res, doi:10.1002/ptr.7314
Bakhshi, Gholami, Mahboubi, Jaafari, Namdari, Combination Therapy with 1% Nanocurcumin Gel and 0.1% Triamcinolone Acetonide Mouth Rinse for Oral Lichen Planus: A Randomized Double-Blind Placebo Controlled Clinical Trial, Dermatol Res. Pract, doi:10.1155/2020/4298193
Bakhshi, Mahboubi, Jaafari, Ebrahimi, Tofangchiha et al., Comparative Efficacy of 1% Curcumin Nanomicelle Gel and 2% Curcumin Gel for Treatment of Recurrent Aphthous Stomatitis: A Double-Blind Randomized Clinical Trial, J. Evid Based Dent Pract, doi:10.1016/j.jebdp.2022.101708
Banerjee, Pal, Penmetsa, Kathi, Girish et al., Novel Bioenhanced Curcumin With Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-to-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-controlled Pilot Study, J. Clin Gastroenterol, doi:10.1097/MCG.0000000000001416
Banik, Khatoon, Hegde, Thakur, Puppala et al., A novel bioavailable curcumin-galactomannan complex modulates the genes responsible for the development of chronic diseases in mice: A RNA sequence analysis, Life Sci, doi:10.1016/j.lfs.2021.120074
Bateni, Behrouz, Rahimi, Hedayati, Afsharian et al., Effects of nano-curcumin supplementation on oxidative stress, systemic inflammation, adiponectin, and NF-κB in patients with metabolic syndrome: A randomized, double-blind clinical trial, Journal of Herbal Medicine, doi:10.1016/j.hermed.2021.100531
Bateni, Rahimi, Hedayati, Afsharian, Goudarzi et al., The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: A randomized, double-blind clinical trial, Phytother Res, doi:10.1002/ptr.7109
Belcaro, Cesarone, Dugall, Pellegrini, Ledda et al., Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients, Altern Med. Rev
Belcaro, Cesarone, Dugall, Pellegrini, Ledda et al., Product-evaluation registry of Meriva(R), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis, Panminerva Med
Belcaro, Hosoi, Pellegrini, Appendino, Ippolito et al., A controlled study of a lecithinized delivery system of curcumin (Meriva(R)) to alleviate the adverse effects of cancer treatment, Phytother Res, doi:10.1002/ptr.5014
Bertrand, Wu, Xu, Kamaly, Farokhzad, Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology, Adv. Drug Deliv Rev, doi:10.1016/j.addr.2013.11.009
Bilia, Bergonzi, Isacchi, Antiga, Caproni, Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels, J. Pharm. Pharmacol, doi:10.1111/jphp.12910
Birudaraju, Cherukuri, Kinninger, Chaganti, Shaikh et al., A combined effect of Cavacurcumin, Eicosapentaenoic acid (Omega-3s), Astaxanthin and Gamma -linoleic acid (Omega-6) (CEAG) in healthy volunteers-a randomized, double-blind, placebo-controlled study, Clin Nutr ESPEN, doi:10.1016/j.clnesp.2019.09.011
Bisht, Feldmann, Soni, Ravi, Karikar et al., Polymeric nanoparticle-encapsulated curcumin (″nano-curcumin″): a novel strategy for human cancer therapy, J. Nanobiotechnology, doi:10.1186/1477-3155-5-3
Biswas, Sinha, Mukherjee, Roy, Siddiqi et al., Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal, Hum Exp Toxicol, doi:10.1177/0960327109359020
Bordoloi, Kunnumakkara, The potential of curcumin: a multitargeting agent in cancer cell chemosensitization
Boutry-Regard, Vinyes-Pares, Breuille, Moritani, Supplementation with Whey Protein, Omega-3 Fatty Acids and Polyphenols Combined with Electrical Muscle Stimulation Increases Muscle Strength in Elderly Adults with Limited Mobility: A Randomized Controlled Trial, Nutrients, doi:10.3390/nu12061866
Briata, Paleari, Rutigliani, Petrera, Caviglia et al., A Presurgical Study of Curcumin Combined with Anthocyanin Supplements in Patients with Colorectal Adenomatous Polyps, Int. J. Mol. Sci, doi:10.3390/ijms222011024
Campbell, Ouyang, Charnigo, Westgate, Fleenor, Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial, Nutrition, doi:10.1016/j.nut.2019.01.002
Cardaci, Machek, Wilburn, Hwang, Willoughby, Ubiquitin Proteasome System Activity is Suppressed by Curcumin following Exercise-Induced Muscle Damage in Human Skeletal Muscle, J. Am. Coll Nutr, doi:10.1080/07315724.2020.1783721
Cerletti, Colucci, Storto, Semeraro, Ammollo et al., Randomised trial of chronic supplementation with a nutraceutical mixture in subjects with non-alcoholic fatty liver disease, Br. J. Nutr, doi:10.1017/S0007114519002484
Chandrashekar, Annigeri, Va, Thimmasetty, A clinicobiochemical evaluation of curcumin as gel and as buccal mucoadhesive patches in the management of oral submucous fibrosis. Oral Surg Oral, Med. Oral Pathol Oral Radiol, doi:10.1016/j.oooo.2020.12.020
Chashmniam, Mirhafez, Dehabeh, Hariri, Azimi Nezhad et al., A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebocontrolled trial, Eur. J. Clin Nutr, doi:10.1038/s41430-018-0386-5
Cicero, Sahebkar, Fogacci, Bove, Giovannini et al., Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and nonalcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial, Eur. J. Nutr, doi:10.1007/s00394-019-01916-7
Cosentino, Fratter, Cosentino, Anti-inflammatory effects exerted by Killox(R), an innovative formulation of food supplement with curcumin, in, urology. Eur. Rev. Med. Pharmacol Sci
Cox, Pipingas, Scholey, Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population, J. Psychopharmacol, doi:10.1177/0269881114552744
Cox, White, Pipingas, Poorun, Scholey, Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study, Nutrients, doi:10.3390/nu12061678
Cruz-Correa, Shoskes, Sanchez, Zhao, Hylind et al., Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis, Clin Gastroenterol Hepatol, doi:10.1016/j.cgh.2006.03.020
Cuomo, Appendino, Dern, Schneider, Mckinnon et al., Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation, J. Nat. Prod, doi:10.1021/np1007262?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Db, Sreedharan, Mahadik, Role of piperine as an effective bioenhancer in drug absorption, Pharm. Anal Acta, doi:10.4172/2153-2435.1000591
Delavarian, Pakfetrat, Ghazi, Jaafari, Homaei Shandiz et al., Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers, Spec Care Dentist, doi:10.1111/scd.12358
Derosa, D'angelo, Vanelli, Maffioli, An Evaluation of a Nutraceutical with Berberine, Curcumin, Inositol, Banaba and Chromium Picolinate in Patients with Fasting Dysglycemia, Diabetes Metab Syndr Obes, doi:10.2147/DMSO.S232791
Dhillon, Aggarwal, Newman, Wolff, Kunnumakkara et al., Phase II trial of curcumin in patients with advanced pancreatic cancer, Clin. Cancer Res, doi:10.1158/1078-0432.CCR-08-0024
Di Mario, Cavallaro, Nouvenne, Stefani, Cavestro et al., A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure?, Helicobacter, doi:10.1111/j.1523-5378.2007.00497.x
Di Pierro, Zacconi, Bertuccioli, Togni, Eggenhoffner et al., A naturally-inspired, curcumin-based lecithin formulation (Meriva(R) formulated as the finished product Algocur(R)) alleviates the osteo-muscular pain conditions in rugby players, Eur. Rev. Med. Pharmacol Sci
Disilvestro, Joseph, Zhao, Bomser, Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people, Nutr J, doi:10.1186/1475-2891-11-79
Dizaji, Rivandi, Javandoost, Saberi Karimian, Raei et al., Association of genetic polymorphisms of PON1 and CETP with the presence of metabolic syndrome; the effects of genotypes on their serum activity and concentrations, Egyptian Journal of Medical Human Genetics, doi:10.1016/j.ejmhg.2017.12.001
Djalali, Abdolahi, Hosseini, Miraghajani, Mohammadi et al., The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: A randomized controlled clinical trial, Complement Ther Clin Pract, doi:10.1016/j.ctcp.2020.101256
Djalali, Djalali, Abdolahi, Mohammadi, Heidari et al., The Effect of Nano-Curcumin Supplementation on Pentraxin 3 Gene Expression and Serum Level in Migraine Patients, Rep. Biochem Mol. Biol, doi:10.29252/rbmb.9.1.1
Dolati, Aghebati-Maleki, Ahmadi, Marofi, Babaloo et al., Nanocurcumin restores aberrant miRNA expression profile in multiple sclerosis, randomized, double-blind, placebocontrolled trial, J. Cell Physiol, doi:10.1002/jcp.26301
Dolati, Ahmadi, Rikhtegar, Babaloo, Ayromlou et al., Changes in Th17 cells function after nanocurcumin use to treat multiple sclerosis, Int. Immunopharmacol, doi:10.1016/j.intimp.2018.05.018
Dolati, Babaloo, Ayromlou, Ahmadi, Rikhtegar et al., Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis, J. Neuroimmunol, doi:10.1016/j.jneuroim.2019.01.007
Dominiak, Mckinney, Heilbrun, Sarkar, Critical need for clinical trials: an example of a pilot human intervention trial of a mixture of natural agents protecting lymphocytes against TNF-alpha induced activation of NF-kappaB, Pharm. Res, doi:10.1007/s11095-010-0113-y
Donovan, Kekes-Szabo, Lin, Massey, Cobb et al., A Placebo-Controlled, Pseudo-Randomized, Crossover Trial of Botanical Agents for Gulf War Illness: Curcumin (Curcuma longa), Boswellia (Boswellia serrata), and French Maritime Pine Bark (Pinus pinaster), Int. J. Environ. Res. Public Health, doi:10.3390/ijerph18052468
Duan, Zhang, Han, Chen, Li et al., Synthesis and in vitro/in vivo anticancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles, Int. J. Pharm, doi:10.1016/j.ijpharm.2010.08.033
Durgaprasad, Pai, Vasanthkumar; Alvres, Namitha, A pilot study of the antioxidant effect of curcumin in tropical pancreatitis, Indian J. Med. Res
Dutzmann, Schiborr, Kocher, Pilatus, Hattingen et al., Intratumoral Concentrations and Effects of Orally Administered Micellar Curcuminoids in Glioblastoma Patients, Nutr Cancer, doi:10.1080/01635581.2016.1187281
Elad, Meidan, Sellam, Simaan, Zeevi et al., Topical curcumin for the prevention of oral mucositis in pediatric patients: case series, Altern Ther Health Med
Farhadi, Bakhshandeh, Shafiei, Mahmoudzadeh, Hosseinimehr, The radioprotective effects of nano-curcumin against genotoxicity induced by iodine-131 in patients with differentiated thyroid carcinoma (DTC) by micronucleus assay, International Journal of Cancer Management, doi:10.5812/ijcm.14193?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Farshbaf-Khalili, Farajnia, Pourzeinali, Shakouri, Salehi-Pourmehr, The effect of nanomicelle curcumin supplementation and Nigella sativa oil on the expression level of miRNA-21, miRNA-422a, and miRNA-503 gene in postmenopausal women with low bone mass density: A randomized, triple-blind, placebo-controlled clinical trial with factorial design, Phytother Res, doi:10.1002/ptr.7259
Ferguson, Stojanovski, Macdonald-Wicks, Garg, Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals. A randomised controlled trial, Metabolism, doi:10.1016/j.metabol.2017.12.009
Ferguson, Wolska, Remaley, Stojanovski, Macdonald-Wicks et al., Bread enriched with phytosterols with or without curcumin modulates lipoprotein profiles in hypercholesterolaemic individuals. A randomised controlled trial, Food Funct, doi:10.1039/C8FO02512F
