Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases
et al., International Journal of Nanomedicine, doi:10.2147/ijn.s451206, Feb 2024
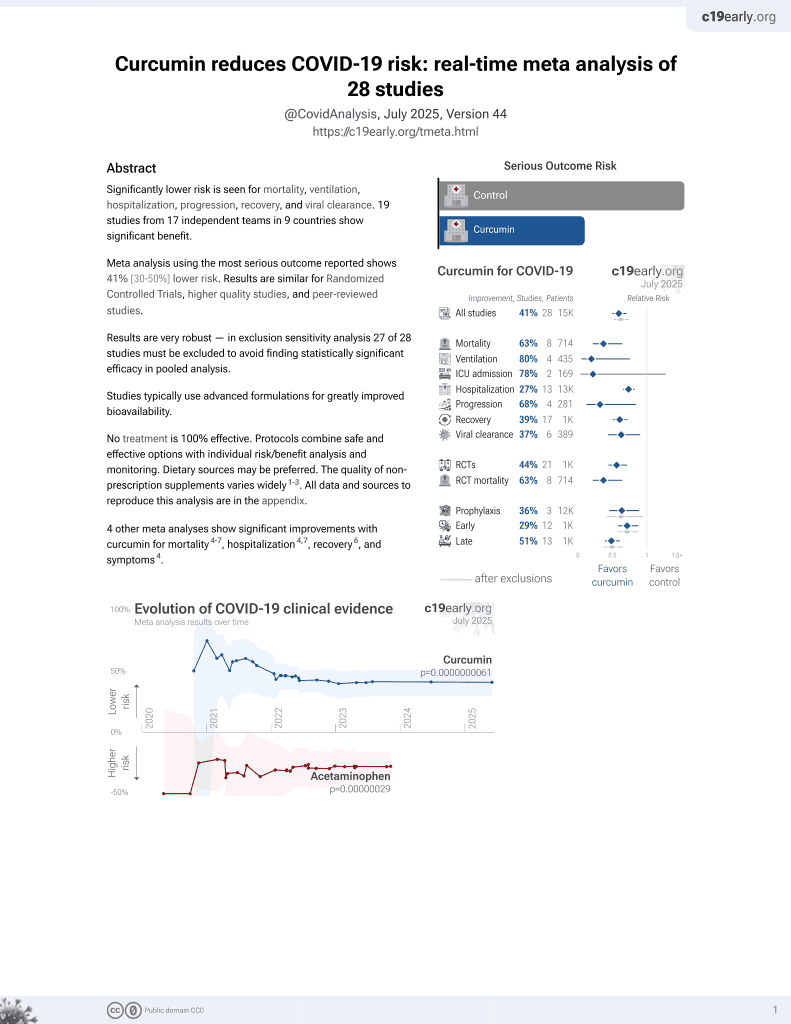
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of nanoformulations for inhaled therapeutics for respiratory diseases including potential for COVID-19. Inhaled formulations deliver treatment directly to both the upper and lower respiratory tract, enabling higher local concentrations while minimizing systemic side effects. They also provide solutions for bioavailability challenges like instability and low solubility. This review discusses recent advances in nano- and microparticles made from lipids, polymers, and nanocomposites to encapsulate natural compounds like curcumin and enhance delivery to lung tissues. Though not specific to COVID-19, properly designed inhaled formulations could fight early viral replication in the upper airways as well as later stage infection in lungs.
Review covers curcumin and quercetin.
1.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
2.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
3.
Duan et al., Bioactive compounds,quercetin, curcumin and β-glucan,regulate innate immunity via the gut-liver-brain axis, Trends in Food Science & Technology, doi:10.1016/j.tifs.2024.104864.
4.
Rajak et al., Antiallergic Implications of Curcumin During COVID-19: Current Status and Perspectives, Biotechnology of Medicinal Plants with Antiallergy Properties, doi:10.1007/978-981-97-1467-4_4.
5.
Kali et al., Curcumin as a Promising Therapy for COVID-19: A Review, Global Journal of Medical, Pharmaceutical, and Biomedical Update, doi:10.25259/GJMPBU_78_2023.
6.
Vajdi et al., Effect of polyphenols against complications of COVID-19: current evidence and potential efficacy, Pharmacological Reports, doi:10.1007/s43440-024-00585-6.
7.
Yong et al., Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases, International Journal of Nanomedicine, doi:10.2147/ijn.s451206.
8.
Halma et al., Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology, Endocrine and Metabolic Science, doi:10.1016/j.endmts.2024.100163.
9.
Arab et al., Immunoregulatory effects of nanocurcumin in inflammatory milieu: Focus on COVID-19, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2024.116131.
10.
Daskou et al., The Role of the NRF2 Pathway in the Pathogenesis of Viral Respiratory Infections, Pathogens, doi:10.3390/pathogens13010039.
11.
Law et al., Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review, Pharmaceuticals, doi:10.3390/ph17010034.
12.
Donzelli, A., Neglected Effective Early Therapies against COVID-19: Focus on Functional Foods and Related Active Substances. A Review, MDPI AG, doi:10.20944/preprints202312.1178.v1.
13.
Hulscher et al., Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination, Cureus, doi:10.7759/cureus.49204.
14.
Hegde et al., Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far?, ACS Omega, doi:10.1021/acsomega.2c07326.
Yong et al., 29 Feb 2024, peer-reviewed, 8 authors.
Contact: hh1643689264@163.com.
Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases
International Journal of Nanomedicine, doi:10.2147/ijn.s451206
Given the unique physiological and pathological characteristics of the lung, the direct, inhalable route is more conducive to pulmonary drug delivery and disease control than traditional systemic drug delivery, significantly circumventing drug loss, off-target effects, systemic and organ toxicity, etc., and is widely regarded as the preferred regimen for pulmonary drug delivery. However, very few lung diseases are currently treated with the preferred inhaled formulations, such as asthma, chronic obstructive pulmonary disease and pulmonary hypertension. And there is a lack of appropriate inhaled formulations for other critical lung diseases, such as lung cancer and pulmonary fibrosis, due to the fact that the physicochemical properties of the drugs and their pharmacokinetic profiles do not match the physiology of the lung, and conventional inhalation devices are unable to deliver them to the specific parts of the lung. Phytochemicals of natural origin, due to their wide availability and clear safety profile, hold great promise for the preparation of inhalable formulations to improve the current dilemma in the treatment of lung diseases. In particular, the preparation of inhalable formulations based on nano-and microparticulate carriers for drug delivery to deep lung tissues, which overcome the shortcomings of conventional inhalation therapies while targeting the drug activity directly to a specific part of the lung, may be the best approach to change the current dilemma of lung disease treatment. In this review, we discuss recent advances in nano-and micron-carrier-based inhalation formulations for the delivery of natural products for the treatment of pulmonary diseases, which may represent an opportunity for practical clinical translation of natural products.
Disclosure The authors report no conflicts of interest in this work.
