Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination
et al., Cureus, doi:10.7759/cureus.49204, Nov 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
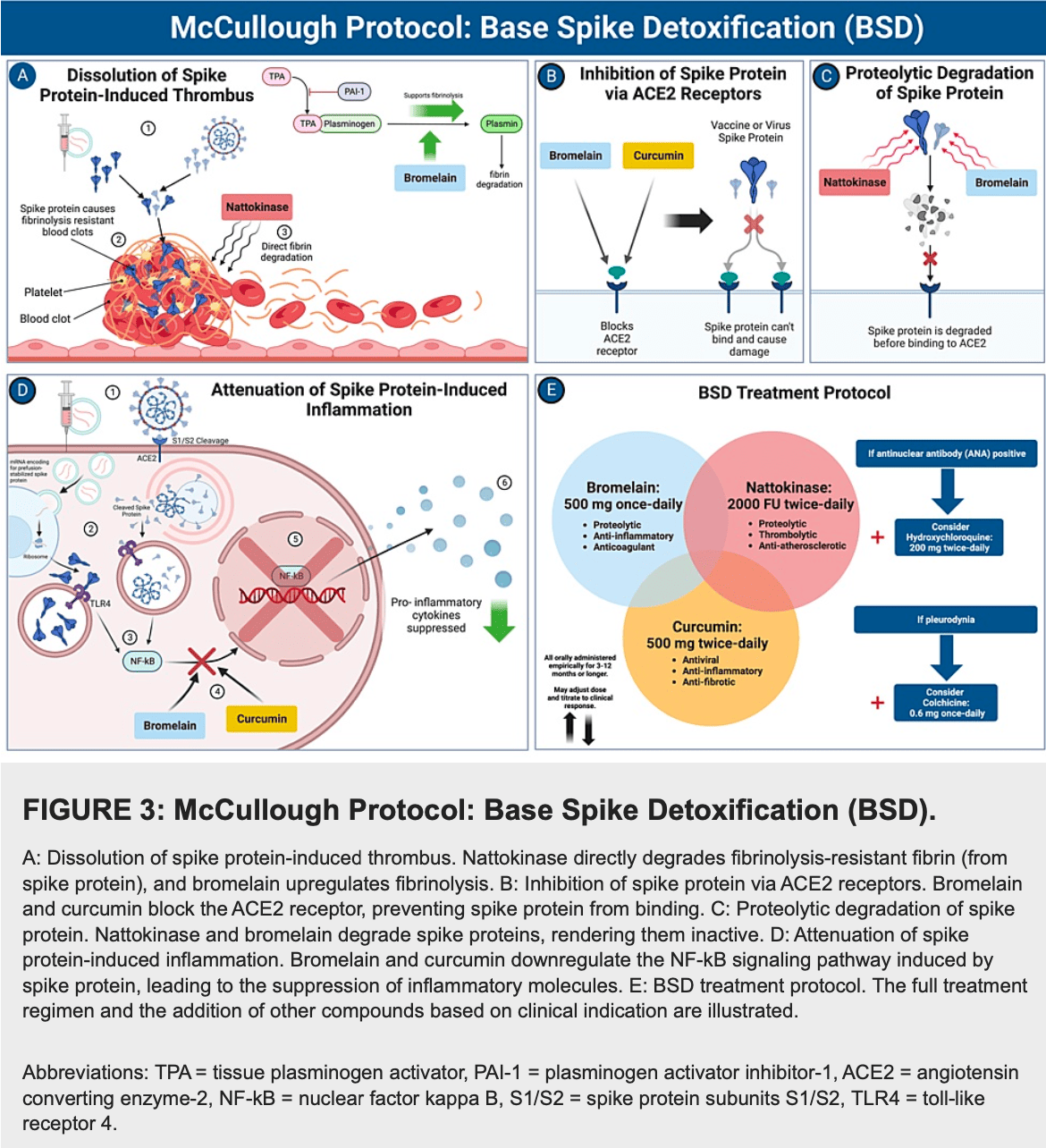
Review of evidence that the SARS-CoV-2 spike protein can damage cardiovascular, hematological, neurological, respiratory, gastrointestinal, and immunological systems, and may be a primary cause of long COVID symptoms. Authors introduce a base spike detoxification protocol including oral nattokinase, bromelain, and curcumin to degrade spike protein, inhibit inflammation, dissolve microthrombi, and provide anticoagulation. Authors discuss other treatments that may also be beneficial including HCQ, colchicine, ivermectin, quercetin, glutathione, apigenin, nicotine, emodin, fisetin, rutin, silymarin, and NAC. Authors note that the safety and efficacy of this protocol warrants formal study in large clinical trials.