Funamoto, Shimizu, Sunagawa, Katanasaka, Miyazaki et al., Effects of Highly Absorbable Curcumin in Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus, J. Diabetes Res, doi:10.1155/2019/8208237
Funamoto, Sunagawa, Katanasaka, Miyazaki, Imaizumi et al., Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD, Int. J. Chron Obstruct Pulmon Dis, doi:10.2147/COPD.S104490
Ganjali, Sahebkar, Mahdipour, Jamialahmadi, Torabi et al., Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial, ScientificWorldJournal, doi:10.1155/2014/898361
Garcea, Berry, Jones, Singh, Dennison et al., Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences, Cancer Epidemiol Biomarkers Prev, doi:10.1158/1055-9965.120.14.1
Gbolahan, O'neil, Mcree, Sanoff, Fallon et al., A phase I evaluation of the effect of curcumin on doselimiting toxicity and pharmacokinetics of irinotecan in participants with solid tumors, Clin Transl Sci, doi:10.1111/cts.13250
Ghazimoradi, Saberi-Karimian, Mohammadi, Sahebkar, Tavallaie et al., The Effects of Curcumin and Curcumin-Phospholipid Complex on the Serum Prooxidant-Antioxidant Balance in Subjects with Metabolic Syndrome, Phytother Res, doi:10.1002/ptr.5899
Ghodsi, Rahimi, Aghili, Saberi, Shoeibi, Evaluation of curcumin as add-on therapy in patients with Parkinson's disease: A pilot randomized, triple-blind, placebo-controlled trial, Clin Neurol Neurosurg, doi:10.1016/j.clineuro.2022.107300
Gopi, Jacob, Varma, Jude, Amalraj et al., Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study, Phytother Res, doi:10.1002/ptr.5931
Gosangari, Watkin, Effect of preparation techniques on the properties of curcumin liposomes: characterization of size, release and cytotoxicity on a squamous oral carcinoma cell line, Pharm. Dev Technol, doi:10.3109/10837450.2010.522583
Greil, Greil-Ressler, Weiss, Schonlieb, Magnes et al., A phase 1 doseescalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc()) in patients with locally advanced or metastatic cancer, Cancer Chemother Pharmacol, doi:10.1007/s00280-018-3654-0
Gupte, Giramkar, Harke, Kulkarni, Deshmukh et al., Evaluation of the efficacy and safety of Capsule Longvida, doi:10.2147/JIR.S205390
Guru, Reddy, Rao, Padmanabhan, Guru et al., Comparative evaluation of 2% turmeric extract with nanocarrier and 1% chlorhexidine gel as an adjunct to scaling and root planing in patients with chronic periodontitis: A pilot randomized controlled clinical trial, J. Indian Soc. Periodontol, doi:10.4103/jisp.jisp_207_19
Hajialilo, Dolati, Abdolmohammadi-Vahid, Ahmadi, Kamrani et al., Nanocurcumin: A novel strategy in treating ankylosing spondylitis by modulating Th17 cells frequency and function, J. Cell Biochem, doi:10.1002/jcb.28488
Hariri, Gholami, Mirhafez, Bidkhori, Sahebkar, A pilot study of the effect of curcumin on epigenetic changes and DNA damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled, clinical trial, Complement Ther Med, doi:10.1016/j.ctim.2020.102447
Haroyan, Mukuchyan, Mkrtchyan, Minasyan, Gasparyan et al., Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blind, placebo-controlled study, BMC Complement Altern Med, doi:10.1186/s12906-017-2062-z
Hassaniazad, Eftekhar, Inchehsablagh, Kamali, Tousi et al., A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytother Res, doi:10.1002/ptr.7294
Hatab, Abdel Hamid, Soliman, Al-Shafie, None
Hazarey, Sakrikar, Ganvir, Efficacy of curcumin in the treatment for oral submucous fibrosis -A randomized clinical trial, J. Oral Maxillofac Pathol, doi:10.4103/0973-029X.164524
Hellou, Mohsin, Elemy, Hakim, Mustafa-Hellou et al., Effect of ArtemiC in patients with COVID-19: A Phase II prospective study, J. Cell Mol. Med, doi:10.1111/jcmm.17337
Hemmati, Rajaee, Houshmand, Fakhroddin, Dargahi-Malamir et al., Study the effects of antiinflammatory curcumex capsules containing three plants (ginger, curcumin and black pepper) in patients with active rheumatoid arthritis, IIOAB Journal
Heng, Song, Harker, Heng, Druginduced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters, Br J. Dermatol, doi:10.1046/j.1365-2133.2000.03767.x
Henrotin, Gharbi, Dierckxsens, Priem, Marty et al., Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial, BMC Complement Altern Med, doi:10.1186/1472-6882-14-159
Hodaei, Adibian, Nikpayam, Hedayati, Sohrab, The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: a randomized, double-blind clinical trial, Diabetol Metab Syndr, doi:10.1186/s13098-019-0437-7
Honarkar Shafie, Taheri, Alijani, Okhovvat, Goudarzi et al., Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: A randomized double-blind placebo-controlled trial, Phytother Res, doi:10.1002/ptr.7374
Honarvar, Soveid, Abdolahi, Djalali, Hatami et al., Anti-Neuroinflammatory Properties of n-3 Fatty Acids and Nano-Curcumin on Migraine Patients from Cellular to Clinical Insight: A Randomized, Double-Blind and Placebo-Controlled Trial, Endocr Metab Immune Disord Drug Targets, doi:10.2174/1871530320666200729144430
Hosseininasab, Zarghami, Mazhari, Salehifar, Moosazadeh et al., Nanocurcumin as an Add-on to Antipsychotic Drugs for Treatment of Negative Symptoms in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study, J. Clin Psychopharmacol, doi:10.1097/JCP.0000000000001324
Imaizumi, Highly bioavailable curcumin (Theracurmin): Its development and clinical application, PharmaNutrition, doi:10.1016/j.phanu.2015.08.002
Ismail, El-Houseini, A combined treatment of curcumin, piperine, and taurine alters the circulating levels of IL-10 and miR-21 in hepatocellular carcinoma patients: a pilot study, J. Gastrointest Oncol, doi:10.21037/jgo.2019.03.07
Jacob, Amalraj, Raj, Divya, Kunnumakkara et al., A novel bioavailable hydrogenated curcuminoids formulation (CuroWhite) improves symptoms and diagnostic indicators in rheumatoid arthritis patients -A randomized, double blind and placebo controlled study, J. Tradit Complement Med, doi:10.1016/j.jtcme.2018.06.001
Jager, Lowery, Calvanese, Joy, Purpura et al., Comparative absorption of curcumin formulations, Nutr J, doi:10.1186/1475-2891-13-11
Jamwal, Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers, J. Integr Med, doi:10.1016/j.joim.2018.07.001
Javadi, Khadem Haghighian, Goodarzy, Abbasi, Nassiri-Asl, Effect of curcumin nanomicelle on the clinical symptoms of patients with rheumatoid arthritis: A randomized, double-blind, controlled trial, Int. J. Rheum Dis, doi:10.1111/1756-185X.13688
Jazayeri-Tehrani, Rezayat, Mansouri, Qorbani, Alavian et al., Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebocontrolled clinical trial, Nutr Metab (Lond), doi:10.1186/s12986-019-0331-1
Kamaly, Yameen, Wu, Farokhzad, Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release, Chem. Rev, doi:10.1021/acs.chemrev.5b00346?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Kanai, Imaizumi, Otsuka, Sasaki, Hashiguchi et al., Doseescalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers, Cancer Chemother Pharmacol, doi:10.1007/s00280-011-1673-1
Kanai, Otsuka, Otsuka, Sato, Nishimura et al., A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients, Cancer Chemother Pharmacol, doi:10.1007/s00280-013-2151-8
Kanai, Yoshimura, Asada, Imaizumi, Suzuki et al., A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer, Cancer Chemother Pharmacol, doi:10.1007/s00280-010-1470-2
Karandish, Mozaffari-Khosravi, Mohammadi, Cheraghian, Azhdari, Curcumin and zinc co-supplementation along with a loss-weight diet can improve lipid profiles in subjects with prediabetes: a multi-arm, parallel-group, randomized, doubleblind placebo-controlled phase 2 clinical trial, Diabetol Metab Syndr, doi:10.1186/s13098-022-00792-2
Karandish, Mozaffari-Khosravi, Mohammadi, Cheraghian, Azhdari, The effect of curcumin and zinc cosupplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design, Phytother Res, doi:10.1002/ptr.7136
Karimi, Mahmoodpoor, Kooshki, Niazkar, Shoorei et al., Effects of nanocurcumin on inflammatory factors and clinical outcomes in critically ill patients with sepsis: A pilot randomized clinical trial, European Journal of Integrative Medicine, doi:10.1016/j.eujim.2020.101122
Karimi, Naeini, Niazkar, Tutunchi, Musazadeh et al., Nano-curcumin supplementation in critically ill patients with sepsis: a randomized clinical trial investigating the inflammatory biomarkers, oxidative stress indices, endothelial function, clinical outcomes and nutritional status, Food Funct, doi:10.1039/D1FO03746C
Khanna, Das, Kannan, Swick, Matthewman et al., The effects of oral administration of curcumin-galactomannan complex on brain waves are consistent with brain penetration: a randomized, double-blinded, placebo-controlled pilot study, Nutr Neurosci, doi:10.1080/1028415X.2020.1853410
Khanna, Das, Smina, Thomas, Kunnumakkara et al., Curcumagalactomannoside/Glucosamine Combination Improved Joint Health Among Osteoarthritic Subjects as Compared to Chondroitin Sulfate/Glucosamine: Double-Blinded, Randomized Controlled Study, J. Altern Complement Med, doi:10.1089/acm.2020.0128
Khdair, Abdulridha, Shafek, The effect of curcumin adjuvant therapy on pulmonary function and levels of interleukin-6 (IL-6) and superoxide dismutase-3 (EC-SOD3) in patients with chronic bronchial asthma, Indonesian Journal of Pharmacy, doi:10.22146/ijp.1136
Kia, Basirat, Mortezaie, Moosavi, Comparison of oral Nano-Curcumin with oral prednisolone on oral lichen planus: a randomized double-blinded clinical trial, BMC Complement Med. Ther, doi:10.1186/s12906-020-03128-7
Kia, Basirat, Saedi, Arab, Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: a randomized clinical trial, BMC Complement Med. Ther, doi:10.1186/s12906-021-03400-4
Kishimoto, Imaizumi, Wada, Yamakage, Satoh-Asahara et al., Newly Developed Highly Bioavailable Curcumin Formulation, curcuRouge(TM), Reduces Neutrophil/Lymphocyte Ratio in the Elderly: A Double-Blind, Placebo-Controlled Clinical Trial, J. Nutr Sci. Vitaminol, doi:10.3177/jnsv.67.249
Kiso, Suzuki, Watanabe, Oshima, Hikino, Antihepatotoxic principles of Curcuma longa rhizomes, Planta Med, doi:10.1055/s-2007-969845
Klickovic, Doberer, Gouya, Aschauer, Weisshaar et al., Human pharmacokinetics of high dose oral curcumin and its effect on heme oxygenase-1 expression in healthy male subjects, Biomed Res. Int, doi:10.1155/2014/458592
Kocaadam, Sanlier, Curcumin, an active component of turmeric (Curcuma longa), and its effects on health, Crit Rev. Food Sci. Nutr, doi:10.1080/10408398.2015.1077195
Kocher, Bohnert, Schiborr, Frank, Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals, Mol. Nutr Food Res, doi:10.1002/mnfr.201501034
Kothaplly, Alukapally, Nagula, Maddela, Superior Bioavailability of a Novel Curcumin Formulation in Healthy Humans Under Fasting Conditions, Adv. Ther, doi:10.1007/s12325-022-02081-w