References
Abdelaziz, Gaber, Abd-Elwakil, Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates, J Control Release, doi:10.1016/j.jconrel.2017.11.036
Adel, Elmeligy, Abdelrahim, Design and Characterization of Spray-Dried Proliposomes for the Pulmonary Delivery of Curcumin, Int J Nanomed, doi:10.2147/IJN.S306831
Agel, Baghdan, Pinnapireddy, Curcumin loaded nanoparticles as efficient photoactive formulations against gram-positive and gram-negative bacteria, Colloids Surf B Biointerfaces, doi:10.1016/j.ejpb.2019.07.023
Ahmed, Mansour, Ishak, Customizable resveratrol spray-dried micro-composites for inhalation as a promising contender for treatment of idiopathic pulmonary fibrosis, Int J Pharm, doi:10.1016/j.ijpharm.2023.123117
Alhajj, Reilly, Cathcart, Nano-in-Microparticles for Pulmonary Drug Delivery
Altube, Perez, Romero, Inhaled lipid nanocarriers for pulmonary delivery of glucocorticoids: previous strategies, recent advances and key factors description, Int J Pharm, doi:10.1016/j.ijpharm.2023.123146
Arbain, Salim, Masoumi, In vitro evaluation of the inhalable quercetin loaded nanoemulsion for pulmonary delivery, Drug Deliv Transl Res, doi:10.1007/s13346-018-0509-5
Armstrong, Ashby, Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial, Lancet Respir Med, doi:10.1016/S2213-2600(15)00245-3
Ashaolu, Nanoemulsions for health, food, and cosmetics: a review, Environ Chem Lett, doi:10.1007/s10311-021-01216-9
Asmawi, Salim, Abdulmalek, Modeling the Effect of Composition on Formation of Aerosolized Nanoemulsion System Encapsulating Docetaxel and Curcumin Using D-Optimal Mixture Experimental Design, Int J Mol Sci, doi:10.3390/ijms21124357
Asmawi, Salim, Abdulmalek, Size-Controlled Preparation of Docetaxel-and Curcumin-Loaded Nanoemulsions for Potential Pulmonary Delivery, Pharmaceutics, doi:10.3390/pharmaceutics15020652
Asmawi, Salim, Ngan, Excipient selection and aerodynamic characterization of nebulized lipid-based nanoemulsion loaded with docetaxel for lung cancer treatment, Drug Deliv Transl Res, doi:10.1007/s13346-018-0526-4
Baghdan, Duse, Schüer, Development of inhalable curcumin loaded Nano-in-Microparticles for bronchoscopic photodynamic therapy, Eur J Pharm Sci, doi:10.1016/j.ejps.2019.02.025
Bai, Zhao, Chen, Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge, Sci Adv, doi:10.1126/sciadv.abn7162
Bassetti, Vena, Russo, Inhaled Liposomal Antimicrobial Delivery in Lung Infections, Drugs, doi:10.1007/s40265-020-01359-z
Borghardt, Kloft, Sharma, Inhaled Therapy in Respiratory Disease: the Complex Interplay of Pulmonary Kinetic Processes, Can Respir J, doi:10.1155/2018/2732017
Chen, Zhang, Wang, A novel inhalable quercetin-alginate nanogel as a promising therapy for acute lung injury, J Nanobiotechnology, doi:10.1186/s12951-022-01452-3
Chennakesavulu, Pulmonary delivery of liposomal dry powder inhaler formulation for effective treatment of idiopathic pulmonary fibrosis, Asian J Pharm Sci, doi:10.1016/j.ajps.2017.08.005
Costa, Moreira, Lobo, Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies, Acta Pharm Sin B, doi:10.1016/j.apsb.2021.02.012
Cui, Zhang, Zhao, A novel ligand-modified nanocomposite microparticles improved efficiency of quercetin and paclitaxel delivery in the non-small cell lung cancer, International Journal of Nanomedicine Dovepress
Danaei, Dehghankhold, Ataei, Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems, Pharmaceutics
Dang, Lin, Xie, Quantitative determination of myricetin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and its absolute bioavailability, Drug Res (Stuttg), doi:10.1055/s-0033-1363220
Densmore, Kleinerman, Gautam, Growth suppression of established human osteosarcoma lung metastases in mice by aerosol gene therapy with PEI-p53 complexes, Cancer Gene Ther, doi:10.1038/sj.cgt.7700343
El-Moslemany, El-Kamel, Allam, Tanshinone IIA loaded bioactive nanoemulsion for alleviation of lipopolysaccharide induced acute lung injury via inhibition of endothelial glycocalyx shedding, Biomed Pharmacother, doi:10.1016/j.biopha.2022.113666
El-Sherbiny, Smyth, Controlled release pulmonary administration of curcumin using swellable biocompatible microparticles, Mol Pharm, doi:10.1021/mp200351y
Fazio, Gandhi, Flattery, Silicosis Among Immigrant Engineered Stone (Quartz) Countertop Fabrication Workers in California, JAMA Intern Med, doi:10.1001/jamainternmed.2023.3295
Fu, Wang, Wu, Inhalable Biomineralized Liposomes for Cyclic Ca 2+ -Burst-Centered Endoplasmic Reticulum Stress Enhanced Lung Cancer Ferroptosis Therapy, ACS Nano, doi:10.1021/acsnano.2c10830
Gandhi, Roy, Lipid-Based Inhalable Micro-and Nanocarriers of Active Agents for Treating Non-Small-Cell Lung Cancer, Pharmaceutics
Gao, Zhang, Xu, Pulmonary delivery of liposomes co-loaded with SN38 prodrug and curcumin for the treatment of lung cancer, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2022.08.021
Garbuzenko, Kuzmov, Taratula, Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo-and gene therapy, Theranostics, doi:10.7150/thno.39816
García-Fernández, Sancenón, Martínez-Máñez, Mesoporous silica nanoparticles for pulmonary drug delivery, Adv Drug Deliv Rev, doi:10.1016/j.addr.2021.113953
Gautam, Densmore, Waldrep, Inhibition of experimental lung metastasis by aerosol delivery of PEI-p53 complexes, Mol Ther, doi:10.1006/mthe.2000.0138
Gautam, Waldrep, Densmore, Growth inhibition of established B16-F10 lung metastases by sequential aerosol delivery of p53 gene and 9-nitrocamptothecin, Gene Ther, doi:10.1038/sj.gt.3301662
Guagliardo, Pérez-Gil, Smedt, Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins, J Control Release, doi:10.1016/j.jconrel.2018.10.012
Guo, Bera, Shi, Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines, Acta Pharm Sin B, doi:10.1016/j.apsb.2021.05.015
Gupta, Jaipuria, Gupta, Inhalable Formulations to Treat Non-Small Cell Lung Cancer (NSCLC): recent Therapies and Developments, Pharmaceutics
Hald Albertsen, Kulkarni, Witzigmann, The role of lipid components in lipid nanoparticles for vaccines and gene therapy, Adv Drug Deliv Rev, doi:10.1016/j.addr.2022.114416
Han, Huang, Liu, Peptide-conjugated PAMAM for targeted doxorubicin delivery to transferrin receptor overexpressed tumors, Mol Pharm, doi:10.1021/mp100185f
Han, Sheng, Zhang, Bioresponsive Immunotherapeutic Materials, Adv Mater, doi:10.1002/adma.202209778
Hassan, Lau, Effect of particle shape on dry particle inhalation: study of flowability, aerosolization, and deposition properties, AAPS Pharm Sci Tech, doi:10.1208/s12249-009-9313-3
Hatipoglu, Hickey, Garcia-Contreras, Pharmacokinetics and pharmacodynamics of high doses of inhaled dry powder drugs, Int J Pharm, doi:10.1016/j.ijpharm.2018.07.050
Hickey, Emerging trends in inhaled drug delivery, Adv Drug Deliv Rev, doi:10.1016/j.addr.2020.07.006
Hu, Yang, Liao, Docetaxel-Loaded Cholesterol-PEG Co-Modified Poly (n-Butyl) Cyanoacrylate Nanoparticles for Antitumor Drug Pulmonary Delivery: preparation, Characterization, and in vivo Evaluation, Int J Nanomed, doi:10.2147/IJN.S249511
Hureaux, Lagarce, Gagnadoux, Lipid nanocapsules: ready-to-use nanovectors for the aerosol delivery of paclitaxel, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2009.06.013
Iba, Levy, Hirota, Protection of the endothelial glycocalyx by antithrombin in an endotoxin-induced rat model of sepsis, Thromb Res, doi:10.1016/j.thromres.2018.09.042
Ji, Yu, Liu, Naringenin-loaded solid lipid nanoparticles: preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics, International Journal of Nanomedicine, doi:10.2147/DDDT.S97738
Jiang, Li, Yu, A dry powder inhalable formulation of salvianolic acids for the treatment of pulmonary fibrosis: safety, lung deposition, and pharmacokinetic study, Drug Deliv Transl Res, doi:10.1007/s13346-020-00857-7
Jin, Gao, Wu, Harnessing inhaled nanoparticles to overcome the pulmonary barrier for respiratory disease therapy, Adv Drug Deliv Rev