1.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
2.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
3.
Duan et al., Bioactive compounds,quercetin, curcumin and β-glucan,regulate innate immunity via the gut-liver-brain axis, Trends in Food Science & Technology, doi:10.1016/j.tifs.2024.104864.
4.
Rajak et al., Antiallergic Implications of Curcumin During COVID-19: Current Status and Perspectives, Biotechnology of Medicinal Plants with Antiallergy Properties, doi:10.1007/978-981-97-1467-4_4.
5.
Kali et al., Curcumin as a Promising Therapy for COVID-19: A Review, Global Journal of Medical, Pharmaceutical, and Biomedical Update, doi:10.25259/GJMPBU_78_2023.
6.
Vajdi et al., Effect of polyphenols against complications of COVID-19: current evidence and potential efficacy, Pharmacological Reports, doi:10.1007/s43440-024-00585-6.
7.
Yong et al., Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases, International Journal of Nanomedicine, doi:10.2147/ijn.s451206.
8.
Halma et al., Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology, Endocrine and Metabolic Science, doi:10.1016/j.endmts.2024.100163.
9.
Arab et al., Immunoregulatory effects of nanocurcumin in inflammatory milieu: Focus on COVID-19, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2024.116131.
10.
Daskou et al., The Role of the NRF2 Pathway in the Pathogenesis of Viral Respiratory Infections, Pathogens, doi:10.3390/pathogens13010039.
11.
Law et al., Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review, Pharmaceuticals, doi:10.3390/ph17010034.
12.
Donzelli, A., Neglected Effective Early Therapies against COVID-19: Focus on Functional Foods and Related Active Substances. A Review, MDPI AG, doi:10.20944/preprints202312.1178.v1.
13.
Hulscher et al., Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination, Cureus, doi:10.7759/cureus.49204.
14.
Hegde et al., Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far?, ACS Omega, doi:10.1021/acsomega.2c07326.
Hulscher et al., 21 Nov 2023, peer-reviewed, 4 authors.
Contact: nichulscher@gmail.com.
Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination
Cureus, doi:10.7759/cureus.49204
The spike protein of SARS-CoV-2 has been found to exhibit pathogenic characteristics and be a possible cause of post-acute sequelae after SARS-CoV-2 infection or COVID-19 vaccination. COVID-19 vaccines utilize a modified, stabilized prefusion spike protein that may share similar toxic effects with its viral counterpart. The aim of this study is to investigate possible mechanisms of harm to biological systems from SARS-CoV-2 spike protein and vaccine-encoded spike protein and to propose possible mitigation strategies. We searched PubMed, Google Scholar, and 'grey literature' to find studies that (1) investigated the effects of the spike protein on biological systems, (2) helped differentiate between viral and vaccine-generated spike proteins, and (3) identified possible spike protein detoxification protocols and compounds that had signals of benefit and acceptable safety profiles. We found abundant evidence that SARS-CoV-2 spike protein may cause damage in the cardiovascular, hematological, neurological, respiratory, gastrointestinal, and immunological systems. Viral and vaccine-encoded spike proteins have been shown to play a direct role in cardiovascular and thrombotic injuries from both SARS-CoV-2 and vaccination. Detection of spike protein for at least 6-15 months after vaccination and infection in those with post-acute sequelae indicates spike protein as a possible primary contributing factor to long COVID. We rationalized that these findings give support to the potential benefit of spike protein detoxification protocols in those with long-term postinfection and/or vaccine-induced complications. We propose a base spike detoxification protocol, composed of oral nattokinase, bromelain, and curcumin. This approach holds immense promise as a base of clinical care, upon which additional therapeutic agents are applied with the goal of aiding in the resolution of postacute sequelae after SARS-CoV-2 infection and COVID-19 vaccination. Large-scale, prospective, randomized, double-blind, placebo-controlled trials are warranted in order to determine the relative risks and benefits of the base spike detoxification protocol.