Krishnakumar, Maliakel, Gopakumar, Kumar, Maliakel et al., Improved blood−brain-barrier permeability and tissue distribution following the oral administration of a foodgrade formulation of curcumin with fenugreek fibre, Journal of functional foods, doi:10.1016/j.jff.2015.01.049
Kumar, Harsha, Parama, Girisa, Daimary et al., Current clinical developments in curcumin-based therapeutics for cancer and chronic diseases, Phytother Res, doi:10.1002/ptr.7264
Kunnumakkara, Bordoloi, Harsha, Banik, Gupta et al., Curcumin mediates anticancer effects by modulating multiple cell signaling pathways, Clin Sci. (Lond), doi:10.1042/CS20160935
Kunnumakkara, Harsha, Banik, Vikkurthi, Sailo et al., Is curcumin bioavailability a problem in humans: lessons from clinical trials, Expert Opin Drug Metab Toxicol, doi:10.1080/17425255.2019.1650914
Kuszewski, Howe, Wong, Evaluation of Cognitive Performance following Fish-Oil and Curcumin Supplementation in Middle-Aged and Older Adults with Overweight or Obesity, J. Nutr, doi:10.1093/jn/nxaa299
Kuszewski, Wong, Howe, Fish oil supplementation reduces osteoarthritis-specific pain in older adults with overweight/obesity, Rheumatol Adv. Pract, doi:10.1093/rap/rkaa036
Kuszewski, Wong, Wood, Howe, Effects of fish oil and curcumin supplementation on cerebrovascular function in older adults: A randomized controlled trial, Nutr Metab Cardiovasc Dis, doi:10.1016/j.numecd.2019.12.010
Lao, Ruffin, Normolle, Heath, Murray et al., Dose escalation of a curcuminoid formulation, BMC Complement Altern Med, doi:10.1186/1472-6882-6-10
Ledda, Belcaro, Dugall, Luzzi, Scoccianti et al., a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: a pilot, product evaluation registry study, Panminerva Med
Lee, Loo, Young, Traini, Mason et al., Recent advances in curcumin nanoformulation for cancer therapy, Expert Opin Drug Deliv, doi:10.1517/17425247.2014.916686
Lin, Thomas, Chen, Shen, Yang et al., In vitro suppression of oral squamous cell carcinoma growth by ultrasound-mediated delivery of curcumin microemulsions, Int. J. Nanomedicine, doi:10.2147/IJN.S28510
Liu, Robbins, Eyles, Fedorova, Virk et al., Efficacy and safety of a supplement combination on hand pain among people with symptomatic hand osteoarthritis an internet-based, randomised clinical trial the RADIANT study, Osteoarthritis Cartilage, doi:10.1016/j.joca.2021.01.011
Ma, Haddadi, Molavi, Lavasanifar, Lai et al., Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin, J. Biomed Mater. Res, doi:10.1002/jbm.a.31584
Maes, Leunis, Attenuation of autoimmune responses to oxidative specific epitopes, but not nitroso-adducts, is associated with a better clinical outcome in Myalgic Encephalomyelitis/chronic fatigue syndrome, Neuro Endocrinol Lett
Malekzadeh, Kia, Mashaei, Moosavi, Oral nano-curcumin on gingival inflammation in patients with gingivitis and mild periodontitis, Clin Exp Dent Res, doi:10.1002/cre2.330
Mallard, Briskey, Richards, Rao, Curcumin Improves Delayed Onset Muscle Soreness and Postexercise Lactate Accumulation, J. Diet Suppl, doi:10.1080/19390211.2020.1796885
Marczylo, Verschoyle, Cooke, Morazzoni, Steward et al., Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine, Cancer Chemother Pharmacol, doi:10.1007/s00280-006-0355-x
Martinez, Herrera, Frias, Provencio, Perez-Carrion et al., A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: results of a pilot study, Clin Transl Oncol, doi:10.1007/s12094-018-1950-0
Masoodi, Mahdiabadi, Mokhtare, Agah, Kashani et al., The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients' self-reported well-being: A randomized double-blind controlled trial, J. Cell Biochem, doi:10.1002/jcb.27273
Maulina, Diana, Cahyanto, Amaliya, The efficacy of curcumin in managing acute inflammation pain on the post-surgical removal of impacted third molars patients: A randomised controlled trial, J. Oral Rehabil, doi:10.1111/joor.12679
Mazzolani, Togni, Giacomelli, Eggenhoffner, Franceschi, Oral administration of a curcumin-phospholipid formulation (Meriva(R)) for treatment of chronic diabetic macular edema: a pilot study, Eur. Rev. Med. Pharmacol Sci, doi:10.26355/eurrev_201806_15189
Mikirova, Kesari, Ichim, Riordan, Effect of Infla-Kine supplementation on the gene expression of inflammatory markers in peripheral mononuclear cells and on C-reactive protein in blood, J. Transl Med, doi:10.1186/s12967-017-1315-4
Miodownik, Lerner, Kudkaeva, Lerner, Pashinian et al., Curcumin as Add-On to Antipsychotic Treatment in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study, Clin Neuropharmacol, doi:10.1097/WNF.0000000000000344
Miranda-Castro, Aidar, De Moura, Marcucci-Barbosa, Lobo et al., The Curcumin Supplementation with Piperine Can Influence the Acute Elevation of Exercise-Induced Cytokines: Double-Blind Crossover Study, Biology, doi:10.3390/biology11040573
Mirhafez, Farimani, Dehhabe, Bidkhori, Hariri et al., Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial, J. Gastrointestin Liver Dis, doi:10.15403/jgld-179
Mirhafez, Farimani, Gholami, Hooshmand, Tavallaie et al., The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: a randomized, doubleblind, placebo-controlled trial, Drug Metab Pers Ther, doi:10.1515/dmpt-2018-0040
Mirzaei, Shakeri, Rashidi, Jalili, Banikazemi et al., Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies, Biomed Pharmacother, doi:10.1016/j.biopha.2016.11.098
Mogharrabi, Rahimi, Hasanzadeh, Dastani, Kazemi-Oskuee et al., The effects of nanomicelle of curcumin on the matrix metalloproteinase (MMP-2, 9) activity and expression in patients with coronary artery disease (CAD): A randomized controlled clinical trial, ARYA Atheroscler, doi:10.22122/arya.v16i3.1938
Mohajer, Ghayour-Mobarhan, Parizadeh, Tavallaie, Rajabian et al., Effects of supplementation with curcuminoids on serum copper and zinc concentrations and superoxide dismutase enzyme activity in obese subjects, Trace Elem Electrolytes, doi:10.5414/TEX01363
Mohammadi, Ghazi-Moradi, Ghayour-Mobarhan, Esmaeili, Moohebati et al., The Effects of Curcumin on Serum Heat Shock Protein 27 Antibody Titers in Patients with Metabolic Syndrome, J. Diet Suppl, doi:10.1080/19390211.2018.1472710
Mohammadi, Sadeghnia, Saberi-Karimian, Safarian, Ferns et al., Effects of Curcumin on Serum Vitamin E Concentrations in Individuals with Metabolic Syndrome, Phytother Res, doi:10.1002/ptr.5779
Mohammadi, Sahebkar, Iranshahi, Amini, Khojasteh et al., Effects of supplementation with curcuminoids on dyslipidemia in obese patients: a randomized crossover trial, Phytother Res, doi:10.1002/ptr.4715
Mohanty, Sahoo, The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation, Biomaterials, doi:10.1016/j.biomaterials.2010.04.062
Mokhtari, Razzaghi, Momen-Heravi, The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebocontrolled trial, Phytother Res, doi:10.1002/ptr.6957
Moradi Kelardeh, Rahmati-Ahmadabad, Farzanegi, Helalizadeh, Azarbayjani, Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease, J. Bodyw Mov Ther, doi:10.1016/j.jbmt.2020.02.021
Morgia, Russo, Urzi, Privitera, Castelli et al., A phase II, randomized, single-blinded, placebo-controlled clinical trial on the efficacy of Curcumina and Calendula suppositories for the treatment of patients with chronic prostatitis/chronic pelvic pain syndrome type III, Arch Ital Urol Androl, doi:10.4081/aiua.2017.2.110
Morimoto, Sunagawa, Katanasaka, Hirano, Namiki et al., Drinkable preparation of Theracurmin exhibits high absorption efficiency-a single-dose, double-blind, 4-way crossover study, Biol. Pharm. Bull, doi:10.1248/bpb.b13-00150
Mustafa, Ungphaiboon, Phadoongsombut, Pangsomboon, Chelae et al., Effectiveness of an Alcohol-Free Chitosan-Curcuminoid Mouthwash Compared with Chlorhexidine Mouthwash in Denture Stomatitis Treatment: A Randomized Trial, J. Altern Complement Med, doi:10.1089/acm.2018.0459
Naeini, Tutunchi, Razmi, Mahmoodpoor, Vajdi et al., Does nano-curcumin supplementation improve hematological indices in critically ill patients with sepsis? A randomized controlled clinical trial, J. Food Biochem, doi:10.1111/jfbc.14093
Nair, Thulasidasan, Deepa, Anto, Kumar, Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier, Int. J. Pharm, doi:10.1016/j.ijpharm.2012.01.003
Nakagawa, Mori, Yamada, Mukai, Hirose et al., The Oral Administration of Highly-Bioavailable Curcumin for One Year Has Clinical and Chondro-Protective Effects: A Randomized, Double-Blinded, Placebo-Controlled Prospective Study, Arthrosc Sports Med. Rehabil, doi:10.1016/j.asmr.2021.10.016
Nerkar Rajbhoj, Kulkarni, Shete, Shete, Gore et al., A Comparative Study to Evaluate Efficacy of Curcumin and Aloe Vera Gel along with Oral Physiotherapy in the Management of Oral Submucous Fibrosis: A Randomized Clinical Trial. Asian Pac, J. Cancer Prev, doi:10.31557/APJCP.2021.22.S1.107
Ngolab, Donohue, Belsha, Salazar, Cohen et al., Feasibility study for detection of retinal amyloid in clinical trials: The Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) trial, Alzheimers Dement (Amst), doi:10.1002/dad2.12199
Nivetha, Rahmathunisha, Lokeshwari, Kumaresan, Nikkitha et al., Efficacy of nanocurcumin with application of iontophoresis on inflammatory arthritis patients, Research Journal of Pharmacy and Technology, doi:10.52711/0974-360X.2022.00137
O'rawe, Wickremesekera, Pandey, Young, Sim et al., Treatment of glioblastoma with re-purposed renin-angiotensin system modulators: Results of a phase I clinical trial, J. Clin Neurosci, doi:10.1016/j.jocn.2021.11.023
Osali, Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome, Diabetol Metab Syndr, doi:10.1186/s13098-020-00532-4
Pal, Sung, Bhanu Prasad, Schuber, Jr et al., Curcumin glucuronides: assessing the proliferative activity against human cell lines, Bioorg. Med. Chem, doi:10.1016/j.bmc.2013.11.006
Pan, Huang, Lin, Biotransformation of curcumin through reduction and glucuronidation in mice, Drug Metab. Dispos
Panahi, Alishiri, Parvin, Sahebkar, Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled Trial, J. Diet Suppl, doi:10.3109/19390211.2015.1008611
Panahi, Ghanei, Bashiri, Hajihashemi, Sahebkar, Short-term Curcuminoid Supplementation for Chronic Pulmonary Complications due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-blind Placebo-controlled Trial, Drug Res. (Stuttg), doi:10.1055/s-0034-1389986
Panahi, Ghanei, Hajhashemi, Sahebkar, Effects of Curcuminoids-Piperine Combination on Systemic Oxidative Stress, Clinical Symptoms and Quality of Life in Subjects with Chronic Pulmonary Complications Due to Sulfur Mustard: A Randomized Controlled Trial, J. Diet Suppl, doi:10.3109/19390211.2014.952865
Panahi, Hosseini, Khalili, Naimi, Majeed et al., Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis, Clin Nutr, doi:10.1016/j.clnu.2014.12.019