Jin, Liu, Zhang, Catechin-functionalized Cationic Lipopolymer Based Multicomponent Nanomicelles for Lung-Targeting Delivery, Adv Mater, doi:10.1002/adma.202302985
Kakran, Shegokar, Sahoo, Fabrication of quercetin nanocrystals: comparison of different methods, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2011.08.006
Kanwal, Shahid, Ahmad, Sustainable, economical and rapid treatment of multiple lung diseases using therapeutic potential of curcumin nanoparticles, Environ Res, doi:10.1016/j.envres.2023.116477
Karvouniaris, Makris, Zygoulis, Nebulised colistin for ventilator-associated pneumonia prevention, International Journal of Nanomedicine, doi:10.1183/13993003.02235-2014
Kaur, Mishra, Shunmugaperumal, Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer, J Drug Delivery Sci Technol, doi:10.1016/j.jddst.2020.101502
Ke, Chang, Chan, Engineering the right formulation for enhanced drug delivery, Adv Drug Deliv Rev, doi:10.1016/j.addr.2022.114561
Khan, Apostolou, Bnyan, Paclitaxel-loaded micro or nano transfersome formulation into novel tablets for pulmonary drug delivery via nebulization, Int J Pharm, doi:10.1016/j.ijpharm.2019.118919
Khutoryanskiy, Beyond PEGylation: alternative surface-modification of nanoparticles with mucus-inert biomaterials, Adv Drug Deliv Rev, doi:10.1016/j.addr.2017.07.015
Knight, Kleinerman, Waldrep, 9-Nitrocamptothecin liposome aerosol treatment of human cancer subcutaneous xenografts and pulmonary cancer metastases in mice, Ann N Y Acad Sci, doi:10.1111/j.1749-6632.2000.tb07033.x
Koshkina, Gilbert, Waldrep, Distribution of camptothecin after delivery as a liposome aerosol or following intramuscular injection in mice, Cancer Chemother Pharmacol, doi:10.1007/s002800050966
Koshkina, Kleinerman, Waidrep, 9-Nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice, Clin Cancer Res
Koshkina, Waldrep, Roberts, Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model, Clin Cancer Res
Kotta, Aldawsari, Badr-Eldin, Aerosol Delivery of Surfactant Liposomes for Management of Pulmonary Fibrosis: an Approach Supporting Pulmonary Mechanics, Pharmaceutics
Lababidi, Montefusco-Pereira, De, Carvalho-Wodarz, Spray-dried multidrug particles for pulmonary co-delivery of antibiotics with N-acetylcysteine and curcumin-loaded PLGA-nanoparticles, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2020.10.010
Lai, Wang, Hanes, Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues, International Journal of Nanomedicine, doi:10.1016/j.addr.2008.11.002
Lam, Schreiner, Leung, Optimizing Lipid Nanoparticles for Delivery in Primates, Adv Mater, doi:10.1002/adma.202211420
Lee, Loo, Traini, Development and Evaluation of Paclitaxel and Curcumin Dry Powder for Inhalation Lung Cancer Treatment, Pharmaceutics, doi:10.3390/pharmaceutics13010009
Lee, Loo, Traini, Nano-and micro-based inhaled drug delivery systems for targeting alveolar macrophages, Expert Opin Drug Deliv, doi:10.1517/17425247.2015.1039509
Leiter, Veluswamy, Wisnivesky, The global burden of lung cancer: current status and future trends, Nat Rev Clin Oncol, doi:10.1038/s41571-023-00798-3
Li, Guo, Zheng, Preparation of honokiol-loaded chitosan microparticles via spray-drying method intended for pulmonary delivery, Drug Deliv, doi:10.1080/10717540902738341
Li, Xiao, Zhang, Pulmonary Delivery of Specialized Pro-Resolving Mediators-Based Nanotherapeutics Attenuates Pulmonary Fibrosis in Preclinical Animal Models, ACS Nano
Li, Zhang, Zhu, Liposomal andrographolide dry powder inhalers for treatment of bacterial pneumonia via anti-inflammatory pathway, Int J Pharm, doi:10.1016/j.ijpharm.2017.06.005
Li, Zhu, Liu, Tea tree oil nanoemulsions for inhalation therapies of bacterial and fungal pneumonia, Colloids Surf B Biointerfaces, doi:10.1016/j.colsurfb.2016.02.017
Liang, Duan, Lu, Engineering exosomes for targeted drug delivery, Theranostics, doi:10.7150/thno.52570
Liao, Li, Wang, Natural Products-Based Nanoformulations: a New Approach Targeting CSCs to Cancer Therapy, Int J Nanomed, doi:10.2147/IJN.S380697
Liao, Zhang, Chen, Targeting cancer stem cells and signalling pathways through phytochemicals: a promising approach against colorectal cancer, Phytomedicine, doi:10.1016/j.phymed.2022.154524
Lim, Banerjee, Önyüksel, Improvement of drug safety by the use of lipid-based nanocarriers, J Control Release, doi:10.1016/j.jconrel.2012.06.002
Lin, Li, Du, Pulmonary delivery of triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX antibody for lung cancer therapy, Sci Rep, doi:10.1038/s41598-017-00957-4
Liu, Crowe, Wang, An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases, Nat Commun, doi:10.1038/s41467-019-13094-5
Liu, Xue, Zhang, The influence of a biomimetic pulmonary surfactant modification on the in vivo fate of nanoparticles in the lung, Acta Biomater, doi:10.1016/j.actbio.2022.05.038
Liu, Yang, Paclitaxel and quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery, Int J Nanomed, doi:10.2147/IJN.S147028
Lu, Li, Liu, Salvianolic acid B dry powder inhaler for the treatment of idiopathic pulmonary fibrosis, Asian J Pharm Sci, doi:10.1016/j.ajps.2022.04.004
Luo, Sun, Liu, Self-Assembled Redox Dual-Responsive Prodrug-Nanosystem Formed by Single Thioether-Bridged Paclitaxel-Fatty Acid Conjugate for Cancer Chemotherapy, Nano Lett, doi:10.1021/acs.nanolett.6b01632
Ma, Pulmonary delivery of size-transformable nanoparticles improves tumor accumulation and penetration for chemo-sonodynamic combination therapy, J Control Release, doi:10.1016/j.jconrel.2022.08.003
Mahajan, Mahajan, Cox, Development of grafted xyloglucan micelles for pulmonary delivery of curcumin: in vitro and in vivo studies, Int J Biol Macromol, doi:10.1038/s41568-020-00329-7
Meenach, Anderson, Hilt, Characterization and aerosol dispersion performance of advanced spray-dried chemotherapeutic PEGylated phospholipid particles for dry powder inhalation delivery in lung cancer, Eur J Pharm Sci, doi:10.1016/j.ejps.2013.05.012
Mehta, Bothiraja, Mahadik, Phytoconstituent based dry powder inhalers as biomedicine for the management of pulmonary diseases, Biomed Pharmacother, doi:10.1016/j.biopha.2018.09.094
Middleton, Robinson, Mckay, A pilot study of inhaled dry-powder mannitol during cystic fibrosis-related pulmonary exacerbation, Eur Respir J, doi:10.1183/09031936.00137814
Mirchandani, Patravale, Solid lipid nanoparticles for hydrophilic drugs, J Control Release, doi:10.1016/j.jconrel.2021.05.032
Mohtar, Taylor, Sheikh, Design and development of dry powder sulfobutylether-β-cyclodextrin complex for pulmonary delivery of fisetin, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2016.11.036
Muller, Jiang, Sun, Characterization of Lipid-Protein Interactions and Lipid-Mediated Modulation of Membrane Protein Function through Molecular Simulation, Chem Rev, doi:10.1021/acs.chemrev.8b00608
Muraoka, Harada, Shiku, Self-assembled polysaccharide nanogel delivery system for overcoming tumor immune resistance, J Control Release, doi:10.1016/j.jconrel.2022.05.004
Muta, Parikh, Kathawala, Quality-by-Design Approach for the Development of Nano-Sized Tea Tree Oil Formulation-Impregnated Biocompatible Gel with Antimicrobial Properties, Pharmaceutics, doi:10.3390/pharmaceutics12111091
Nafee, Gaber, Elzoghby, Promoted Antitumor Activity of Myricetin against Lung Carcinoma Via Nanoencapsulated Phospholipid Complex in Respirable Microparticles, Pharm Res, doi:10.1007/s11095-020-02794-z
Nelson, Dahlin, Bisson, The Essential Medicinal Chemistry of Curcumin, J Med Chem, doi:10.1021/acs.jmedchem.6b00975
Panahi, Ghanei, Rahimi, Evaluation the efficacy and safety of N-acetylcysteine inhalation spray in controlling the symptoms of patients with COVID-19: an open-label randomized controlled clinical trial, J Med Virol, doi:10.1002/jmv.28393
Pasqua, Hamblin, Edwards, Developing inhaled drugs for respiratory diseases: a medicinal chemistry perspective, Drug Discov Today, doi:10.1016/j.drudis.2021.09.005