Additional Information Author Contributions All authors have reviewed the final version to be published and agreed to be accountable for all aspects of the work.
Concept and design:
References
Ait-Belkacem, García, Millet-Wallisky, SARS-CoV-2 spike protein induces a differential monocyte activation that may contribute to age bias in COVID-19 severity, Sci Rep, doi:10.1038/s41598-022-25259-2
Ajala, Azhar, Kalaji, A rare case of pleurodynia after the COVID-19 vaccine, Chest, doi:10.1016/j.chest.2022.08.2056
Akhter, Quéromès, Pillai, The combination of bromelain and acetylcysteine (Bromac) synergistically inactivates SARS-CoV-2, Viruses, doi:10.3390/v13030425
Almehdi, Khoder, Alchakee, Alsayyid, Sarg et al., SARS-CoV-2 spike protein: pathogenesis, vaccines, and potential therapies, Infection, doi:10.1007/s15010-021-01677-8
Avila, Long, Holladay, Gottlieb, Thrombotic complications of COVID-19, Am J Emerg Med, doi:10.1016/j.ajem.2020.09.065
Avolio, Carrabba, Milligan, The SARS-CoV-2 spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease, Clin Sci (Lond), doi:10.1042/CS20210735
Barati, Motavallihaghi, Nikfar, Chaichian, Momtazi-Borojeni, Potential therapeutic effects of ivermectin in COVID-19, Exp Biol Med, doi:10.1177/15353702221099579
Baumeier, Aleshcheva, Harms, Intramyocardial Inflammation after COVID-19 vaccination: an endomyocardial biopsy-proven case series, Int J Mol Sci, doi:10.3390/ijms23136940
Brogna, Cristoni, Marino, Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: possible molecular mechanisms, Proteomics Clin Appl, doi:10.1002/prca.202300048
Chakraborty, Mitra, Tallei, Bromelain a potential bioactive compound: a comprehensive overview from a pharmacological perspective, Life, doi:10.3390/life11040317
Cheng, Hsu, Lin, Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions, Anticancer Res
Clemens, Ye, Zhou, SARS-CoV-2 spike protein-mediated cardiomyocyte fusion may contribute to increased arrhythmic risk in COVID-19, PLoS One, doi:10.1371/journal.pone.0282151
Corbett, Edwards, Leist, SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness, Nature, doi:10.1038/s41586-020-2622-0
Craddock, Mahajan, Spikes, Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19, J Med Virol, doi:10.1002/jmv.28568
Dabbagh, Negahdaripour, Berenjian, Nattokinase: production and application, Appl Microbiol Biotechnol, doi:10.1007/s00253-014-6135-3
Davis, Mccorkell, Vogel, Topol, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol, doi:10.1038/s41579-022-00846-2
De Michele, Amati, Leopizzi, Evidence of SARS-CoV-2 spike protein on retrieved thrombi from COVID-19 patients, J Hematol Oncol, doi:10.1186/s13045-022-01329-w
Debnath, Dewaker, Prabhakar, Bhattacharyya, Mandal, Conformational perturbation of SARS-CoV-2 spike protein using N-acetyl cysteine, a molecular scissor: a probable strategy to combat COVID-19, PREPRINT, doi:10.26434/chemrxiv.12687923.v1
Deore, Tran, Andrews, Ramirez, Galie, SARS-CoV-2 spike protein disrupts blood-brain barrier integrity via RhoA activation, J Neuroimmune Pharmacol, doi:10.1007/s11481-021-10029-0
Dormoy, Perotin, Gosset, Maskos, Polette et al., Nicotinic receptors as SARS-CoV-2 spike co-receptors?, Med Hypotheses, doi:10.1016/j.mehy.2021.110741
Engin, Engin, Engin, Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking, Environ Toxicol Pharmacol, doi:10.1016/j.etap.2020.103411
Espinoza, Emmady, Histology, monocytes
Finterer, Scorza, A retrospective analysis of clinically confirmed long post-COVID vaccination syndrome, J Clin Transl Res