Panahi, Hosseini, Khalili, Naimi, Simental-Mendía et al., Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A posthoc analysis of a randomized controlled trial, Biomedicine & pharmacotherapy, doi:10.1016/j.biopha.2016.05.037
Panahi, Hosseini, Khalili, Naimi, Soflaei et al., Effects of supplementation with curcumin on serum adipokine concentrations: A randomized controlled trial, Nutrition, doi:10.1016/j.nut.2016.03.018
Panahi, Khalili, Hosseini, Abbasinazari, Sahebkar, Lipid-modifying effects of adjunctive therapy with curcuminoids-piperine combination in patients with metabolic syndrome: results of a randomized controlled trial, Complement Ther Med, doi:10.1016/j.ctim.2014.07.006
Panahi, Khalili, Sahebi, Namazi, Atkin et al., Curcuminoids Plus Piperine Modulate Adipokines in Type 2 Diabetes Mellitus, Curr. Clin Pharmacol, doi:10.2174/1574884713666180104095641
Panahi, Khalili, Sahebi, Namazi, Karimian et al., Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial, Inflammopharmacology, doi:10.1007/s10787-016-0301-4
Panahi, Khalili, Sahebi, Namazi, Reiner et al., Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial, Complement Ther Med, doi:10.1016/j.ctim.2017.05.006
Panahi, Khalili, Sahebi, Namazi, Simental-Mendia et al., Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial, Drug Res. (Stuttg), doi:10.1055/s-0044-101752
Panahi, Kianpour, Mohtashami, Jafari, Simental-Mendia et al., Curcumin Lowers Serum Lipids and Uric Acid in Subjects With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial, J. Cardiovasc Pharmacol, doi:10.1097/FJC.0000000000000406
Panahi, Kianpour, Mohtashami, Jafari, Simental-Mendia et al., Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial, Drug Res. (Stuttg), doi:10.1055/s-0043-100019
Panahi, Kianpour, Mohtashami, Soflaei, Sahebkar, Efficacy of phospholipidated curcumin in nonalcoholic fatty liver disease: a clinical study, J. Asian Nat. Prod Res, doi:10.1080/10286020.2018.1505873
Panahi, Saadat, Beiraghdar, Sahebkar, Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial, Phytother Res, doi:10.1002/ptr.5149
Panahi, Sahebkar, Amiri, Davoudi, Beiraghdar et al., Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: results of a randomised, double-blind, placebocontrolled trial, Br. J. Nutr, doi:10.1017/S0007114511006544
Panahi, Sahebkar, Parvin, Saadat, A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications, Ann. Clin Biochem, doi:10.1258/acb.2012.012040
Panahi, Valizadegan, Ahamdi, Ganjali, Majeed et al., Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial, J. Cell Biochem, doi:10.1002/jcb.28877
Pancholi, Smina, Kunnumakkara, Maliakel, Krishnakumar, Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study, Toxicol Rep, doi:10.1016/j.toxrep.2021.06.008
Pandaran Sudheeran, Jacob, Natinga Mulakal, Gopinathan Nair, Maliakel et al., Tolerance, and Enhanced Efficacy of a Bioavailable Formulation of Curcumin With Fenugreek Dietary Fiber on Occupational Stress: A Randomized, Double-Blind, Placebo-Controlled Pilot Study, J. Clin Psychopharmacol, doi:10.1097/JCP.0000000000000508
Parohan, Sarraf, Javanbakht, Foroushani, Ranji-Burachaloo et al., The synergistic effects of nanocurcumin and coenzyme Q10 supplementation in migraine prophylaxis: a randomized, placebo-controlled, double-blind trial, Nutr Neurosci, doi:10.1080/1028415X.2019.1627770
Parravano, Allegrini, Carnevali, Costanzo, Giannaccare et al., Effectiveness of a Hydrophilic Curcumin-Based Formulation in Coadjuvating the Therapeutic Effect of Intravitreal Dexamethasone in Subjects With Diabetic Macular Edema, Front Pharmacol, doi:10.3389/fphar.2021.726104
Pastorelli, Fabricio, Giovanis, D'ippolito, Fiduccia et al., Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial, Pharmacol. Res, doi:10.1016/j.phrs.2018.03.013
Pawar, Mastud, Pawar, Pawar, Bhoite et al., Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial, Front Pharmacol, doi:10.3389/fphar.2021.669362
Perez-Pacheco, Fernandes, Primo, Tedesco, Bellile et al., Local application of curcumin-loaded nanoparticles as an adjunct to scaling and root planing in periodontitis: Randomized, placebo-controlled, double-blind splitmouth clinical trial, Clin Oral Investig, doi:10.1007/s00784-020-03652-3
Petracca, Quarantelli, Moccia, Vacca, Satelliti et al., ProspeCtive study to evaluate efficacy, safety and tOlerability of dietary supplemeNT of Curcumin (BCM95) in subjects with Active relapsing MultIple Sclerosis treated with subcutaNeous Interferon beta 1a 44 mcg TIW (CONTAIN): A randomized, controlled trial, Mult Scler Relat Disord, doi:10.1016/j.msard.2021.103274
Pivari, Mingione, Piazzini, Ceccarani, Ottaviano et al., Curcumin Supplementation (Meriva, doi:10.3390/nu14010231
Piyush, Mahajan, Singh, Ghosh, Gupta, Comparison of therapeutic response of lycopene and curcumin in oral submucous fibrosis: A randomized controlled trial, Oral Dis, doi:10.1111/odi.12947
Portincasa, Bonfrate, Scribano, Kohn, Caporaso et al., Curcumin and Fennel Essential Oil Improve Symptoms and Quality of Life in Patients with Irritable Bowel Syndrome, J. Gastrointestin Liver Dis, doi:10.15403/jgld.2014.1121.252.ccm
Prasad, Tyagi, Aggarwal, Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice, Cancer Res. Treat, doi:10.4143/crt.2014.46.1.2
Priyadarsini, The chemistry of curcumin: from extraction to therapeutic agent, Molecules, doi:10.3390/molecules191220091
Purpura, Lowery, Wilson, Mannan, Munch et al., Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects, Eur. J. Nutr, doi:10.1007/s00394-016-1376-9
Radkar, Lakshmanan, Mary, Chaudhary, Durairaj, A Novel Multi-Ingredient Supplement Reduces Inflammation of the Eye and Improves Production and Quality of Tears in Humans, Ophthalmol Ther, doi:10.1007/s40123-021-00357-y
Rahimnia, Panahi, Alishiri, Sharafi, Sahebkar, Impact of Supplementation with Curcuminoids on Systemic Inflammation in Patients with Knee Osteoarthritis: Findings from a Randomized Double-Blind Placebo-Controlled Trial, Drug Res, doi:10.1055/s-0034-1384536
Rahman, Islam, Akash, Harun-Or-Rashid, Ray et al., Recent advancements of nanoparticles application in cancer and neurodegenerative disorders: At a glance, Biomed Pharmacother, doi:10.1016/j.biopha.2022.113305
Rahmani, Asgary, Askari, Keshvari, Hatamipour et al., Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial, Phytother Res, doi:10.1002/ptr.5659
Rai, Kaur, Gombra, Hasan, Kumar, Comparative evaluation of curcumin and antioxidants in the management of oral submucous fibrosis, J. Investig Clin Dent, doi:10.1111/jicd.12464
Ravindranath, Chandrasekhara, Absorption and tissue distribution of curcumin in rats, Toxicology, doi:10.1016/0300-483X(80)90122-5
Ravindranath, Chandrasekhara, Metabolism of curcumnstudies with [3H] curcumin, Toxicology, doi:10.1016/0300-483X(81)90027-5
Ross, Curcuma longa (Theracumin(R)): A Bioavailable Form of Curcumin and Its Cognitive Benefits, Holist Nurs Pract, doi:10.1097/HNP.0000000000000281
Saadati, Sadeghi, Mansour, Yari, Poustchi et al., Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial, BMC Gastroenterol, doi:10.1186/s12876-019-1055-4
Saadipoor, Razzaghdoust, Simforoosh, Mahdavi, Bakhshandeh et al., Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy, Phytother Res, doi:10.1002/ptr.6230
Saber-Moghaddam, Salari, Hejazi, Amini, Taherzadeh et al., Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial, Phytother Res, doi:10.1002/ptr.7004
Saberi-Karimian, Ghazizadeh, Mohammadzadeh, Ferns, Ghayour-Mobarhan et al., Does curcumin have an effect on sleep duration in metabolic syndrome patients? Avicenna, J. Phytomed
Saberi-Karimian, Keshvari, Ghayour-Mobarhan, Salehizadeh, Rahmani et al., Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial, Complement Ther Med, doi:10.1016/j.ctim.2020.102322
Saberi-Karimian, Parizadeh, Ghayour-Mobarhan, Salahshooh, Dizaji et al., Evaluation of the effects of curcumin in patients with metabolic syndrome, Comparative Clinical Pathology, doi:10.1007/s00580-017-2624-y
Safarian, Parizadeh, Saberi-Karimain, Darroudi, Javandoost et al., The Effect of Curcumin on Serum Copper and Zinc and Zn/Cu Ratio in Individuals with Metabolic Syndrome: A Double-Blind Clinical Trial, J. Diet Suppl, doi:10.1080/19390211.2018.1472711
Saghatelyan, Tananyan, Janoyan, Tadevosyan, Petrosyan et al., Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebocontrolled clinical trial, Phytomedicine, doi:10.1016/j.phymed.2020.153218
Sahebkar, Mohammadi, Atabati, Rahiman, Tavallaie et al., Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals, Phytother Res, doi:10.1002/ptr.4952
Sandoughdaran, Razzaghdoust, Tabibi, Basiri, Simforoosh et al., Double-blind Pilot Study of Nanocurcumin in Bladder Cancer Patients Receiving Induction Chemotherapy, Urol J, doi:10.22037/uj.v0i0.5719
Santos-Parker, Strahler, Bassett, Bispham, Chonchol et al., Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress, Aging, doi:10.18632/aging.101149
Santosa, Suharti, Riwanto, Dharmana, Pangarsa et al., Curcumin as adjuvant therapy to improve remission in myeloma patients: A pilot randomized clinical trial, Caspian J. Intern Med, doi:10.22088/cjim.13.2.9
Sasaki, Sunagawa, Takahashi, Imaizumi, Fukuda et al., Innovative preparation of curcumin for improved oral bioavailability, Biol. Pharm. Bull, doi:10.1248/bpb.34.660
Schiborr, Kocher, Behnam, Jandasek, Toelstede et al., The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes, Mol. Nutr Food Res, doi:10.1002/mnfr.201300724
Sedighiyan, Abdolahi, Jafari, Vahabi, Sohrabi Athar et al., The effects of nano-curcumin supplementation on adipokines levels in obese and overweight patients with migraine: a double blind clinical trial study, BMC Res. Notes, doi:10.1186/s13104-022-06074-4
Shabnam, Harsha, Thakur, Khatoon, Kunnumakkara, Curcumin: a potential molecule for the prevention and treatment of inflammatory diseases, The Chemistry and Bioactive Components of Turmeric; Royal Society of Chemistry
Shadnoush, Zahedi, Norouzy, Sahebkar, Sadeghi et al., Effects of supplementation with curcuminoids on serum adipokines in critically ill patients: a randomized doubleblind placebo-controlled trial, Phytother Res, doi:10.1002/ptr.6749
Shafabakhsh, Asemi, Reiner, Soleimani, Aghadavod et al., The Effects of Nano-curcumin on Metabolic Status in Patients With Diabetes on Hemodialysis, a Randomized, Double Blind, Placebo-controlled Trial. Iran, J. Kidney Dis
Shah, Rath, Sharma, Senapati, Mishra, Effectiveness of curcumin mouthwash on radiation-induced oral mucositis among head and neck cancer patients: A triple-blind, pilot randomised controlled trial, Indian J. Dent Res, doi:10.4103/ijdr.IJDR_822_18