Pavelić, Pavelić, Bulog, Nanoparticles in Medicine: current Status in Cancer Treatment, Int J Mol Sci, doi:10.3390/ijms241612827
Peng, Cai, Wang, Carboxymethyl Chitosan Modified Oxymatrine Liposomes for the Alleviation of Emphysema in Mice via Pulmonary Administration, Molecules
Peng, Wang, Guo, Development of Inhalable Chitosan-Coated Oxymatrine Liposomes to Alleviate RSV-Infected Mice, Int J Mol Sci
Petros, Desimone, Strategies in the design of nanoparticles for therapeutic applications, Nat Rev Drug Discov, doi:10.1038/nrd2591
Pommier, Topoisomerase I inhibitors: camptothecins and beyond, Nat Rev Cancer, doi:10.1038/nrc1977
Prota, Santoro, Bifulco, Leucine enhances aerosol performance of naringin dry powder and its activity on cystic fibrosis airway epithelial cells, Int J Pharm, doi:10.1016/j.ijpharm.2011.03.055
Pápay, Kósa, Böddi, Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment, J Aerosol Med Pulm Drug Deliv, doi:10.1016/j.ijbiomac.2021.12.073
Qi, Jia, Peng, Baicalin/ambroxol hydrochloride combined dry powder inhalation formulation targeting lung delivery for treatment of idiopathic pulmonary fibrosis: fabrication, characterization, pharmacokinetics, and pharmacodynamics, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2023.05.017
Rezazadeh, Davatsaz, Emami, Preparation and Characterization of Spray-Dried Inhalable Powders Containing Polymeric Micelles for Pulmonary Delivery of Paclitaxel in Lung Cancer, J Pharm Pharm Sci, doi:10.18433/jpps30048
Riaz, Zhang, Wong, Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy, Int J Nanomed, doi:10.2147/IJN.S192219
Rosière, Van Woensel, Gelbcke, New Folate-Grafted Chitosan Derivative To Improve Delivery of Paclitaxel-Loaded Solid Lipid Nanoparticles for Lung Tumor Therapy by Inhalation, Mol Pharm, doi:10.1021/acs.molpharmaceut.7b00846
Said-Elbahr, Nasr, Alhnan, Nebulizable colloidal nanoparticles co-encapsulating a COX-2 inhibitor and a herbal compound for treatment of lung cancer, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2016.03.025
Said-Elbahr, Nasr, Alhnan, Simultaneous pulmonary administration of celecoxib and naringin using a nebulization-friendly nanoemulsion: a device-targeted delivery for treatment of lung cancer, Expert Opin Drug Deliv, doi:10.1080/17425247.2022.2076833
Sansone, Aquino, Gaudio, Physical characteristics and aerosol performance of naringin dry powders for pulmonary delivery prepared by spray-drying, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2008.10.007
Scalia, Haghi, Losi, Quercetin solid lipid microparticles: a flavonoid for inhalation lung delivery, Eur J Pharm Sci, doi:10.1016/j.ejps.2013.03.009
Scalia, Trotta, Traini, Incorporation of quercetin in respirable lipid microparticles: effect on stability and cellular uptake on A549 pulmonary alveolar epithelial cells, Colloids Surf B Biointerfaces, doi:10.1016/j.colsurfb.2013.07.067
Shanmugam, Joshi, Ahamad, Enhanced absorption, and efficacy of oral self-assembled paclitaxel nanocochleates in multi-drug resistant colon cancer, Int J Pharm, doi:10.1016/j.ijpharm.2020.119482
Shanmugam, Joshi, Kaviratna, Aerosol Delivery of Paclitaxel-Containing Self-Assembled Nanocochleates for Treating Pulmonary Metastasis: an Approach Supporting Pulmonary Mechanics, ACS Biomater Sci Eng, doi:10.1021/acsbiomaterials.0c01126
Shen, Minko, Pharmacokinetics of inhaled nanotherapeutics for pulmonary delivery, J Control Release, doi:10.1016/j.jconrel.2020.07.011
Shi, Kantoff, Wooster, Cancer nanomedicine: progress, challenges and opportunities, Nat Rev Cancer, doi:10.1038/nrc.2016.108
Singh, Meher, Raval, Nanoemulsion: concepts, development and applications in drug delivery, J Control Release, doi:10.1016/j.jconrel.2017.03.008
Soriano, Kendrick, Paulson, Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017, Lancet Respir Med, doi:10.1016/S2213-2600(20)30105-3
Taki, Tagami, Fukushige, Fabrication of nanocomposite particles using a two-solution mixing-type spray nozzle for use in an inhaled curcumin formulation, Int J Pharm, doi:10.1016/j.ijpharm.2016.06.134
Taratula, Kuzmov, Shah, Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA, J Control Release, doi:10.1016/j.jconrel.2013.04.018
Tayab, Hochhaus, Pharmacokinetic/pharmacodynamic evaluation of inhalation drugs: application to targeted pulmonary delivery systems, Expert Opin Drug Deliv, doi:10.1517/17425247.2.3.519
Tenchov, Bird, Curtze, Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement, ACS Nano, doi:10.1021/acsnano.1c04996
Tian, Bera, Guo, Pulmonary Delivery of Reactive Oxygen Species/Glutathione-Responsive Paclitaxel Dimeric Nanoparticles Improved Therapeutic Indices against Metastatic Lung Cancer, ACS Appl Mater Interfaces, doi:10.1021/acsami.1c16351
Trigo-Gutierrez, Vega-Chacón, Soares, Antimicrobial Activity of Curcumin in Nanoformulations: a Comprehensive Review, Int J Mol Sci, doi:10.3390/ijms22137130
Trotta, Lee, Loo, Co-spray dried resveratrol and budesonide inhalation formulation for reducing inflammation and oxidative stress in rat alveolar macrophages, Eur J Pharm Sci, doi:10.1016/j.ejps.2016.02.018
Trotta, Lee, Loo, In vitro biological activity of resveratrol using a novel inhalable resveratrol spray-dried formulation, Int J Pharm, doi:10.1016/j.ijpharm.2015.06.033
Van Drooge, Hinrichs, Dickhoff, Spray freeze drying to produce a stable Delta(9)-tetrahydrocannabinol containing inulinbased solid dispersion powder suitable for inhalation, Eur J Pharm Sci, doi:10.1016/j.ejps.2005.06.007
Wang, Chen, Zhang, Matrix metalloproteinase 2/9-triggered-release micelles for inhaled drug delivery to treat lung cancer: preparation and in vitro/in vivo studies, Int J Nanomed, doi:10.2147/IJN.S166584
Wang, Inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles for enhanced efficacy against non-small cell lung cancer, Int J Biol Macromol, doi:10.1016/j.ijbiomac.2020.07.124
Wang, Zhang, Li, Research Status of Dendrimer Micelles in Tumor Therapy for Drug Delivery, Small, doi:10.1002/smll.202304006
Waxman, Restrepo-Jaramillo, Thenappan, Long-term inhaled treprostinil for pulmonary hypertension due to interstitial lung disease: INCREASE open-label extension study, Eur Respir J, doi:10.1183/13993003.02414-2022
West, Chaudhuri, Barczyk, Inhaled pirfenidone solution (AP01) for IPF: a randomised, open-label, dose-response trial, Thorax, doi:10.1136/thorax-2022-219391
Wilson, Welte, Polverino, Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: a Phase II randomised study, Eur Respir J, doi:10.1183/09031936.00071312
Wu, Lin, Hung, Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application, J Pharm Biomed Anal, doi:10.1016/j.jpba.2007.06.026
Xiao, Zhuang, Zhang, Pulmonary delivery of cationic liposomal hydroxycamptothecin and 5-aminolevulinic acid for chemosonodynamic therapy of metastatic lung cancer, Int J Pharm, doi:10.1016/j.ijpharm.2021.120572
Xu, Lu, Zhu, Formulation and Characterization of Spray-Dried Powders Containing Vincristine-Liposomes for Pulmonary Delivery and Its Pharmacokinetic Evaluation From In Vitro and In Vivo, J Pharm Sci, doi:10.1016/j.xphs.2019.05.009
Yao, Li, Meng, Enhancement of suppression oxidative stress and inflammation of quercetin by nano-decoration for ameliorating silica-induced pulmonary fibrosis, Environ Toxicol: Int J, doi:10.1002/tox.23781
Zhang, Chen, Ge, Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers, Acta Pharm Sin B, doi:10.1016/j.apsb.2018.03.004
Zhang, Li, Du, Paclitaxel-in-liposome-in-bacteria for inhalation treatment of primary lung cancer, Int J Pharm, doi:10.1016/j.ijpharm.2020.119177
Zhang, Lu, Qin, Traditional Chinese medicine combined with pulmonary drug delivery system and idiopathic pulmonary fibrosis: rationale and therapeutic potential, Biomed Pharmacother, doi:10.1016/j.biopha.2020.111072
Zhang, Lv, Jiang, Evaluation of High-Performance Curcumin Nanocrystals for Pulmonary Drug Delivery Both In Vitro and In Vivo, Nanoscale Res Lett, doi:10.1186/s11671-015-1085-y