Fiolet, Opstal, Mosterd, Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials, Eur Heart J, doi:10.1093/eurheartj/ehab115
Fontes-Dantas, Fernandes, Gutman, SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice, Cell Rep, doi:10.1016/j.celrep.2023.112189
Forsyth, Zhang, Bhushan, The SARS-CoV-2 S1 spike protein promotes MAPK and NF-kB activation in human lung cells and inflammatory cytokine production in human lung and intestinal epithelial cells, Microorganisms, doi:10.3390/microorganisms10101996
Grobbelaar, Venter, Vlok, SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19, Biosci Rep, doi:10.1042/BSR20210611
Groff, Sun, Ssentongo, Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.28568
Hassaniazad, Eftekhar, Inchehsablagh, A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytother Res, doi:10.1002/ptr.7294
Hegde, Girisa, Bharathwajchetty, Vishwa, Kunnumakkara, Curcumin formulations for better bioavailability: what we learned from clinical trials thus far?, ACS Omega, doi:10.1021/acsomega.2c07326
Hillary, Ceasar, An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines, Heliyon, doi:10.1016/j.heliyon.2023.e13952
Ho, Wu, Chen, Li, Hsiang, Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction, Antiviral Res, doi:10.1016/j.antiviral.2006.04.014
Hulscher, Alexander, Amerling, A systematic review of autopsy findings in deaths after COVID-19 vaccination, PREPRINT, doi:10.5281/zenodo.8120771
Huynh, Rethi, Lee, Higa, Kao et al., Spike protein impairs mitochondrial function in human cardiomyocytes: mechanisms underlying cardiac injury in COVID-19, Cells, doi:10.3390/cells12060877
Karatza, Ismailos, Karalis, Colchicine for the treatment of COVID-19 patients: efficacy, safety, and model informed dosage regimens, Xenobiotica, doi:10.1080/00498254.2021.1909782
Kim, Jeon, Kim, Lee, Kim et al., Spike proteins of SARS-CoV-2 induce pathological changes in molecular delivery and metabolic function in the brain endothelial cells, Viruses, doi:10.3390/v13102021
Kritis, Karampela, Kokoris, Dalamaga, The combination of bromelain and curcumin as an immuneboosting nutraceutical in the prevention of severe COVID-19, Metabol Open, doi:10.1016/j.metop.2020.100066
Kucia, Ratajczak, Bujko, An evidence that SARS-CoV-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner, Leukemia, doi:10.1038/s41375-021-01332-z
Kurosawa, Nirengi, Homma, Esaki, Ohta et al., A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles, Sci Rep, doi:10.1038/srep11601
Lotz-Winter, On the pharmacology of bromelain: an update with special regard to animal studies on dosedependent effects, Planta Med, doi:10.1055/s-2006-960949
Maurer, Bromelain: biochemistry, pharmacology and medical use, Cell Mol Life Sci, doi:10.1007/PL00000936
Mayordomo-Colunga, Vivanco-Allende, López-Alonso, López-Martínez, Vega et al., SARS-CoV-2 spike protein in intestinal cells of a patient with coronavirus disease 2019 multisystem inflammatory syndrome, J Pediatr, doi:10.1016/j.jpeds.2021.11.058
Mccullough, Wynn, Procter, Clinical rationale for SARS-CoV-2 base spike protein detoxification in post COVID-19 and vaccine injury syndromes, J Am Physicians Surg
Murae, Shimizu, Yamamoto, The function of SARS-CoV-2 spike protein is impaired by disulfide-bond disruption with mutation at cysteine-488 and by thiol-reactive N-acetyl-cysteine and glutathione, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2022.01.106
Nag, Banerjee, Paul, Kundu, Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Comput Biol Med, doi:10.1016/j.compbiomed.2022.105552