Shapira, Leshno, Katz, Maharshak, Hevroni et al., Of mice and men: a novel dietary supplement for the treatment of ulcerative colitis, Therap Adv. Gastroenterol, doi:10.1177/1756283X17741864
Sharma, Euden, Platton, Cooke, Shafayat et al., Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance, Clin. Cancer Res, doi:10.1158/1078-0432.CCR-04-0744
Sharma, Jain, Bahudar, Oberoi, Efficacy of Curcumin and Piperine as Antioxidant Adjuvant to Intralesional Dexamethasone Injection for Management of Oral Submucous Fibrosis: A Clinical Trial, Journal of Orofacial Sciences, doi:10.4103/jofs.jofs_218_21
Shirmohammadi, Ghayour-Mobarhan, Saberi-Karimian, Iranshahi, Tavallaie et al., Effect of Curcumin on Serum Cathepsin D in Patients with Metabolic Syndrome, Cardiovasc Hematol Disord Drug Targets, doi:10.2174/1871529X19666190919110652
Shoba, Joy, Joseph, Majeed, Rajendran et al., Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers, Planta Med, doi:10.1055/s-2006-957450
Sitzia, Meregalli, Belicchi, Farini, Arosio et al., Preliminary Evidences of Safety and Efficacy of Flavonoids-and Omega 3-Based Compound for Muscular Dystrophies Treatment: A Randomized Double-Blind Placebo Controlled Pilot Clinical Trial, Front Neurol, doi:10.3389/fneur.2019.00755
Small, Siddarth, Li, Miller, Ercoli et al., Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial, Am. J. Geriatr Psychiatry, doi:10.1016/j.jagp.2017.10.010
Soveyd, Abdolahi, Djalali, Hatami, Tafakhori et al., The Combined Effects of omega −3 Fatty Acids and Nano-Curcumin Supplementation on Intercellular Adhesion Molecule-1 (ICAM-1) Gene Expression and Serum Levels in Migraine Patients, CNS Neurol Disord Drug Targets, doi:10.2174/1871527317666171213154749
Srinivasan, Effect of curcumin on blood sugar as seen in a diabetic subject, Indian J. Med. Sci
Stancioiu, Mihai, Papadakis, Tsatsakis, Spandidos et al., Treatment for benign thyroid nodules with a combination of natural extracts, Mol. Med. Rep, doi:10.3892/mmr.2019.10453
Steigerwalt, Nebbioso, Appendino, Belcaro, Ciammaichella et al., a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy, Panminerva Med
Sterzi, Giordani, Morrone, Lena, Magrone et al., The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: a randomized, double-blind, placebocontrolled study, Eur. J. Phys. Rehabil Med
Stohs, Chen, Ray, Ji, Bucci et al., Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review, Molecules, doi:10.3390/molecules25061397
Stohs, Ji, Bucci, Preuss, A Comparative Pharmacokinetic Assessment of a Novel Highly Bioavailable Curcumin Formulation with 95% Curcumin: A Randomized, Double-Blind, Crossover Study, J. Am. Coll Nutr, doi:10.1080/07315724.2017.1358118
Stohs, Ray, Issues with human bioavailability determinations of bioactive curcumin, Biomedical Journal of Scientific & Technical Research, doi:10.26717/BJSTR.2019.12.002289
Sugawara, Akazawa, Miyaki, Choi, Tanabe et al., Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study, Am. J. Hypertens, doi:10.1038/ajh.2012.24
Sugimoto, Ikeya, Bamba, Andoh, Yamasaki et al., Highly Bioavailable Curcumin Derivative Ameliorates Crohn's Disease Symptoms: A Randomized, Double-Blind, Multicenter Study, J. Crohns Colitis, doi:10.1093/ecco-jcc/jjaa097
Sunagawa, Hirano, Katanasaka, Miyazaki, Funamoto et al., Colloidal submicron-particle curcumin exhibits high absorption efficiency-a double-blind, 3-way crossover study, J. Nutr Sci. Vitaminol, doi:10.3177/jnsv.61.37
Sunagawa, Miyazaki, Funamoto, Shimizu, Shimizu et al., A novel amorphous preparation improved curcumin bioavailability in healthy volunteers: A single-dose, double-blind, twoway crossover study, Journal of Functional Foods, doi:10.1016/j.jff.2021.104443
Tahmasebi, El-Esawi, Mahmoud, Timoshin, Valizadeh et al., Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients, J. Cell Physiol, doi:10.1002/jcp.30233
Tahmasebi, Saeed, Temirgalieva, Yumashev, El-Esawi et al., Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2, Life Sci, doi:10.1016/j.lfs.2021.119437
Takahashi, Suzuki, Kim, Otsuka, Imaizumi et al., Effects of curcumin supplementation on exercise-induced oxidative stress in humans, Int. J. Sports Med, doi:10.1055/s-0033-1357185
Talakesh, Tabatabaee, Atoof, Aliasgharzadeh, Sarvizade et al., Effect of Nano-Curcumin on Radiotherapy-Induced Skin Reaction in Breast Cancer Patients: A Randomized, Triple-Blind, Placebo-Controlled Trial, Curr. Radiopharm, doi:10.2174/1874471015666220623104316
Tanabe, Chino, Ohnishi, Ozawa, Sagayama et al., Effects of oral curcumin ingested before or after eccentric exercise on markers of muscle damage and inflammation, Scand J. Med. Sci. Sports, doi:10.1111/sms.13373
Tanabe, Chino, Sagayama, Lee, Ozawa et al., Effective Timing of Curcumin Ingestion to Attenuate Eccentric Exercise-Induced Muscle Soreness in Men, J. Nutr Sci. Vitaminol, doi:10.3177/jnsv.65.82
Tanabe, Maeda, Akazawa, Zempo-Miyaki, Choi et al., Attenuation of indirect markers of eccentric exercise-induced muscle damage by curcumin, Eur. J. Appl. Physiol, doi:10.1007/s00421-015-3170-4
Teiten, Dicato, Diederich, Hybrid curcumin compounds: a new strategy for cancer treatment, Molecules, doi:10.3390/molecules191220839
Thomas, Smina, Khanna, Kunnumakkara, Maliakel et al., Influence of a lowdose supplementation of curcumagalactomannoside complex (CurQfen) in knee osteoarthritis: A randomized, open-labeled, activecontrolled clinical trial, Phytother Res, doi:10.1002/ptr.6907
Thota, Dias, Abbott, Acharya, Garg, Curcumin alleviates postprandial glycaemic response in healthy subjects: A cross-over, randomized controlled study, Sci. Rep, doi:10.1038/s41598-018-32032-x
Thota, Rosato, Dias, Burrows, Martins et al., Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3beta and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer's Disease, Nutrients, doi:10.3390/nu12041032
Tiekou Lorinczova, Begum, Temouri, Renshaw, Zariwala, Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study, Nutrients, doi:10.3390/nu14030712
Tonnesen, Masson, Loftsson, Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability, Int. J. Pharm, doi:10.1016/S0378-5173(02)00323-X
Vafadar-Afshar, Rasmi, Yaghmaei, Khadem-Ansari, Makhdoomi et al., The effects of nanocurcumin supplementation on inflammation in hemodialysis patients: A randomized controlled trial, Hemodial Int, doi:10.1111/hdi.12911
Valizadeh, Abdolmohammadi-Vahid, Danshina, Ziya Gencer, Ammari et al., Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients, Int. Immunopharmacol, doi:10.1016/j.intimp.2020.107088
Varma, Amalraj, Divya, Gopi, The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study, J. Med. Food, doi:10.1089/jmf.2020.4778
Venkatesan, Curcumin attenuation of acute adriamycin myocardial toxicity in rats, Br. J. Pharmacol, doi:10.1038/sj.bjp.0701877
Venkatesan, Punithavathi, Arumugam, Curcumin prevents adriamycin nephrotoxicity in rats, Br. J. Pharmacol, doi:10.1038/sj.bjp.0703067
Volak, Ghirmai, Cashman, Court, Curcuminoids inhibit multiple human cytochromes P450, UDPglucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor, Drug Metab. Dispos, doi:10.1124/dmd.108.020552
Volak, Hanley, Masse, Hazarika, Harmatz et al., Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers, Br. J. Clin. Pharmacol, doi:10.1111/j.1365-2125.2012.04364.x
Wang, Pan, Cheng, Lin, Ho et al., Stability of curcumin in buffer solutions and characterization of its degradation products, J. Pharm. Biomed Anal, doi:10.1016/S0731-7085(96)02024-9
Wolf, Klang, Stojcic, Fuchs, Wolzt et al., NLC versus nanoemulsions: Effect on physiological skin parameters during regular in vivo application and impact on drug penetration, Int. J. Pharm, doi:10.1016/j.ijpharm.2018.08.007
Yang, Lin, Tseng, Wang, Tsai, Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS, J. Chromatogr B Analyt Technol. Biomed Life Sci, doi:10.1016/j.jchromb.2007.03.010
Yeung, Horbanczuk, Tzvetkov, Mocan, Carradori et al., Curcumin: Total-Scale Analysis of the Scientific Literature, Molecules, doi:10.3390/molecules24071393
Zahedi, Hosseinzadeh-Attar, Shadnoush, Sahebkar, Barkhidarian et al., Effects of curcuminoids on inflammatory and oxidative stress biomarkers and clinical outcomes in critically ill patients: A randomized double-blind placebo-controlled trial, Phytother Res, doi:10.1002/ptr.7179
Zamani, Rezagholizadeh, Effect of eight-week curcumin supplementation with endurance training on glycemic indexes in middle age women with type 2 diabetes in Iran, A preliminary study, Diabetes Metab Syndr, doi:10.1016/j.dsx.2021.04.002
Zheng, Mcclements, Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability, Molecules, doi:10.3390/molecules25122791
Zupi, Lazzeri, Centini, Endometriosis and pain: postsurgical alternative treatment in patients desiring pregnancy, Journal of Endometriosis and Pelvic Pain Disorders, doi:10.5301/je.5000226
DOI record:
{
"DOI": "10.1021/acsomega.2c07326",
"ISSN": [
"2470-1343",
"2470-1343"
],
"URL": "http://dx.doi.org/10.1021/acsomega.2c07326",
"alternative-id": [
"10.1021/acsomega.2c07326"
],
"author": [
{
"affiliation": [
{
"name": "Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Assam 781039, India"
}
],
"family": "Hegde",
"given": "Mangala",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Assam 781039, India"
}
],
"family": "Girisa",
"given": "Sosmitha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Assam 781039, India"
}
],
"family": "BharathwajChetty",
"given": "Bandari",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5532-1387",
"affiliation": [
{
"name": "Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Assam 781039, India"
}
],
"authenticated-orcid": true,
"family": "Vishwa",
"given": "Ravichandran",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9121-6816",
"affiliation": [
{
"name": "Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Assam 781039, India"
}
],
"authenticated-orcid": true,
"family": "Kunnumakkara",
"given": "Ajaikumar B.",
"sequence": "additional"
}
],
"container-title": "ACS Omega",
"container-title-short": "ACS Omega",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
3,
13
]
],
"date-time": "2023-03-13T20:25:33Z",
"timestamp": 1678739133000
},
"deposited": {
"date-parts": [
[
2023,
3,
28
]
],
"date-time": "2023-03-28T09:42:43Z",
"timestamp": 1679996563000
},
"funder": [
{
"DOI": "10.13039/501100001843",
"award": [
"PDF/2021/004053"
],
"doi-asserted-by": "publisher",
"name": "Science and Engineering Research Board"
},
{
"DOI": "10.13039/501100001407",
"award": [
"BT/556/NE/U-Excel/2016"
],
"doi-asserted-by": "publisher",
"name": "Department of Biotechnology , Ministry of Science and Technology"
}
],
"indexed": {
"date-parts": [
[
2023,
3,
29
]
],
"date-time": "2023-03-29T04:30:43Z",
"timestamp": 1680064243755
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
3,
13
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2023,