Zhang, Sun, Bai, RBMS1 regulates lung cancer ferroptosis through translational control of SLC7A11, J Clin Invest
Zheng, Zhu, Tang, Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer, J Transl Med, doi:10.1186/s12967-023-04206-3
Zhou, Peterson, Fan, The Commonly Used Stabilizers for Phytochemical-Based Nanoparticles: stabilization Effects, Mechanisms, and Applications, Nutrients, doi:10.3390/nu15183881
Zhu, Chen, Jiang, Sequential Targeting Hybrid Nanovesicles Composed of Chimeric Antigen Receptor T-Cell-Derived Exosomes and Liposomes for Enhanced Cancer Immunochemotherapy, ACS Nano, doi:10.1021/acsnano.3c03456
Zhu, Kong, Liu, Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy, Pulm Pharmacol Ther, doi:10.1016/j.pupt.2019.02.001
Zhu, Yu, Feng, Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer, Carbohydr Polym, doi:10.1016/j.carbpol.2021.118237
DOI record:
{
"DOI": "10.2147/ijn.s451206",
"ISSN": [
"1178-2013"
],
"URL": "http://dx.doi.org/10.2147/ijn.s451206",
"author": [
{
"affiliation": [],
"family": "Yong",
"given": "Jiangyan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shu",
"given": "Hongli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Kun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Guining",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Jiaqi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Hong",
"sequence": "additional"
}
],
"container-title": "International Journal of Nanomedicine",
"container-title-short": "IJN",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
2,
22
]
],
"date-time": "2024-02-22T17:05:10Z",
"timestamp": 1708621510000
},
"deposited": {
"date-parts": [
[
2024,
2,
22
]
],
"date-time": "2024-02-22T17:05:21Z",
"timestamp": 1708621521000
},
"indexed": {
"date-parts": [
[
2024,
2,
23
]
],
"date-time": "2024-02-23T00:45:53Z",
"timestamp": 1708649153552
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=96980",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=96980",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1723-1748",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2024,
2
]
]
},
"published-online": {
"date-parts": [
[
2024,
2
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/S2213-2600(20)30105-3",
"author": "Soriano",
"doi-asserted-by": "publisher",
"first-page": "585",
"journal-title": "Lancet Respir Med",
"key": "ref1",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1038/s41571-023-00798-3",
"author": "Leiter",
"doi-asserted-by": "publisher",
"first-page": "624",
"journal-title": "Nat Rev Clin Oncol",
"key": "ref2",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1126/sciadv.abn7162",
"author": "Bai",
"doi-asserted-by": "publisher",
"first-page": "eabn7162",
"journal-title": "Sci Adv",
"key": "ref3",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/j.jconrel.2020.07.011",
"author": "Shen",
"doi-asserted-by": "publisher",
"first-page": "222",
"journal-title": "J Control Release",
"key": "ref4",
"volume": "326",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2018.09.094",
"author": "Mehta",
"doi-asserted-by": "publisher",
"first-page": "828",
"journal-title": "Biomed Pharmacother",
"key": "ref5",
"volume": "108",
"year": "2018"
},
{
"DOI": "10.1016/j.addr.2020.07.006",
"author": "Hickey",
"doi-asserted-by": "publisher",
"first-page": "63",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref6",
"volume": "157",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2020.111072",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "111072",
"journal-title": "Biomed Pharmacother",
"key": "ref7",
"volume": "133",
"year": "2021"
},
{
"DOI": "10.1016/j.envres.2023.116477",
"author": "Kanwal",
"doi-asserted-by": "publisher",
"first-page": "116477",
"journal-title": "Environ Res",
"key": "ref8",
"volume": "233",
"year": "2023"
},
{
"DOI": "10.1016/j.ijpharm.2023.123146",
"author": "Julia Altube",
"doi-asserted-by": "publisher",
"first-page": "123146",
"journal-title": "Int J Pharm",
"key": "ref9",
"volume": "642",
"year": "2023"
},
{
"DOI": "10.1155/2018/2732017",
"author": "Borghardt",
"doi-asserted-by": "publisher",
"first-page": "2732017",
"journal-title": "Can Respir J",
"key": "ref10",
"volume": "2018",
"year": "2018"
},
{
"DOI": "10.3390/pharmaceutics10020057",
"author": "Danaei",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "Pharmaceutics",
"key": "ref11",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.3390/ph15050548",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "548",
"journal-title": "Pharmaceutics",
"key": "ref12",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/j.drudis.2021.09.005",
"author": "Pasqua",
"doi-asserted-by": "publisher",
"first-page": "134",
"journal-title": "Drug Discov Today",
"key": "ref13",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1016/j.ijpharm.2018.07.050",
"author": "Kukut Hatipoglu",
"doi-asserted-by": "publisher",
"first-page": "306",
"journal-title": "Int J Pharm",
"key": "ref14",
"volume": "549",
"year": "2018"
},
{
"DOI": "10.1016/j.apsb.2021.05.015",
"author": "Guo",
"doi-asserted-by": "publisher",
"first-page": "2565",
"journal-title": "Acta Pharm Sin B",
"key": "ref15",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.2147/IJN.S380697",
"author": "Liao",
"doi-asserted-by": "publisher",
"first-page": "4163",
"journal-title": "Int J Nanomed",
"key": "ref16",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1038/nrc.2016.108",
"author": "Shi",
"doi-asserted-by": "publisher",
"first-page": "20",
"journal-title": "Nat Rev Cancer",
"key": "ref17",
"volume": "17",
"year": "2017"
},
{
"DOI": "10.1038/nrd2591",
"author": "Petros",
"doi-asserted-by": "publisher",
"first-page": "615",
"journal-title": "Nat Rev Drug Discov",
"key": "ref18",
"volume": "9",
"year": "2010"
},
{
"DOI": "10.1002/adma.202209778",
"author": "Han",
"doi-asserted-by": "publisher",
"first-page": "e2209778",
"journal-title": "Adv Mater",
"key": "ref19",
"year": "2023"
},
{
"DOI": "10.3390/pharmaceutics15051457",
"author": "Gandhi",
"doi-asserted-by": "crossref",
"first-page": "658",
"journal-title": "Pharmaceutics",
"key": "ref20",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.addr.2022.114561",
"author": "Ke",
"doi-asserted-by": "publisher",
"first-page": "114561",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref21",
"volume": "191",
"year": "2022"
},
{
"DOI": "10.1517/17425247.2015.1039509",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "1009",
"journal-title": "Expert Opin Drug Deliv",
"key": "ref22",
"volume": "12",
"year": "2015"
},
{
"DOI": "10.1208/s12249-009-9313-3",
"author": "Hassan",
"doi-asserted-by": "publisher",
"first-page": "1252",
"journal-title": "AAPS Pharm Sci Tech",
"key": "ref23",
"volume": "10",
"year": "2009"
},
{
"DOI": "10.1016/j.addr.2017.07.015",
"author": "Khutoryanskiy",
"doi-asserted-by": "publisher",
"first-page": "140",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref24",
"volume": "124",
"year": "2018"
},
{
"DOI": "10.1016/j.addr.2021.113953",
"author": "García-Fernández",
"doi-asserted-by": "publisher",
"first-page": "113953",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref25",
"volume": "177",
"year": "2021"
},
{
"DOI": "10.1016/j.jconrel.2018.10.012",
"author": "Guagliardo",
"doi-asserted-by": "publisher",
"first-page": "116",
"journal-title": "J Control Release",
"key": "ref26",
"volume": "291",
"year": "2018"
},
{
"DOI": "10.1016/j.addr.2023.115111",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "115111",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref27",
"year": "2023"
},
{
"DOI": "10.1016/j.carbpol.2021.118237",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "118237",
"journal-title": "Carbohydr Polym",
"key": "ref28",
"volume": "268",
"year": "2021"
},
{
"DOI": "10.1016/j.jconrel.2017.11.036",
"author": "Abdelaziz",
"doi-asserted-by": "publisher",
"first-page": "374",
"journal-title": "J Control Release",
"key": "ref29",
"volume": "269",
"year": "2018"
},
{
"DOI": "10.1002/jmv.28393",
"author": "Panahi",