Oba, Rongduo, Saito, Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2021.07.034
Oh, Cho, Barcelon, Kim, Hong et al., SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death, Sci Rep, doi:10.1038/s41598-022-09410-7
Omoboyowa, Balogun, Chukwudozie, SARS-CoV-2 spike glycoprotein as inhibitory target for Insilico screening of natural compounds, Biointerface Res Appl Chem, doi:10.33263/BRIAC116.1497414985
Palestra, Poto, Ciardi, SARS-CoV-2 spike protein activates human lung macrophages, Int J Mol Sci, doi:10.3390/ijms24033036
Pandey, Rane, Chatterjee, Kumar, Khan et al., Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an in silico study for drug development, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1796811
Parry, Lefringhausen, Turni, Neil, Cosford et al., Spikeopathy': COVID-19 spike protein is pathogenic, from both virus and vaccine mRNA, Biomedicines, doi:10.3390/biomedicines11082287
Patel, Kaki, Potluri, Kahar, Khanna, A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson, Hum Vaccin Immunother, doi:10.1080/21645515.2021.2002083
Patterson, Francisco, Yogendra, Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection, Front Immunol, doi:10.3389/fimmu.2021.746021
Perico, Morigi, Pezzotta, SARS-CoV-2 spike protein induces lung endothelial cell dysfunction and thrombo-inflammation depending on the C3a/C3a receptor signalling, Sci Rep, doi:10.1038/s41598-023-38382-5
Praditya, Kirchhoff, Brüning, Rachmawati, Steinmann et al., Anti-infective properties of the golden spice curcumin, Front Microbiol, doi:10.3389/fmicb.2019.00912
Rabbani, Parikh, Rafique, Colchicine for the treatment of myocardial injury in patients with coronavirus disease 2019 (COVID-19)-an old drug with new life?, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.13556
Robles, Zamora, Castro, Siqueiros-Marquez, De La Escalera et al., The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling, J Biol Chem, doi:10.1016/j.jbc.2022.101695
Rowan, Buttle, Barrett, The cysteine proteinases of the pineapple plant, Biochem J
Sadeghizadeh, Asadollahi, Jahangiri, Promising clinical outcomes of nano-curcumin treatment as an adjunct therapy in hospitalized COVID-19 patients: a randomized, double-blinded, placebo-controlled trial, Phytother Res, doi:10.1002/ptr.7844
Said, Al-Otaibi, Aljaloud, Al-Anazi, Alsolami et al., The frequency and patterns of post-COVID-19 vaccination syndrome reveal initially mild and potentially Immunocytopenic signs in primarily young Saudi women, Vaccines, doi:10.3390/vaccines10071015
Schroeder, Bieneman, The S1 subunit of the SARS-CoV-2 spike protein activates human monocytes to produce cytokines linked to COVID-19: relevance to galectin-3, Front Immunol, doi:10.3389/fimmu.2022.831763
Sheng, Yang, Wang, Sun, Yan, Microbial nattokinase: from synthesis to potential application, Food Funct, doi:10.1039/d2fo03389e
Shrestha, Venkataraman, The prevalence of post-COVID-19 vaccination syndrome and quality of life among COVID-19-vaccinated individuals, IN PRESS, doi:10.1016/j.vacun.2023.10.002
Sogut, Can, Guven, Safety and efficacy of hydroxychloroquine in 152 outpatients with confirmed COVID-19: a pilot observational study, Am J Emerg Med, doi:10.1016/j.ajem.2020.12.014
Soni, Mehta, Ratre, Curcumin, a traditional spice component, can hold the promise against COVID-19?, Eur J Pharmacol, doi:10.1016/j.ejphar.2020.173551
Speciale, Muscarà, Molonia, Cimino, Saija et al., Silibinin as potential tool against SARS-CoV-2: in silico spike receptor-binding domain and main protease molecular docking analysis, and in vitro endothelial protective effects, Phytother Res, doi:10.1002/ptr.7107