3,
28
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
13
]
],
"date-time": "2023-03-13T00:00:00Z",
"timestamp": 1678665600000
}
}
],
"link": [
{
"URL": "https://pubs.acs.org/doi/pdf/10.1021/acsomega.2c07326",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "unspecified"
},
{
"URL": "https://pubs.acs.org/doi/pdf/10.1021/acsomega.2c07326",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "316",
"original-title": [],
"page": "10713-10746",
"prefix": "10.1021",
"published": {
"date-parts": [
[
2023,
3,
13
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
13
]
]
},
"published-print": {
"date-parts": [
[
2023,
3,
28
]
]
},
"publisher": "American Chemical Society (ACS)",
"reference": [
{
"DOI": "10.1126/science.1230720",
"doi-asserted-by": "publisher",
"key": "ref1/cit1"
},
{
"DOI": "10.3390/molecules27030781",
"doi-asserted-by": "publisher",
"key": "ref2/cit2"
},
{
"DOI": "10.1002/ijc.26442",
"doi-asserted-by": "publisher",
"key": "ref3/cit3"
},
{
"DOI": "10.1182/blood-2007-01-067256",
"doi-asserted-by": "publisher",
"key": "ref4/cit4"
},
{
"DOI": "10.1158/1940-6207.CAPR-13-0058-T",
"doi-asserted-by": "publisher",
"key": "ref5/cit5"
},
{
"DOI": "10.3389/fonc.2022.962066",
"doi-asserted-by": "publisher",
"key": "ref6/cit6"
},
{
"DOI": "10.1007/s10555-022-10068-w",
"doi-asserted-by": "publisher",
"key": "ref7/cit7"
},
{
"DOI": "10.3390/biom9120836",
"doi-asserted-by": "publisher",
"key": "ref8/cit8"
},
{
"DOI": "10.1038/s41392-021-00658-5",
"doi-asserted-by": "publisher",
"key": "ref9/cit9"
},
{
"DOI": "10.1016/j.semcancer.2019.12.008",
"doi-asserted-by": "publisher",
"key": "ref10/cit10"
},
{
"DOI": "10.1002/med.21876",
"doi-asserted-by": "publisher",
"key": "ref11/cit11"
},
{
"DOI": "10.1016/j.phymed.2022.154520",
"doi-asserted-by": "publisher",
"key": "ref12/cit12"
},
{
"DOI": "10.2174/187152012803833035",
"doi-asserted-by": "publisher",
"key": "ref13/cit13"
},
{
"DOI": "10.1039/9781839165894-00001",
"doi-asserted-by": "publisher",
"key": "ref14/cit14"
},
{
"DOI": "10.1142/7150",
"author": "Aggarwal B. B.",
"doi-asserted-by": "crossref",
"key": "ref15/cit15",
"volume-title": "Molecular Targets and Therapeutic Uses of Spices: Modern Uses for Ancient Medicine",
"year": "2009"
},
{
"DOI": "10.1002/mnfr.201100741",
"doi-asserted-by": "publisher",
"key": "ref16/cit16"
},
{
"DOI": "10.1016/j.jddst.2020.102082",
"doi-asserted-by": "publisher",
"key": "ref17/cit17"
},
{
"DOI": "10.1021/mp700113r",
"doi-asserted-by": "publisher",
"key": "ref18/cit18"
},
{
"DOI": "10.1016/j.tifs.2017.11.009",
"doi-asserted-by": "publisher",
"key": "ref19/cit19"
},
{
"DOI": "10.1002/anie.201107724",
"doi-asserted-by": "publisher",
"key": "ref20/cit20"
},
{
"DOI": "10.3390/medicina56070336",
"doi-asserted-by": "publisher",
"key": "ref21/cit21"
},
{
"DOI": "10.1208/s12248-015-9811-z",
"doi-asserted-by": "publisher",
"key": "ref22/cit22"
},
{
"DOI": "10.1016/j.drudis.2011.09.009",
"doi-asserted-by": "publisher",
"key": "ref23/cit23"
},
{
"DOI": "10.1208/s12248-012-9432-8",
"doi-asserted-by": "publisher",
"key": "ref24/cit24"
},
{
"DOI": "10.1016/B978-0-12-815461-8.00006-2",
"author": "Amalraj A.",
"doi-asserted-by": "crossref",
"first-page": "105",
"key": "ref25/cit25",
"volume-title": "Curcumin for Neurological and Psychiatric Disorders",
"year": "2019"
},
{
"DOI": "10.1111/bph.13621",
"doi-asserted-by": "publisher",
"key": "ref26/cit26"
},
{
"DOI": "10.1021/acsptsci.1c00017",
"doi-asserted-by": "publisher",
"key": "ref27/cit27"
},
{
"DOI": "10.1002/ptr.7264",
"doi-asserted-by": "publisher",
"key": "ref28/cit28"
},
{
"DOI": "10.1039/9781788015936-00150",
"author": "Shabnam B.",
"doi-asserted-by": "crossref",
"first-page": "150",
"key": "ref29/cit29",
"volume-title": "The Chemistry and Bioactive Components of Turmeric",
"year": "2020"
},
{
"DOI": "10.1016/j.jtcme.2018.06.001",
"doi-asserted-by": "publisher",
"key": "ref30/cit30"
},
{
"DOI": "10.1055/s-2007-969845",
"doi-asserted-by": "publisher",
"key": "ref31/cit31"
},
{
"DOI": "10.1038/sj.bjp.0701877",
"doi-asserted-by": "publisher",
"key": "ref32/cit32"
},
{
"DOI": "10.1038/sj.bjp.0703067",
"doi-asserted-by": "publisher",
"key": "ref33/cit33"
},
{
"author": "Srinivasan M.",
"first-page": "269",
"issue": "4",
"journal-title": "Indian J. Med. Sci.",
"key": "ref34/cit34",
"volume": "26",
"year": "1972"
},
{
"DOI": "10.1023/A:1013106527829",
"doi-asserted-by": "publisher",
"key": "ref35/cit35"
},
{
"DOI": "10.1042/CS20160935",
"doi-asserted-by": "publisher",
"key": "ref36/cit36"
},
{
"DOI": "10.1016/B978-0-12-812373-7.00002-4",
"author": "Bordoloi D.",
"doi-asserted-by": "crossref",
"first-page": "31",
"key": "ref37/cit37",
"volume-title": "Role of nutraceuticals in cancer chemosensitization",
"year": "2018"
},
{
"DOI": "10.1080/10408398.2015.1077195",
"doi-asserted-by": "publisher",
"key": "ref38/cit38"
},
{
"DOI": "10.3390/molecules24071393",
"doi-asserted-by": "publisher",
"key": "ref39/cit39"
},
{
"DOI": "10.1155/2014/458592",
"doi-asserted-by": "publisher",
"key": "ref40/cit40"
},
{
"DOI": "10.1111/cts.13250",
"doi-asserted-by": "publisher",
"key": "ref41/cit41"
},
{
"DOI": "10.3390/molecules25061397",
"doi-asserted-by": "publisher",
"key": "ref42/cit42"
},
{
"DOI": "10.1517/17425247.2014.916686",
"doi-asserted-by": "publisher",
"key": "ref43/cit43"
},
{
"DOI": "10.1016/j.toxrep.2021.06.008",
"doi-asserted-by": "publisher",
"key": "ref44/cit44"
},
{
"DOI": "10.3390/molecules191220091",
"doi-asserted-by": "publisher",
"key": "ref45/cit45"
},
{
"DOI": "10.1016/S0731-7085(96)02024-9",
"doi-asserted-by": "publisher",
"key": "ref46/cit46"
},
{
"DOI": "10.1016/j.joim.2018.07.001",
"doi-asserted-by": "publisher",
"key": "ref47/cit47"
},
{
"DOI": "10.1080/17425255.2019.1650914",
"doi-asserted-by": "publisher",
"key": "ref48/cit48"
},
{
"DOI": "10.1016/j.phanu.2015.08.002",
"doi-asserted-by": "publisher",
"key": "ref49/cit49"
},
{
"DOI": "10.1186/1472-6882-6-10",
"doi-asserted-by": "publisher",
"key": "ref50/cit50"
},
{
"DOI": "10.1158/1078-0432.CCR-04-0744",
"doi-asserted-by": "publisher",
"key": "ref51/cit51"
},
{
"DOI": "10.1007/s00280-010-1470-2",
"doi-asserted-by": "publisher",
"key": "ref52/cit52"
},
{
"DOI": "10.3390/molecules25122791",
"doi-asserted-by": "publisher",
"key": "ref53/cit53"
},
{
"DOI": "10.4143/crt.2014.46.1.2",
"doi-asserted-by": "publisher",
"key": "ref54/cit54"
},
{
"DOI": "10.1055/s-2006-957450",
"doi-asserted-by": "publisher",
"key": "ref55/cit55"
},
{
"DOI": "10.1016/j.jchromb.2007.03.010",
"doi-asserted-by": "publisher",
"key": "ref56/cit56"
},
{
"author": "Pan M. H.",
"first-page": "486",
"issue": "4",
"journal-title": "Drug Metab. Dispos.",
"key": "ref57/cit57",
"volume": "27",
"year": "1999"
},
{
"DOI": "10.1016/0300-483X(81)90027-5",
"doi-asserted-by": "publisher",
"key": "ref58/cit58"
},
{
"DOI": "10.1016/0300-483X(80)90122-5",
"doi-asserted-by": "publisher",
"key": "ref59/cit59"
},
{
"DOI": "10.1007/s00280-006-0355-x",
"doi-asserted-by": "publisher",
"key": "ref60/cit60"
},
{
"DOI": "10.1158/1055-9965.120.14.1",
"doi-asserted-by": "publisher",
"key": "ref61/cit61"
},
{
"DOI": "10.1158/1078-0432.CCR-08-0024",
"doi-asserted-by": "publisher",
"key": "ref62/cit62"
},
{
"DOI": "10.3390/molecules191220839",
"doi-asserted-by": "publisher",
"key": "ref63/cit63"
},
{
"DOI": "10.1016/j.biopha.2016.11.098",
"doi-asserted-by": "publisher",
"key": "ref64/cit64"
},
{
"DOI": "10.4172/2153-2435.1000591",
"doi-asserted-by": "publisher",
"key": "ref65/cit65"
},
{
"DOI": "10.1124/dmd.108.020552",
"doi-asserted-by": "publisher",
"key": "ref66/cit66"
},
{
"DOI": "10.1016/j.bmc.2013.11.006",
"doi-asserted-by": "publisher",
"key": "ref67/cit67"
},
{
"DOI": "10.26717/BJSTR.2019.12.002289",
"doi-asserted-by": "publisher",
"key": "ref68/cit68"
},
{
"DOI": "10.1080/07315724.2020.1783721",
"doi-asserted-by": "publisher",
"key": "ref69/cit69"
},
{
"DOI": "10.3390/biology11040573",
"doi-asserted-by": "publisher",
"key": "ref70/cit70"
},
{
"DOI": "10.1177/0960327109359020",
"doi-asserted-by": "publisher",
"key": "ref71/cit71"
},
{
"DOI": "10.22146/ijp.1136",
"doi-asserted-by": "publisher",
"key": "ref72/cit72"
},
{
"DOI": "10.3389/fphar.2021.669362",
"doi-asserted-by": "publisher",
"key": "ref73/cit73"
},
{
"DOI": "10.1186/s13063-022-06375-w",
"doi-asserted-by": "publisher",
"key": "ref74/cit74"
},
{
"DOI": "10.1111/odi.12947",
"doi-asserted-by": "publisher",
"key": "ref75/cit75"
},
{
"author": "Durgaprasad S.",
"first-page": "315",
"issue": "4",
"journal-title": "Indian J. Med. Res.",
"key": "ref76/cit76",
"volume": "122",
"year": "2005"
},
{
"DOI": "10.2174/1574884713666180104095641",
"doi-asserted-by": "publisher",
"key": "ref77/cit77"
},
{
"author": "Hemmati A. A.",
"first-page": "389",
"issue": "5",
"journal-title": "IIOAB Journal",
"key": "ref78/cit78",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.21037/jgo.2019.03.07",
"doi-asserted-by": "publisher",
"key": "ref79/cit79"
},
{
"DOI": "10.1016/j.jocn.2021.11.023",
"doi-asserted-by": "publisher",
"key": "ref80/cit80"
},
{
"DOI": "10.3892/mmr.2019.10453",
"doi-asserted-by": "publisher",
"key": "ref81/cit81"
},
{
"DOI": "10.15403/jgld.2014.1121.252.ccm",
"doi-asserted-by": "publisher",
"key": "ref82/cit82"
},
{
"DOI": "10.1016/j.cgh.2006.03.020",
"doi-asserted-by": "publisher",
"key": "ref83/cit83"
},
{
"DOI": "10.1111/j.1523-5378.2007.00497.x",
"doi-asserted-by": "publisher",
"key": "ref84/cit84"
},
{
"DOI": "10.4081/aiua.2017.2.110",
"doi-asserted-by": "publisher",
"key": "ref85/cit85"
},
{
"DOI": "10.1097/WNF.0000000000000344",
"doi-asserted-by": "publisher",
"key": "ref86/cit86"
},
{
"DOI": "10.1089/acm.2018.0459",
"doi-asserted-by": "publisher",
"key": "ref87/cit87"
},
{
"DOI": "10.4103/ijdr.IJDR_662_17",
"doi-asserted-by": "publisher",
"key": "ref88/cit88"
},
{
"DOI": "10.1055/s-0034-1389986",
"doi-asserted-by": "publisher",
"key": "ref89/cit89"
},
{
"DOI": "10.1055/s-0044-101752",
"doi-asserted-by": "publisher",
"key": "ref90/cit90"
},
{
"DOI": "10.3109/19390211.2015.1008611",
"doi-asserted-by": "publisher",
"key": "ref91/cit91"
},
{
"DOI": "10.1016/j.ctim.2014.07.006",
"doi-asserted-by": "publisher",
"key": "ref92/cit92"
},
{
"DOI": "10.1016/j.clnu.2014.12.019",
"doi-asserted-by": "publisher",
"key": "ref93/cit93"
},
{
"DOI": "10.1016/j.biopha.2016.05.037",
"doi-asserted-by": "publisher",
"key": "ref94/cit94"
},
{
"DOI": "10.1016/j.nut.2016.03.018",
"doi-asserted-by": "publisher",
"key": "ref95/cit95"
},
{
"DOI": "10.1002/jcb.28877",
"doi-asserted-by": "publisher",
"key": "ref96/cit96"
},
{
"DOI": "10.1016/j.ctim.2020.102322",
"doi-asserted-by": "publisher",
"key": "ref97/cit97"
},
{
"DOI": "10.1002/ptr.4715",
"doi-asserted-by": "publisher",
"key": "ref98/cit98"
},
{
"DOI": "10.1017/S0007114511006544",
"doi-asserted-by": "publisher",