"doi-asserted-by": "publisher",
"first-page": "e28393",
"journal-title": "J Med Virol",
"key": "ref30",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1183/13993003.02414-2022",
"author": "Waxman",
"doi-asserted-by": "publisher",
"first-page": "2202414",
"journal-title": "Eur Respir J",
"key": "ref31",
"volume": "61",
"year": "2023"
},
{
"DOI": "10.1136/thorax-2022-219391",
"author": "West",
"doi-asserted-by": "publisher",
"first-page": "882",
"journal-title": "Thorax",
"key": "ref32",
"volume": "78",
"year": "2023"
},
{
"DOI": "10.1183/13993003.02235-2014",
"author": "Karvouniaris",
"doi-asserted-by": "publisher",
"first-page": "1732",
"journal-title": "Eur Respir J",
"key": "ref33",
"volume": "46",
"year": "2015"
},
{
"DOI": "10.1183/09031936.00137814",
"author": "Middleton",
"doi-asserted-by": "publisher",
"first-page": "541",
"journal-title": "Eur Respir J",
"key": "ref34",
"volume": "45",
"year": "2015"
},
{
"DOI": "10.1183/09031936.00071312",
"author": "Wilson",
"doi-asserted-by": "publisher",
"first-page": "1107",
"journal-title": "Eur Respir J",
"key": "ref35",
"volume": "41",
"year": "2013"
},
{
"DOI": "10.1517/17425247.2.3.519",
"author": "Tayab",
"doi-asserted-by": "publisher",
"first-page": "519",
"journal-title": "Expert Opin Drug Deliv",
"key": "ref36",
"volume": "2",
"year": "2005"
},
{
"DOI": "10.1016/j.phymed.2022.154524",
"author": "Liao",
"doi-asserted-by": "publisher",
"first-page": "154524",
"journal-title": "Phytomedicine",
"key": "ref37",
"volume": "108",
"year": "2023"
},
{
"DOI": "10.1016/j.ejpb.2011.08.006",
"author": "Kakran",
"doi-asserted-by": "publisher",
"first-page": "113",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref38",
"volume": "80",
"year": "2012"
},
{
"DOI": "10.1016/j.jpba.2007.06.026",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "635",
"journal-title": "J Pharm Biomed Anal",
"key": "ref39",
"volume": "45",
"year": "2007"
},
{
"DOI": "10.1021/acs.chemrev.8b00608",
"author": "Muller",
"doi-asserted-by": "publisher",
"first-page": "6086",
"journal-title": "Chem Rev",
"key": "ref40",
"volume": "119",
"year": "2019"
},
{
"DOI": "10.1021/acsnano.1c04996",
"author": "Tenchov",
"doi-asserted-by": "publisher",
"first-page": "16982",
"journal-title": "ACS Nano",
"key": "ref41",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1016/j.addr.2022.114416",
"author": "Hald Albertsen",
"doi-asserted-by": "publisher",
"first-page": "114416",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref42",
"volume": "188",
"year": "2022"
},
{
"DOI": "10.1002/adma.202211420",
"author": "Lam",
"doi-asserted-by": "publisher",
"first-page": "e2211420",
"journal-title": "Adv Mater",
"key": "ref43",
"volume": "35",
"year": "2023"
},
{
"DOI": "10.1016/j.ijpharm.2017.06.005",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "163",
"journal-title": "Int J Pharm",
"key": "ref44",
"volume": "528",
"year": "2017"
},
{
"DOI": "10.3390/ijms232415909",
"author": "Peng",
"doi-asserted-by": "crossref",
"first-page": "15909",
"journal-title": "Int J Mol Sci",
"key": "ref45",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3390/molecules27113610",
"author": "Peng",
"doi-asserted-by": "crossref",
"first-page": "3610",
"journal-title": "Molecules",
"key": "ref46",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics13111851",
"author": "Kotta",
"doi-asserted-by": "crossref",
"first-page": "1851",
"journal-title": "Pharmaceutics",
"key": "ref47",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.ajps.2017.08.005",
"author": "Chennakesavulu",
"doi-asserted-by": "publisher",
"first-page": "91",
"journal-title": "Asian J Pharm Sci",
"key": "ref48",
"volume": "13",
"year": "2018"
},
{
"author": "Koshkina",
"first-page": "3258",
"journal-title": "Clin Cancer Res",
"key": "ref49",
"volume": "7",
"year": "2001"
},
{
"DOI": "10.1016/j.ijpharm.2020.119177",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "119177",
"journal-title": "Int J Pharm",
"key": "ref50",
"volume": "578",
"year": "2020"
},
{
"DOI": "10.1016/j.pupt.2019.02.001",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Pulm Pharmacol Ther",
"key": "ref51",
"volume": "55",
"year": "2019"
},
{
"DOI": "10.1016/j.ijpharm.2021.120572",
"author": "Xiao",
"doi-asserted-by": "publisher",
"first-page": "120572",
"journal-title": "Int J Pharm",
"key": "ref52",
"volume": "601",
"year": "2021"
},
{
"DOI": "10.1016/j.xphs.2019.05.009",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "3348",
"journal-title": "J Pharm Sci",
"key": "ref53",
"volume": "108",
"year": "2019"
},
{
"DOI": "10.1007/s002800050966",
"author": "Koshkina",
"doi-asserted-by": "publisher",
"first-page": "187",
"journal-title": "Cancer Chemother Pharmacol",
"key": "ref54",
"volume": "44",
"year": "1999"
},
{
"DOI": "10.1111/j.1749-6632.2000.tb07033.x",
"author": "Knight",
"doi-asserted-by": "publisher",
"first-page": "151",
"journal-title": "Ann N Y Acad Sci",
"key": "ref55",
"volume": "922",
"year": "2000"
},
{
"author": "Koshkina",
"first-page": "2876",
"journal-title": "Clin Cancer Res",
"key": "ref56",
"volume": "6",
"year": "2000"
},
{
"DOI": "10.1038/sj.gt.3301662",
"author": "Gautam",
"doi-asserted-by": "publisher",
"first-page": "353",
"journal-title": "Gene Ther",
"key": "ref57",
"volume": "9",
"year": "2002"
},
{
"DOI": "10.2147/IJN.S306831",
"author": "Adel",
"doi-asserted-by": "publisher",
"first-page": "2667",
"journal-title": "Int J Nanomed",
"key": "ref58",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/j.apsb.2018.03.004",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "440",
"journal-title": "Acta Pharm Sin B",
"key": "ref59",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1016/j.ejpb.2022.08.021",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "156",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref60",
"volume": "179",
"year": "2022"
},
{
"DOI": "10.1021/acsnano.2c10830",
"author": "Fu",
"doi-asserted-by": "publisher",
"first-page": "5486",
"journal-title": "ACS Nano",
"key": "ref61",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.2147/IJN.S192219",
"author": "Riaz",
"doi-asserted-by": "publisher",
"first-page": "2879",
"journal-title": "Int J Nanomed",
"key": "ref62",
"volume": "14",
"year": "2019"
},
{
"DOI": "10.1038/s41598-017-00957-4",
"author": "Lin",
"doi-asserted-by": "publisher",
"first-page": "1097",
"journal-title": "Sci Rep",
"key": "ref63",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1021/acs.molpharmaceut.7b00846",
"author": "Rosière",
"doi-asserted-by": "publisher",
"first-page": "899",
"journal-title": "Mol Pharm",
"key": "ref64",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1007/s11095-020-02794-z",
"author": "Nafee",
"doi-asserted-by": "publisher",
"first-page": "82",
"journal-title": "Pharm Res",
"key": "ref65",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.2147/DDDT.S97738",
"author": "Ji",
"doi-asserted-by": "publisher",
"first-page": "911",
"journal-title": "Drug Des Devel Ther",
"key": "ref66",
"volume": "10",
"year": "2016"
},
{
"DOI": "10.1016/j.jddst.2020.101502",
"author": "Kaur",
"doi-asserted-by": "publisher",
"first-page": "101502",
"journal-title": "J Drug Delivery Sci Technol",
"key": "ref67",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.7150/thno.39816",
"author": "Garbuzenko",
"doi-asserted-by": "publisher",
"first-page": "8362",
"journal-title": "Theranostics",
"key": "ref68",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/j.jconrel.2013.04.018",
"author": "Taratula",
"doi-asserted-by": "publisher",
"first-page": "349",
"journal-title": "J Control Release",
"key": "ref69",
"volume": "171",
"year": "2013"
},
{
"DOI": "10.1016/j.colsurfb.2016.02.017",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "408",
"journal-title": "Colloids Surf B Biointerfaces",
"key": "ref70",
"volume": "141",
"year": "2016"
},
{
"DOI": "10.1016/j.biopha.2022.113666",
"author": "El-Moslemany",
"doi-asserted-by": "publisher",
"first-page": "113666",