Tanikawa, Kiba, Yu, Degradative effect of nattokinase on spike protein of SARS-CoV-2, Molecules, doi:10.3390/molecules27175405
Tardif, Bouabdallaoui, Allier, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00222-8
Tenório, Graciliano, Moura, Oliveira, Goulart, N-acetylcysteine (NAC): impacts on human health, Antioxidants, doi:10.3390/antiox10060967
Theoharides, Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome?, Mol Neurobiol, doi:10.1007/s12035-021-02696-0
Tuli, Sood, Pundir, Molecular docking studies of apigenin, kaempferol, and quercetin as potential target against spike receptor protein of SARS CoV, J Exp Biol Agric Sci, doi:10.18006/2022.10(1).144.149
Turner, Khan, Putrino, Woodcock, Kell et al., Long COVID: pathophysiological factors and abnormalities of coagulation, Trends Endocrinol Metab, doi:10.1016/j.tem.2023.03.002
Vettori, Dima, Henry, Effects of different types of recombinant SARS-CoV-2 spike protein on circulating monocytes' structure, Int J Mol Sci, doi:10.3390/ijms24119373
Wrapp, Wang, Corbett, Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Yasmin, Najeeb, Moeed, Safety and efficacy of colchicine in COVID-19 patients: a systematic review and meta-analysis of randomized control trials, PLoS One, doi:10.1371/journal.pone.0266245
Yonker, Swank, Bartsch, Circulating spike protein detected in post-COVID-19 mRNA vaccine myocarditis, Circulation, doi:10.1161/CIRCULATIONAHA.122.061025
Yuan, Pavel, Wang, Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture, Commun Biol, doi:10.1038/s42003-022-03841-8
Zeng, Li, Deng, SARS-CoV-2 spike spurs intestinal inflammation via VEGF production in enterocytes, EMBO Mol Med, doi:10.15252/emmm.202114844
Zheng, Zhao, Li, SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate, Int J Biol Macromol, doi:10.1016/j.ijbiomac.2021.10.112
DOI record:
{
"DOI": "10.7759/cureus.49204",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.49204",
"author": [
{
"affiliation": [],
"family": "Hulscher",
"given": "Nicolas",
"sequence": "first"
},
{
"affiliation": [],
"family": "Procter",
"given": "Brian C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wynn",
"given": "Cade",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCullough",
"given": "Peter A",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
11,
21
]
],
"date-time": "2023-11-21T23:22:16Z",
"timestamp": 1700608936000
},
"deposited": {
"date-parts": [
[
2023,
11,
21
]
],
"date-time": "2023-11-21T23:22:24Z",
"timestamp": 1700608944000
},
"indexed": {
"date-parts": [
[
2023,
11,
22
]
],
"date-time": "2023-11-22T00:23:30Z",
"timestamp": 1700612610394
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11,
21
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/207654-clinical-approach-to-post-acute-sequelae-after-covid-19-infection-and-vaccination",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2023,
11,
21
]
]
},
"published-print": {
"date-parts": [
[
2023,
11,
21
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.heliyon.2023.e13952",
"article-title": "An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines",
"author": "Hillary VE",
"doi-asserted-by": "publisher",
"journal-title": "Heliyon",
"key": "ref1",
"unstructured": "Hillary VE, Ceasar SA. An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines. Heliyon. 2023, 9:e13952. 10.1016/j.heliyon.2023.e13952",
"volume": "9",
"year": "2023"
},
{
"key": "ref2",
"unstructured": "WHO coronavirus (COVID-19) dashboard. (2023). Accessed. November 22, 2023: https://covid19.who.int/."