"key": "ref99/cit99"
},
{
"DOI": "10.1002/ptr.6749",
"doi-asserted-by": "publisher",
"key": "ref100/cit100"
},
{
"DOI": "10.1002/ptr.7179",
"doi-asserted-by": "publisher",
"key": "ref101/cit101"
},
{
"DOI": "10.1186/s12906-022-03515-2",
"doi-asserted-by": "publisher",
"key": "ref102/cit102"
},
{
"DOI": "10.1002/ptr.7314",
"doi-asserted-by": "publisher",
"key": "ref103/cit103"
},
{
"DOI": "10.1007/s10787-016-0301-4",
"doi-asserted-by": "publisher",
"key": "ref104/cit104"
},
{
"DOI": "10.1002/ptr.4952",
"doi-asserted-by": "publisher",
"key": "ref105/cit105"
},
{
"DOI": "10.5414/TEX01363",
"doi-asserted-by": "publisher",
"key": "ref106/cit106"
},
{
"DOI": "10.1155/2014/898361",
"doi-asserted-by": "publisher",
"key": "ref107/cit107"
},
{
"DOI": "10.1055/s-0034-1384536",
"doi-asserted-by": "publisher",
"key": "ref108/cit108"
},
{
"DOI": "10.1258/acb.2012.012040",
"doi-asserted-by": "publisher",
"key": "ref109/cit109"
},
{
"DOI": "10.3109/19390211.2014.952865",
"doi-asserted-by": "publisher",
"key": "ref110/cit110"
},
{
"DOI": "10.1016/j.ctim.2017.05.006",
"doi-asserted-by": "publisher",
"key": "ref111/cit111"
},
{
"DOI": "10.1515/dmpt-2018-0040",
"doi-asserted-by": "publisher",
"key": "ref112/cit112"
},
{
"DOI": "10.1111/jicd.12464",
"doi-asserted-by": "publisher",
"key": "ref113/cit113"
},
{
"DOI": "10.4103/jofs.jofs_218_21",
"doi-asserted-by": "publisher",
"key": "ref114/cit114"
},
{
"DOI": "10.1111/j.1365-2125.2012.04364.x",
"doi-asserted-by": "publisher",
"key": "ref115/cit115"
},
{
"DOI": "10.3177/jnsv.61.37",
"doi-asserted-by": "publisher",
"key": "ref116/cit116"
},
{
"DOI": "10.4103/0250-474X.44591",
"doi-asserted-by": "publisher",
"key": "ref117/cit117"
},
{
"DOI": "10.22088/cjim.13.2.9",
"doi-asserted-by": "publisher",
"key": "ref118/cit118"
},
{
"DOI": "10.1016/j.msard.2021.103274",
"doi-asserted-by": "publisher",
"key": "ref119/cit119"
},
{
"DOI": "10.1186/s12876-019-1055-4",
"doi-asserted-by": "publisher",
"key": "ref120/cit120"
},
{
"DOI": "10.1186/s13098-022-00792-2",
"doi-asserted-by": "publisher",
"key": "ref121/cit121"
},
{
"DOI": "10.1017/S0007114519002484",
"doi-asserted-by": "publisher",
"key": "ref122/cit122"
},
{
"author": "Sterzi S.",
"first-page": "321",
"issue": "3",
"journal-title": "Eur. J. Phys. Rehabil Med.",
"key": "ref123/cit123",
"volume": "52",
"year": "2016"
},
{
"DOI": "10.1186/s12906-017-2062-z",
"doi-asserted-by": "publisher",
"key": "ref124/cit124"
},
{
"DOI": "10.3290/j.ohpd.b2572979",
"doi-asserted-by": "publisher",
"key": "ref125/cit125"
},
{
"DOI": "10.1002/ptr.7136",
"doi-asserted-by": "publisher",
"key": "ref126/cit126"
},
{
"DOI": "10.1111/jcmm.17337",
"doi-asserted-by": "publisher",
"key": "ref127/cit127"
},
{
"DOI": "10.3390/nu12061866",
"doi-asserted-by": "publisher",
"key": "ref128/cit128"
},
{
"DOI": "10.1016/j.phymed.2020.153218",
"doi-asserted-by": "publisher",
"key": "ref129/cit129"
},
{
"DOI": "10.1177/1756283X17741864",
"doi-asserted-by": "publisher",
"key": "ref130/cit130"
},
{
"author": "Elad S.",
"first-page": "21",
"issue": "3",
"journal-title": "Altern Ther Health Med.",
"key": "ref131/cit131",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.1002/ptr.5659",
"doi-asserted-by": "publisher",
"key": "ref132/cit132"
},
{
"DOI": "10.1055/s-0033-1357185",
"doi-asserted-by": "publisher",
"key": "ref133/cit133"
},
{
"DOI": "10.1089/jmf.2020.4778",
"doi-asserted-by": "publisher",
"key": "ref134/cit134"
},
{
"DOI": "10.1002/ptr.5931",
"doi-asserted-by": "publisher",
"key": "ref135/cit135"
},
{
"DOI": "10.1089/jmf.2019.4533",
"doi-asserted-by": "publisher",
"key": "ref136/cit136"
},
{
"DOI": "10.1007/s00394-016-1376-9",
"doi-asserted-by": "publisher",
"key": "ref137/cit137"
},
{
"DOI": "10.1089/jmf.2017.3930",
"doi-asserted-by": "publisher",
"key": "ref138/cit138"
},
{
"DOI": "10.1002/ptr.6328",
"doi-asserted-by": "publisher",
"key": "ref139/cit139"
},
{
"DOI": "10.1186/s13098-019-0437-7",
"doi-asserted-by": "publisher",
"key": "ref140/cit140"
},
{
"DOI": "10.1046/j.1365-2133.2000.03767.x",
"doi-asserted-by": "publisher",
"key": "ref141/cit141"
},
{
"DOI": "10.31557/APJCP.2021.22.S1.107",
"doi-asserted-by": "publisher",
"key": "ref142/cit142"
},
{
"DOI": "10.1016/j.oooo.2020.12.020",
"doi-asserted-by": "publisher",
"key": "ref143/cit143"
},
{
"DOI": "10.1186/s12967-017-1315-4",
"doi-asserted-by": "publisher",
"key": "ref144/cit144"
},
{
"DOI": "10.5301/je.5000226",
"doi-asserted-by": "publisher",
"key": "ref145/cit145"
},
{
"author": "Cosentino V.",
"first-page": "1390",
"issue": "7",
"journal-title": "Eur. Rev. Med. Pharmacol Sci.",
"key": "ref146/cit146",
"volume": "20",
"year": "2016"
},
{
"DOI": "10.1007/s40123-021-00357-y",
"doi-asserted-by": "publisher",
"key": "ref147/cit147"
},
{
"DOI": "10.1007/s11095-010-0113-y",
"doi-asserted-by": "publisher",
"key": "ref148/cit148"
},
{
"author": "Maes M.",
"first-page": "577",
"issue": "7",
"journal-title": "Neuro Endocrinol Lett.",
"key": "ref149/cit149",
"volume": "35",
"year": "2014"
},
{
"DOI": "10.1007/s12094-018-1950-0",
"doi-asserted-by": "publisher",
"key": "ref150/cit150"
},
{
"DOI": "10.2147/DMSO.S232791",
"doi-asserted-by": "publisher",
"key": "ref151/cit151"
},
{
"DOI": "10.1111/jocd.12890",
"doi-asserted-by": "publisher",
"key": "ref152/cit152"
},
{
"author": "Ablon G.",
"first-page": "558",
"issue": "5",
"journal-title": "J. Drugs Dermatol",
"key": "ref153/cit153",
"volume": "17",
"year": "2018"
},
{
"DOI": "10.1016/j.bcdf.2021.100280",
"doi-asserted-by": "publisher",
"key": "ref154/cit154"
},
{
"DOI": "10.1080/07315724.2017.1358118",
"doi-asserted-by": "publisher",
"key": "ref155/cit155"
},
{
"DOI": "10.1016/j.clnesp.2019.09.011",
"doi-asserted-by": "publisher",
"key": "ref156/cit156"
},
{
"DOI": "10.1186/1475-2891-13-11",
"doi-asserted-by": "publisher",
"key": "ref157/cit157"
},
{
"DOI": "10.3389/fphar.2021.726104",
"doi-asserted-by": "publisher",
"key": "ref158/cit158"
},
{
"DOI": "10.1007/s00394-019-01916-7",
"doi-asserted-by": "publisher",
"key": "ref159/cit159"
},
{
"DOI": "10.1002/mnfr.201501034",
"doi-asserted-by": "publisher",
"key": "ref160/cit160"
},
{
"DOI": "10.1002/mnfr.201300724",
"doi-asserted-by": "publisher",
"key": "ref161/cit161"
},
{
"DOI": "10.1080/01635581.2016.1187281",
"doi-asserted-by": "publisher",
"key": "ref162/cit162"
},
{
"DOI": "10.1016/j.ejphar.2019.172471",
"doi-asserted-by": "publisher",
"key": "ref163/cit163"
},
{
"DOI": "10.3389/fneur.2019.00755",
"doi-asserted-by": "publisher",
"key": "ref164/cit164"
},
{
"DOI": "10.1186/1472-6882-14-159",
"doi-asserted-by": "publisher",
"key": "ref165/cit165"
},
{
"DOI": "10.1016/j.joca.2021.01.011",
"doi-asserted-by": "publisher",
"key": "ref166/cit166"
},
{
"DOI": "10.3390/nu14030712",
"doi-asserted-by": "publisher",
"key": "ref167/cit167"
},
{
"DOI": "10.1080/19390211.2020.1796885",
"doi-asserted-by": "publisher",
"key": "ref168/cit168"
},
{
"DOI": "10.1007/s00280-018-3654-0",
"doi-asserted-by": "publisher",
"key": "ref169/cit169"
},
{
"DOI": "10.1002/ptr.5779",
"doi-asserted-by": "publisher",
"key": "ref170/cit170"
},
{
"DOI": "10.1016/S0378-5173(02)00323-X",
"doi-asserted-by": "publisher",
"key": "ref171/cit171"
},
{
"DOI": "10.1002/jbm.a.31584",
"doi-asserted-by": "publisher",
"key": "ref172/cit172"
},
{
"DOI": "10.1021/np1007262",
"doi-asserted-by": "publisher",
"key": "ref173/cit173"
},
{
"DOI": "10.1002/jcph.806",
"doi-asserted-by": "publisher",
"key": "ref174/cit174"
},
{
"DOI": "10.3390/nu14010231",
"doi-asserted-by": "publisher",
"key": "ref175/cit175"
},
{
"author": "Appendino G.",
"first-page": "43",
"issue": "3",
"journal-title": "Panminerva Med.",
"key": "ref176/cit176",
"volume": "53",
"year": "2011"
},
{
"author": "Steigerwalt R.",
"first-page": "11",
"issue": "1",
"journal-title": "Panminerva Med.",
"key": "ref177/cit177",
"volume": "54",
"year": "2012"
},
{
"DOI": "10.3390/ijerph18052468",
"doi-asserted-by": "publisher",
"key": "ref178/cit178"
},
{
"DOI": "10.1016/j.metabol.2017.12.009",
"doi-asserted-by": "publisher",
"key": "ref179/cit179"
},
{
"DOI": "10.1080/19390211.2018.1472711",
"doi-asserted-by": "publisher",
"key": "ref180/cit180"
},
{
"DOI": "10.1097/FJC.0000000000000406",
"doi-asserted-by": "publisher",
"key": "ref181/cit181"
},
{
"DOI": "10.1055/s-0043-100019",
"doi-asserted-by": "publisher",
"key": "ref182/cit182"
},
{
"DOI": "10.1080/10286020.2018.1505873",
"doi-asserted-by": "publisher",
"key": "ref183/cit183"
},
{
"DOI": "10.1016/j.ctim.2020.102447",
"doi-asserted-by": "publisher",
"key": "ref184/cit184"
},
{
"author": "Belcaro G.",
"first-page": "55",
"issue": "2",
"journal-title": "Panminerva Med.",
"key": "ref185/cit185",
"volume": "52",
"year": "2010"
},
{
"author": "Belcaro G.",
"first-page": "337",
"issue": "4",
"journal-title": "Altern Med. Rev.",
"key": "ref186/cit186",
"volume": "15",
"year": "2010"
},
{
"DOI": "10.1016/j.phrs.2018.03.013",
"doi-asserted-by": "publisher",
"key": "ref187/cit187"
},
{
"author": "Ledda A.",
"first-page": "17",
"issue": "1",
"journal-title": "Panminerva Med.",
"key": "ref188/cit188",
"volume": "54",
"year": "2012"
},
{
"DOI": "10.1155/2015/283634",
"doi-asserted-by": "publisher",
"key": "ref189/cit189"
},
{
"DOI": "10.3390/nu12041032",
"doi-asserted-by": "publisher",
"key": "ref190/cit190"
},
{
"DOI": "10.1002/ptr.5149",
"doi-asserted-by": "publisher",
"key": "ref191/cit191"
},
{
"DOI": "10.1002/ptr.5014",
"doi-asserted-by": "publisher",
"key": "ref192/cit192"
},
{
"DOI": "10.1038/s41430-018-0386-5",
"doi-asserted-by": "publisher",
"key": "ref193/cit193"
},
{
"DOI": "10.15403/jgld-179",
"doi-asserted-by": "publisher",
"key": "ref194/cit194"
},
{
"DOI": "10.26355/eurrev_201806_15189",
"doi-asserted-by": "publisher",
"key": "ref195/cit195"
},
{
"DOI": "10.1038/s41598-018-32032-x",
"doi-asserted-by": "publisher",
"key": "ref196/cit196"
},
{
"DOI": "10.1039/C8FO02512F",
"doi-asserted-by": "publisher",
"key": "ref197/cit197"
},
{
"DOI": "10.3390/ijms222011024",
"doi-asserted-by": "publisher",
"key": "ref198/cit198"
},
{
"author": "Di Pierro F.",
"first-page": "4935",
"issue": "21",
"journal-title": "Eur. Rev. Med. Pharmacol Sci.",
"key": "ref199/cit199",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.1016/j.ijpharm.2018.08.007",
"doi-asserted-by": "publisher",
"key": "ref200/cit200"
},
{
"DOI": "10.1097/MCG.0000000000001416",
"doi-asserted-by": "publisher",
"key": "ref201/cit201"
},
{
"DOI": "10.1089/pho.2009.2637",
"doi-asserted-by": "publisher",
"key": "ref202/cit202"
},
{
"DOI": "10.1016/j.ejmhg.2017.12.001",
"doi-asserted-by": "publisher",
"key": "ref203/cit203"
},
{
"DOI": "10.1002/ptr.5899",
"doi-asserted-by": "publisher",
"key": "ref204/cit204"
},
{
"DOI": "10.1080/19390211.2018.1472710",
"doi-asserted-by": "publisher",
"key": "ref205/cit205"
},
{
"DOI": "10.1007/s00580-017-2624-y",