"journal-title": "Biomed Pharmacother",
"key": "ref71",
"volume": "155",
"year": "2022"
},
{
"DOI": "10.1007/s13346-018-0509-5",
"author": "Arbain",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Drug Deliv Transl Res",
"key": "ref72",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1007/s13346-018-0526-4",
"author": "Asmawi",
"doi-asserted-by": "publisher",
"first-page": "543",
"journal-title": "Drug Deliv Transl Res",
"key": "ref73",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1080/17425247.2022.2076833",
"author": "Said-Elbahr",
"doi-asserted-by": "publisher",
"first-page": "611",
"journal-title": "Expert Opin Drug Deliv",
"key": "ref74",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.3390/ijms21124357",
"author": "Asmawi",
"doi-asserted-by": "publisher",
"first-page": "4357",
"journal-title": "Int J Mol Sci",
"key": "ref75",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.3390/pharmaceutics15020652",
"author": "Asmawi",
"doi-asserted-by": "publisher",
"first-page": "652",
"journal-title": "Pharmaceutics",
"key": "ref76",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1007/s40265-020-01359-z",
"author": "Bassetti",
"doi-asserted-by": "publisher",
"first-page": "1309",
"journal-title": "Drugs",
"key": "ref77",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1038/s41467-019-13094-5",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "5108",
"journal-title": "Nat Commun",
"key": "ref78",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/S2213-2600(15)00245-3",
"author": "Alton",
"doi-asserted-by": "publisher",
"first-page": "684",
"journal-title": "Lancet Respir Med",
"key": "ref79",
"volume": "3",
"year": "2015"
},
{
"DOI": "10.1021/acsnano.2c10388",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "15354",
"journal-title": "ACS Nano",
"key": "ref80",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1016/j.jconrel.2022.08.003",
"author": "Ma",
"doi-asserted-by": "publisher",
"first-page": "132",
"journal-title": "J Control Release",
"key": "ref81",
"volume": "350",
"year": "2022"
},
{
"DOI": "10.1021/acsnano.3c03456",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "16770",
"journal-title": "ACS Nano",
"key": "ref82",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1038/nrc1977",
"author": "Pommier",
"doi-asserted-by": "publisher",
"first-page": "789",
"journal-title": "Nat Rev Cancer",
"key": "ref83",
"volume": "6",
"year": "2006"
},
{
"DOI": "10.1006/mthe.2000.0138",
"author": "Gautam",
"doi-asserted-by": "publisher",
"first-page": "318",
"journal-title": "Mol Ther",
"key": "ref84",
"volume": "2",
"year": "2000"
},
{
"DOI": "10.1038/sj.cgt.7700343",
"author": "Densmore",
"doi-asserted-by": "publisher",
"first-page": "619",
"journal-title": "Cancer Gene Ther",
"key": "ref85",
"volume": "8",
"year": "2001"
},
{
"DOI": "10.1021/acs.jmedchem.6b00975",
"author": "Nelson",
"doi-asserted-by": "publisher",
"first-page": "1620",
"journal-title": "J Med Chem",
"key": "ref86",
"volume": "60",
"year": "2017"
},
{
"author": "Zhang",
"first-page": "548",
"journal-title": "J Clin Invest",
"key": "ref87",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1016/j.jconrel.2012.06.002",
"author": "Lim",
"doi-asserted-by": "publisher",
"first-page": "34",
"journal-title": "J Control Release",
"key": "ref88",
"volume": "163",
"year": "2012"
},
{
"DOI": "10.1021/mp100185f",
"author": "Han",
"doi-asserted-by": "publisher",
"first-page": "2156",
"journal-title": "Mol Pharm",
"key": "ref89",
"volume": "7",
"year": "2010"
},
{
"DOI": "10.1016/j.jconrel.2021.05.032",
"author": "Mirchandani",
"doi-asserted-by": "publisher",
"first-page": "457",
"journal-title": "J Control Release",
"key": "ref90",
"volume": "335",
"year": "2021"
},
{
"DOI": "10.1055/s-0033-1363220",
"author": "Dang",
"doi-asserted-by": "publisher",
"first-page": "516",
"journal-title": "Drug Res (Stuttg)",
"key": "ref91",
"volume": "64",
"year": "2014"
},
{
"DOI": "10.1016/j.apsb.2021.02.012",
"author": "Costa",
"doi-asserted-by": "publisher",
"first-page": "925",
"journal-title": "Acta Pharm Sin B",
"key": "ref92",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.jconrel.2017.03.008",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "28",
"journal-title": "J Control Release",
"key": "ref93",
"volume": "252",
"year": "2017"
},
{
"DOI": "10.1007/s10311-021-01216-9",
"author": "Ashaolu",
"doi-asserted-by": "publisher",
"first-page": "3381",
"journal-title": "Environ Chem Lett",
"key": "ref94",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.3390/pharmaceutics12111091",
"author": "Muta",
"doi-asserted-by": "publisher",
"first-page": "1091",
"journal-title": "Pharmaceutics",
"key": "ref95",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2018.09.042",
"author": "Iba",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Thromb Res",
"key": "ref96",
"volume": "171",
"year": "2018"
},
{
"DOI": "10.1016/j.ejps.2005.06.007",
"author": "van Drooge",
"doi-asserted-by": "publisher",
"first-page": "231",
"journal-title": "Eur J Pharm Sci",
"key": "ref97",
"volume": "26",
"year": "2005"
},
{
"DOI": "10.1016/j.addr.2008.11.002",
"author": "Lai",
"doi-asserted-by": "publisher",
"first-page": "158",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref98",
"volume": "61",
"year": "2009"
},
{
"DOI": "10.1016/j.ejpb.2020.10.010",
"author": "Lababidi",
"doi-asserted-by": "publisher",
"first-page": "200",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref99",
"volume": "157",
"year": "2020"
},
{
"DOI": "10.1016/j.colsurfb.2019.03.027",
"author": "Agel",
"doi-asserted-by": "publisher",
"first-page": "460",
"journal-title": "Colloids Surf B Biointerfaces",
"key": "ref100",
"volume": "178",
"year": "2019"
},
{
"DOI": "10.1016/j.ejpb.2019.07.023",
"author": "Preis",
"doi-asserted-by": "publisher",
"first-page": "531",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref101",
"volume": "142",
"year": "2019"
},
{
"DOI": "10.1002/tox.23781",
"author": "Yao",
"doi-asserted-by": "publisher",
"first-page": "1494",
"journal-title": "Environ Toxicol: Int J",
"key": "ref102",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1016/j.ijbiomac.2020.07.124",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "638",
"journal-title": "Int J Biol Macromol",
"key": "ref103",
"volume": "164",
"year": "2020"
},
{
"DOI": "10.1021/acsami.1c16351",
"author": "Tian",
"doi-asserted-by": "publisher",
"first-page": "56858",
"journal-title": "ACS Appl Mater Interfaces",
"key": "ref104",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.2147/IJN.S249511",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "5361",
"journal-title": "Int J Nanomed",
"key": "ref105",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/j.ejpb.2016.03.025",
"author": "Said-Elbahr",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref106",
"volume": "103",
"year": "2016"
},
{
"DOI": "10.1016/j.ejps.2013.05.012",
"author": "Meenach",
"doi-asserted-by": "publisher",
"first-page": "699",
"journal-title": "Eur J Pharm Sci",
"key": "ref107",
"volume": "49",
"year": "2013"
},
{
"DOI": "10.1016/j.actbio.2022.05.038",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "391",
"journal-title": "Acta Biomater",
"key": "ref108",
"volume": "147",
"year": "2022"
},
{
"DOI": "10.3390/ijms22137130",
"author": "Trigo-Gutierrez",
"doi-asserted-by": "publisher",
"first-page": "7130",
"journal-title": "Int J Mol Sci",
"key": "ref109",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2023.3295",
"author": "Fazio",
"doi-asserted-by": "publisher",
"first-page": "991",
"journal-title": "JAMA Intern Med",
"key": "ref110",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.3390/nu15183881",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "3881",
"journal-title": "Nutrients",
"key": "ref111",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1021/acs.nanolett.6b01632",
"author": "Luo",
"doi-asserted-by": "publisher",
"first-page": "5401",
"journal-title": "Nano Lett",