},
{
"DOI": "10.1080/21645515.2021.2002083",
"article-title": "A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson",
"author": "Patel R",
"doi-asserted-by": "publisher",
"journal-title": "Hum Vaccin Immunother",
"key": "ref3",
"unstructured": "Patel R, Kaki M, Potluri VS, Kahar P, Khanna D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum Vaccin Immunother. 2022, 18:2002083. 10.1080/21645515.2021.2002083",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1007/s15010-021-01677-8",
"article-title": "SARS-CoV-2 spike protein: pathogenesis, vaccines, and potential therapies",
"author": "Almehdi AM",
"doi-asserted-by": "publisher",
"journal-title": "Infection",
"key": "ref4",
"unstructured": "Almehdi AM, Khoder G, Alchakee AS, Alsayyid AT, Sarg NH, Soliman SS. SARS-CoV-2 spike protein: pathogenesis, vaccines, and potential therapies. Infection. 2021, 49:855-76. 10.1007/s15010-021-01677-8",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"article-title": "Long COVID: major findings, mechanisms and recommendations",
"author": "Davis HE",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Microbiol",
"key": "ref5",
"unstructured": "Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023, 21:133-46. 10.1038/s41579-022-00846-2",
"volume": "21",
"year": "2023"
},
{
"article-title": "A retrospective analysis of clinically confirmed long post-COVID vaccination syndrome",
"author": "Finterer J",
"journal-title": "J Clin Transl Res",
"key": "ref6",
"unstructured": "Finterer J, Scorza FA. A retrospective analysis of clinically confirmed long post-COVID vaccination syndrome. J Clin Transl Res. 2022, 8:506-8.",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.28568",
"article-title": "Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review",
"author": "Groff D",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw Open",
"key": "ref7",
"unstructured": "Groff D, Sun A, Ssentongo AE, et al.. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021, 4:e2128568. 10.1001/jamanetworkopen.2021.28568",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.vacun.2023.10.002",
"article-title": "The prevalence of post-COVID-19 vaccination syndrome and quality of life among COVID-19-vaccinated individuals [IN PRESS]",
"author": "Shrestha Y",
"doi-asserted-by": "publisher",
"journal-title": "Vacunas",
"key": "ref8",
"unstructured": "Shrestha Y, Venkataraman R. The prevalence of post-COVID-19 vaccination syndrome and quality of life among COVID-19-vaccinated individuals [IN PRESS]. Vacunas. 2023, 10.1016/j.vacun.2023.10.002",
"year": "2023"
},
{
"DOI": "10.3390/vaccines10071015",
"article-title": "The frequency and patterns of post-COVID-19 vaccination syndrome reveal initially mild and potentially Immunocytopenic signs in primarily young Saudi women",
"author": "Said KB",
"doi-asserted-by": "publisher",
"journal-title": "Vaccines (Basel)",
"key": "ref9",
"unstructured": "Said KB, Al-Otaibi A, Aljaloud L, Al-Anazi B, Alsolami A, Alreshidi FS. The frequency and patterns of post-COVID-19 vaccination syndrome reveal initially mild and potentially Immunocytopenic signs in primarily young Saudi women. Vaccines (Basel). 2022, 10:1015. 10.3390/vaccines10071015",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1007/s12035-021-02696-0",
"article-title": "Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome?",
"author": "Theoharides TC",
"doi-asserted-by": "publisher",
"journal-title": "Mol Neurobiol",