"doi-asserted-by": "publisher",
"key": "ref206/cit206"
},
{
"DOI": "10.2174/1871529X19666190919110652",
"doi-asserted-by": "publisher",
"key": "ref207/cit207"
},
{
"author": "Saberi-Karimian M.",
"first-page": "190",
"issue": "2",
"journal-title": "Avicenna J. Phytomed",
"key": "ref208/cit208",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/1477-3155-5-3",
"doi-asserted-by": "publisher",
"key": "ref209/cit209"
},
{
"DOI": "10.1016/j.ijpharm.2010.08.033",
"doi-asserted-by": "publisher",
"key": "ref210/cit210"
},
{
"DOI": "10.1016/j.ijpharm.2012.01.003",
"doi-asserted-by": "publisher",
"key": "ref211/cit211"
},
{
"DOI": "10.1016/j.biomaterials.2010.04.062",
"doi-asserted-by": "publisher",
"key": "ref212/cit212"
},
{
"DOI": "10.1016/j.addr.2013.11.009",
"doi-asserted-by": "publisher",
"key": "ref213/cit213"
},
{
"DOI": "10.1016/j.biopha.2022.113305",
"doi-asserted-by": "publisher",
"key": "ref214/cit214"
},
{
"DOI": "10.1021/acs.chemrev.5b00346",
"doi-asserted-by": "publisher",
"key": "ref215/cit215"
},
{
"DOI": "10.3109/10837450.2010.522583",
"doi-asserted-by": "publisher",
"key": "ref216/cit216"
},
{
"DOI": "10.2147/IJN.S28510",
"doi-asserted-by": "publisher",
"key": "ref217/cit217"
},
{
"DOI": "10.1007/s13311-018-0606-7",
"doi-asserted-by": "publisher",
"key": "ref218/cit218"
},
{
"DOI": "10.1002/jcb.28488",
"doi-asserted-by": "publisher",
"key": "ref219/cit219"
},
{
"DOI": "10.22122/arya.v16i3.1938",
"doi-asserted-by": "publisher",
"key": "ref220/cit220"
},
{
"DOI": "10.1002/fsn3.2226",
"doi-asserted-by": "publisher",
"key": "ref221/cit221"
},
{
"DOI": "10.1002/ptr.7374",
"doi-asserted-by": "publisher",
"key": "ref222/cit222"
},
{
"DOI": "10.1111/scd.12358",
"doi-asserted-by": "publisher",
"key": "ref223/cit223"
},
{
"DOI": "10.1002/ptr.6230",
"doi-asserted-by": "publisher",
"key": "ref224/cit224"
},
{
"DOI": "10.1111/1756-185X.13688",
"doi-asserted-by": "publisher",
"key": "ref225/cit225"
},
{
"DOI": "10.1016/j.intimp.2020.106607",
"doi-asserted-by": "publisher",
"key": "ref226/cit226"
},
{
"DOI": "10.1111/jfbc.14093",
"doi-asserted-by": "publisher",
"key": "ref227/cit227"
},
{
"DOI": "10.5812/ijcm.14193",
"doi-asserted-by": "publisher",
"key": "ref228/cit228"
},
{
"DOI": "10.1002/ptr.5998",
"doi-asserted-by": "publisher",
"key": "ref229/cit229"
},
{
"DOI": "10.1002/ptr.7109",
"doi-asserted-by": "publisher",
"key": "ref230/cit230"
},
{
"DOI": "10.1016/j.clineuro.2022.107300",
"doi-asserted-by": "publisher",
"key": "ref231/cit231"
},
{
"DOI": "10.1002/jcp.26301",
"doi-asserted-by": "publisher",
"key": "ref232/cit232"
},
{
"DOI": "10.1097/JCP.0000000000001324",
"doi-asserted-by": "publisher",
"key": "ref233/cit233"
},
{
"DOI": "10.1111/joor.12679",
"doi-asserted-by": "publisher",
"key": "ref234/cit234"
},
{
"DOI": "10.1002/jcb.27273",
"doi-asserted-by": "publisher",
"key": "ref235/cit235"
},
{
"DOI": "10.22038/AJP.2021.19380",
"doi-asserted-by": "publisher",
"key": "ref236/cit236"
},
{
"DOI": "10.1016/j.intimp.2021.108237",
"doi-asserted-by": "publisher",
"key": "ref237/cit237"
},
{
"DOI": "10.1016/j.intimp.2020.107088",
"doi-asserted-by": "publisher",
"key": "ref238/cit238"
},
{
"DOI": "10.1016/j.lfs.2021.119437",
"doi-asserted-by": "publisher",
"key": "ref239/cit239"
},
{
"DOI": "10.1002/ptr.7294",
"doi-asserted-by": "publisher",
"key": "ref240/cit240"
},
{
"DOI": "10.1002/jcp.30233",
"doi-asserted-by": "publisher",
"key": "ref241/cit241"
},
{
"DOI": "10.1002/ptr.7375",
"doi-asserted-by": "publisher",
"key": "ref242/cit242"
},
{
"DOI": "10.1002/ptr.7004",
"doi-asserted-by": "publisher",
"key": "ref243/cit243"
},
{
"DOI": "10.1016/j.eujim.2020.101122",
"doi-asserted-by": "publisher",
"key": "ref244/cit244"
},
{
"DOI": "10.1039/D1FO03746C",
"doi-asserted-by": "publisher",
"key": "ref245/cit245"
},
{
"DOI": "10.22037/uj.v0i0.5719",
"doi-asserted-by": "publisher",
"key": "ref246/cit246"
},
{
"DOI": "10.1186/s12906-020-03128-7",
"doi-asserted-by": "publisher",
"key": "ref247/cit247"
},
{
"DOI": "10.1002/ptr.6957",
"doi-asserted-by": "publisher",
"key": "ref248/cit248"
},
{
"author": "Shafabakhsh R.",
"first-page": "290",
"issue": "4",
"journal-title": "Iran J. Kidney Dis",
"key": "ref249/cit249",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.dsx.2021.04.002",
"doi-asserted-by": "publisher",
"key": "ref250/cit250"
},
{
"DOI": "10.1002/ptr.6571",
"doi-asserted-by": "publisher",
"key": "ref251/cit251"
},
{
"DOI": "10.1016/j.ctim.2019.02.014",
"doi-asserted-by": "publisher",
"key": "ref252/cit252"
},
{
"DOI": "10.1016/j.hermed.2021.100531",
"doi-asserted-by": "publisher",
"key": "ref253/cit253"
},
{
"DOI": "10.1186/s12986-019-0331-1",
"doi-asserted-by": "publisher",
"key": "ref254/cit254"
},
{
"DOI": "10.1186/s13104-022-06074-4",
"doi-asserted-by": "publisher",
"key": "ref255/cit255"
},
{
"DOI": "10.1080/1028415X.2019.1627770",
"doi-asserted-by": "publisher",
"key": "ref256/cit256"
},
{
"DOI": "10.1186/s13104-021-05700-x",
"doi-asserted-by": "publisher",
"key": "ref257/cit257"
},
{
"DOI": "10.2174/1871530320666200729144430",
"doi-asserted-by": "publisher",
"key": "ref258/cit258"
},
{
"DOI": "10.1016/j.intimp.2018.05.018",
"doi-asserted-by": "publisher",
"key": "ref259/cit259"
},
{
"DOI": "10.1016/j.jneuroim.2019.01.007",
"doi-asserted-by": "publisher",
"key": "ref260/cit260"
},
{
"DOI": "10.1002/cre2.330",
"doi-asserted-by": "publisher",
"key": "ref261/cit261"
},
{
"DOI": "10.1186/s12906-021-03400-4",
"doi-asserted-by": "publisher",
"key": "ref262/cit262"
},
{
"DOI": "10.1155/2020/4298193",
"doi-asserted-by": "publisher",
"key": "ref263/cit263"
},
{
"DOI": "10.1016/j.jebdp.2022.101708",
"doi-asserted-by": "publisher",
"key": "ref264/cit264"
},
{
"DOI": "10.1186/s13098-020-00532-4",
"doi-asserted-by": "publisher",
"key": "ref265/cit265"
},
{
"DOI": "10.2174/1874471015666220623104316",
"doi-asserted-by": "publisher",
"key": "ref266/cit266"
},
{
"DOI": "10.29252/rbmb.9.1.1",
"doi-asserted-by": "publisher",
"key": "ref267/cit267"
},
{
"DOI": "10.2174/1871527317666180625101643",
"doi-asserted-by": "publisher",
"key": "ref268/cit268"
},
{
"DOI": "10.1016/j.ctcp.2020.101256",
"doi-asserted-by": "publisher",
"key": "ref269/cit269"
},
{
"DOI": "10.52711/0974-360X.2022.00137",
"doi-asserted-by": "publisher",
"key": "ref270/cit270"
},
{
"DOI": "10.2174/1871527317666171213154749",
"doi-asserted-by": "publisher",
"key": "ref271/cit271"
},
{
"DOI": "10.1007/s00251-017-0992-8",
"doi-asserted-by": "publisher",
"key": "ref272/cit272"
},
{
"DOI": "10.2174/1871530319666190212170140",
"doi-asserted-by": "publisher",
"key": "ref273/cit273"
},
{
"DOI": "10.1111/jphp.12910",
"doi-asserted-by": "publisher",
"key": "ref274/cit274"
},
{
"DOI": "10.1002/ptr.7259",
"doi-asserted-by": "publisher",
"key": "ref275/cit275"
},
{
"DOI": "10.4103/ijdr.IJDR_822_18",
"doi-asserted-by": "publisher",
"key": "ref276/cit276"
},
{
"DOI": "10.1016/j.jbmt.2020.02.021",
"doi-asserted-by": "publisher",
"key": "ref277/cit277"
},
{
"DOI": "10.4103/jisp.jisp_207_19",
"doi-asserted-by": "publisher",
"key": "ref278/cit278"
},
{
"DOI": "10.1007/s00784-020-03652-3",
"doi-asserted-by": "publisher",
"key": "ref279/cit279"
},
{
"DOI": "10.1016/j.jagp.2017.10.010",
"doi-asserted-by": "publisher",
"key": "ref280/cit280"
},
{
"DOI": "10.1097/HNP.0000000000000281",
"doi-asserted-by": "publisher",
"key": "ref281/cit281"
},
{
"DOI": "10.1248/bpb.34.660",
"doi-asserted-by": "publisher",
"key": "ref282/cit282"
},
{
"DOI": "10.1007/s00280-011-1673-1",
"doi-asserted-by": "publisher",
"key": "ref283/cit283"
},
{
"DOI": "10.1007/s00421-015-3170-4",
"doi-asserted-by": "publisher",
"key": "ref284/cit284"
},
{
"DOI": "10.1007/s00280-013-2151-8",
"doi-asserted-by": "publisher",
"key": "ref285/cit285"
},
{
"DOI": "10.1248/bpb.b13-00150",
"doi-asserted-by": "publisher",
"key": "ref286/cit286"
},
{
"DOI": "10.3177/jnsv.65.82",
"doi-asserted-by": "publisher",
"key": "ref287/cit287"
},
{
"DOI": "10.1111/sms.13373",
"doi-asserted-by": "publisher",
"key": "ref288/cit288"
},
{
"DOI": "10.1038/ajh.2012.24",
"doi-asserted-by": "publisher",
"key": "ref289/cit289"
},
{
"DOI": "10.1093/ecco-jcc/jjaa097",
"doi-asserted-by": "publisher",
"key": "ref290/cit290"
},
{
"DOI": "10.1111/hdi.12911",
"doi-asserted-by": "publisher",
"key": "ref291/cit291"
},
{
"DOI": "10.1016/j.asmr.2021.10.016",
"doi-asserted-by": "publisher",
"key": "ref292/cit292"
},
{
"DOI": "10.2147/COPD.S104490",
"doi-asserted-by": "publisher",
"key": "ref293/cit293"
},
{
"DOI": "10.1155/2019/8208237",
"doi-asserted-by": "publisher",
"key": "ref294/cit294"
},
{
"DOI": "10.1016/j.lfs.2021.120074",
"doi-asserted-by": "publisher",
"key": "ref295/cit295"
},
{
"DOI": "10.1016/j.jff.2015.01.049",
"doi-asserted-by": "publisher",
"key": "ref296/cit296"
},
{
"DOI": "10.1016/j.jff.2021.104443",
"doi-asserted-by": "publisher",
"key": "ref297/cit297"
},
{
"DOI": "10.1089/acm.2020.0128",
"doi-asserted-by": "publisher",
"key": "ref298/cit298"
},
{
"DOI": "10.1097/JCP.0000000000000508",
"doi-asserted-by": "publisher",
"key": "ref299/cit299"
},
{
"DOI": "10.1016/j.nut.2019.01.002",
"doi-asserted-by": "publisher",
"key": "ref300/cit300"
},
{
"DOI": "10.1002/ptr.6907",
"doi-asserted-by": "publisher",
"key": "ref301/cit301"
},
{
"DOI": "10.1080/1028415X.2020.1853410",
"doi-asserted-by": "publisher",
"key": "ref302/cit302"
},
{
"DOI": "10.3177/jnsv.67.249",
"doi-asserted-by": "publisher",
"key": "ref303/cit303"
},
{
"DOI": "10.1007/s12325-022-02081-w",
"doi-asserted-by": "publisher",
"key": "ref304/cit304"
},
{
"DOI": "10.1177/0269881114552744",
"doi-asserted-by": "publisher",
"key": "ref305/cit305"
},
{
"DOI": "10.3390/nu12061678",
"doi-asserted-by": "publisher",
"key": "ref306/cit306"
},
{
"DOI": "10.18632/aging.101149",
"doi-asserted-by": "publisher",
"key": "ref307/cit307"
},
{
"DOI": "10.1186/1475-2891-11-79",
"doi-asserted-by": "publisher",
"key": "ref308/cit308"
},
{
"DOI": "10.1093/jn/nxaa299",
"doi-asserted-by": "publisher",
"key": "ref309/cit309"
},
{
"DOI": "10.1093/rap/rkaa036",
"doi-asserted-by": "publisher",
"key": "ref310/cit310"
},
{
"DOI": "10.1016/j.numecd.2019.12.010",
"doi-asserted-by": "publisher",
"key": "ref311/cit311"
},
{
"DOI": "10.4103/0973-029X.164524",
"doi-asserted-by": "publisher",
"key": "ref312/cit312"
},
{
"DOI": "10.2147/JIR.S205390",
"doi-asserted-by": "publisher",
"key": "ref313/cit313"
},
{
"DOI": "10.1002/dad2.12199",
"doi-asserted-by": "publisher",
"key": "ref314/cit314"
}
],
"reference-count": 314,
"references-count": 314,
"relation": {},
"resource": {
"primary": {
"URL": "https://pubs.acs.org/doi/10.1021/acsomega.2c07326"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Chemical Engineering",
"General Chemistry"
],
"subtitle": [],
"title": "Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far?",
"type": "journal-article",
"volume": "8"
}