"key": "ref112",
"volume": "16",
"year": "2016"
},
{
"DOI": "10.1002/smll.202304006",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "e2304006",
"journal-title": "Small",
"key": "ref113",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1002/adma.202302985",
"author": "Jin",
"doi-asserted-by": "publisher",
"first-page": "e2302985",
"journal-title": "Adv Mater",
"key": "ref114",
"year": "2023"
},
{
"DOI": "10.18433/jpps30048",
"author": "Rezazadeh",
"doi-asserted-by": "publisher",
"first-page": "200s",
"journal-title": "J Pharm Pharm Sci",
"key": "ref115",
"volume": "21",
"year": "2018"
},
{
"DOI": "10.1016/j.ijbiomac.2015.09.053",
"author": "Mahajan",
"doi-asserted-by": "publisher",
"first-page": "621",
"journal-title": "Int J Biol Macromol",
"key": "ref116",
"volume": "82",
"year": "2016"
},
{
"DOI": "10.1038/s41568-020-00329-7",
"author": "Cox",
"doi-asserted-by": "publisher",
"first-page": "217",
"journal-title": "Nat Rev Cancer",
"key": "ref117",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.2147/IJN.S166584",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "4641",
"journal-title": "Int J Nanomed",
"key": "ref118",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.3390/ijms241612827",
"author": "Pavelić",
"doi-asserted-by": "publisher",
"first-page": "12827",
"journal-title": "Int J Mol Sci",
"key": "ref119",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/j.jconrel.2022.05.004",
"author": "Muraoka",
"doi-asserted-by": "publisher",
"first-page": "175",
"journal-title": "J Control Release",
"key": "ref120",
"volume": "347",
"year": "2022"
},
{
"DOI": "10.1186/s12951-022-01452-3",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "272",
"journal-title": "J Nanobiotechnology",
"key": "ref121",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.7150/thno.52570",
"author": "Liang",
"doi-asserted-by": "publisher",
"first-page": "3183",
"journal-title": "Theranostics",
"key": "ref122",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/s12967-023-04206-3",
"author": "Zheng",
"doi-asserted-by": "publisher",
"first-page": "383",
"journal-title": "J Transl Med",
"key": "ref123",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1016/j.ijpharm.2020.119482",
"author": "Shanmugam",
"doi-asserted-by": "publisher",
"first-page": "119482",
"journal-title": "Int J Pharm",
"key": "ref124",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1021/acsbiomaterials.0c01126",
"author": "Shanmugam",
"doi-asserted-by": "publisher",
"first-page": "144",
"journal-title": "ACS Biomater Sci Eng",
"key": "ref125",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1089/jamp.2016.1316",
"author": "Pápay",
"doi-asserted-by": "publisher",
"first-page": "274",
"journal-title": "J Aerosol Med Pulm Drug Deliv",
"key": "ref126",
"volume": "30",
"year": "2017"
},
{
"DOI": "10.1016/j.ijbiomac.2021.12.073",
"author": "Hanafy",
"doi-asserted-by": "publisher",
"first-page": "101",
"journal-title": "Int J Biol Macromol",
"key": "ref127",
"volume": "198",
"year": "2022"
},
{
"DOI": "10.1016/j.ijpharm.2019.118919",
"author": "Khan",
"doi-asserted-by": "publisher",
"first-page": "118919",
"journal-title": "Int J Pharm",
"key": "ref128",
"volume": "575",
"year": "2020"
},
{
"DOI": "10.1016/j.ejpb.2009.06.013",
"author": "Hureaux",
"doi-asserted-by": "publisher",
"first-page": "239",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref129",
"volume": "73",
"year": "2009"
},
{
"DOI": "10.1016/j.ijpharm.2011.08.023",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "180",
"journal-title": "Int J Pharm",
"key": "ref130",
"volume": "420",
"year": "2011"
},
{
"DOI": "10.1186/s11671-015-1085-y",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "381",
"journal-title": "Nanoscale Res Lett",
"key": "ref131",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1016/j.ejps.2013.03.009",
"author": "Scalia",
"doi-asserted-by": "publisher",
"first-page": "278",
"journal-title": "Eur J Pharm Sci",
"key": "ref132",
"volume": "49",
"year": "2013"
},
{
"DOI": "10.1016/j.colsurfb.2013.07.067",
"author": "Scalia",
"doi-asserted-by": "publisher",
"first-page": "322",
"journal-title": "Colloids Surf B Biointerfaces",
"key": "ref133",
"volume": "112",
"year": "2013"
},
{
"DOI": "10.1016/j.ajps.2022.04.004",
"author": "Lu",
"doi-asserted-by": "publisher",
"first-page": "447",
"journal-title": "Asian J Pharm Sci",
"key": "ref134",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1007/s13346-020-00857-7",
"author": "Jiang",
"doi-asserted-by": "publisher",
"first-page": "1958",
"journal-title": "Drug Deliv Transl Res",
"key": "ref135",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.ejpb.2023.05.017",
"author": "Qi",
"doi-asserted-by": "publisher",
"first-page": "243",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref136",
"volume": "188",
"year": "2023"
},
{
"DOI": "10.1016/j.ejpb.2016.11.036",
"author": "Mohtar",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref137",
"volume": "113",
"year": "2017"
},
{
"DOI": "10.3390/pharmaceutics13010009",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "Pharmaceutics",
"key": "ref138",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1016/j.ijpharm.2015.06.033",
"author": "Trotta",
"doi-asserted-by": "publisher",
"first-page": "190",
"journal-title": "Int J Pharm",
"key": "ref139",
"volume": "491",
"year": "2015"
},
{
"DOI": "10.1016/j.ejps.2016.02.018",
"author": "Trotta",
"doi-asserted-by": "publisher",
"first-page": "20",
"journal-title": "Eur J Pharm Sci",
"key": "ref140",
"volume": "86",
"year": "2016"
},
{
"DOI": "10.1016/j.ejpb.2008.10.007",
"author": "Sansone",
"doi-asserted-by": "publisher",
"first-page": "206",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref141",
"volume": "72",
"year": "2009"
},
{
"DOI": "10.1016/j.ijpharm.2011.03.055",
"author": "Prota",
"doi-asserted-by": "publisher",
"first-page": "8",
"journal-title": "Int J Pharm",
"key": "ref142",
"volume": "412",
"year": "2011"
},
{
"DOI": "10.1021/mp200351y",
"author": "El-Sherbiny",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Mol Pharm",
"key": "ref143",
"volume": "9",
"year": "2012"
},
{
"key": "ref144",
"volume-title": "Pulmonary Drug Delivery Systems: Material and Technological Advances",
"year": "2023"
},
{
"DOI": "10.1016/j.ijpharm.2023.123117",
"author": "Ahmed",
"doi-asserted-by": "publisher",
"first-page": "123117",
"journal-title": "Int J Pharm",
"key": "ref145",
"volume": "642",
"year": "2023"
},
{
"DOI": "10.1016/j.ejps.2019.02.025",
"author": "Baghdan",
"doi-asserted-by": "publisher",
"first-page": "63",
"journal-title": "Eur J Pharm Sci",
"key": "ref146",
"volume": "132",
"year": "2019"
},
{
"DOI": "10.1016/j.ijpharm.2016.06.134",
"author": "Taki",
"doi-asserted-by": "publisher",
"first-page": "104",
"journal-title": "Int J Pharm",
"key": "ref147",
"volume": "511",
"year": "2016"
},
{
"DOI": "10.1080/10717540902738341",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "160",
"journal-title": "Drug Deliv",
"key": "ref148",
"volume": "16",
"year": "2009"
},
{
"DOI": "10.2147/IJN.S147028",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "8239",
"journal-title": "Int J Nanomed",
"key": "ref149",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1080/10717544.2022.2120567",
"author": "Cui",
"doi-asserted-by": "publisher",
"first-page": "3123",
"journal-title": "Drug Deliv",
"key": "ref150",
"volume": "29",
"year": "2022"
}
],
"reference-count": 150,
"references-count": 150,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/natural-products-based-inhaled-formulations-for-treating-pulmonary-dis-peer-reviewed-fulltext-article-IJN"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Organic Chemistry",
"Drug Discovery",
"General Medicine",
"Biomaterials",
"Bioengineering",
"Biophysics",
"Pharmaceutical Science"
],
"subtitle": [],
"title": "Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases",
"type": "journal-article",
"volume": "Volume 19"
}