"key": "ref10",
"unstructured": "Theoharides TC. Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome?. Mol Neurobiol. 2022, 59:1850-61. 10.1007/s12035-021-02696-0",
"volume": "59",
"year": "2022"
},
{
"DOI": "10.1016/j.tem.2023.03.002",
"article-title": "Long COVID: pathophysiological factors and abnormalities of coagulation",
"author": "Turner S",
"doi-asserted-by": "publisher",
"journal-title": "Trends Endocrinol Metab",
"key": "ref11",
"unstructured": "Turner S, Khan MA, Putrino D, Woodcock A, Kell DB, Pretorius E. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab. 2023, 34:321-44. 10.1016/j.tem.2023.03.002",
"volume": "34",
"year": "2023"
},
{
"DOI": "10.3390/biomedicines11082287",
"article-title": "‘Spikeopathy’: COVID-19 spike protein is pathogenic, from both virus and vaccine mRNA",
"author": "Parry PI",
"doi-asserted-by": "publisher",
"journal-title": "Biomedicines",
"key": "ref12",
"unstructured": "Parry PI, Lefringhausen A, Turni C, Neil CJ, Cosford R, Hudson NJ, Gillespie J. ‘Spikeopathy’: COVID-19 spike protein is pathogenic, from both virus and vaccine mRNA. Biomedicines. 2023, 11:2287. 10.3390/biomedicines11082287",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1042/CS20210735",
"article-title": "The SARS-CoV-2 spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease",
"author": "Avolio E",
"doi-asserted-by": "publisher",
"journal-title": "Clin Sci (Lond)",
"key": "ref13",
"unstructured": "Avolio E, Carrabba M, Milligan R, et al.. The SARS-CoV-2 spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin Sci (Lond). 2021, 135:2667-89. 10.1042/CS20210735",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.3390/cells12060877",
"article-title": "Spike protein impairs mitochondrial function in human cardiomyocytes: mechanisms underlying cardiac injury in COVID-19",
"author": "Huynh TV",
"doi-asserted-by": "publisher",
"journal-title": "Cells",
"key": "ref14",
"unstructured": "Huynh TV, Rethi L, Lee TW, Higa S, Kao YH, Chen YJ. Spike protein impairs mitochondrial function in human cardiomyocytes: mechanisms underlying cardiac injury in COVID-19. Cells. 2023, 12:877. 10.3390/cells12060877",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0282151",
"article-title": "SARS-CoV-2 spike protein-mediated cardiomyocyte fusion may contribute to increased arrhythmic risk in COVID-19",
"author": "Clemens DJ",
"doi-asserted-by": "publisher",
"journal-title": "PLoS One",
"key": "ref15",
"unstructured": "Clemens DJ, Ye D, Zhou W, et al.. SARS-CoV-2 spike protein-mediated cardiomyocyte fusion may contribute to increased arrhythmic risk in COVID-19. PLoS One. 2023, 18:e0282151. 10.1371/journal.pone.0282151",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1161/CIRCULATIONAHA.122.061025",
"article-title": "Circulating spike protein detected in post-COVID-19 mRNA vaccine myocarditis",
"author": "Yonker LM",
"doi-asserted-by": "publisher",
"journal-title": "Circulation",
"key": "ref16",
"unstructured": "Yonker LM, Swank Z, Bartsch YC, et al.. Circulating spike protein detected in post-COVID-19 mRNA vaccine myocarditis. Circulation. 2023, 147:867-76. 10.1161/CIRCULATIONAHA.122.061025",
"volume": "147",
"year": "2023"
},
{
"DOI": "10.3390/ijms23136940",
"article-title": "Intramyocardial Inflammation after COVID-19 vaccination: an endomyocardial biopsy-proven case series",
"author": "Baumeier C",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "ref17",
"unstructured": "Baumeier C, Aleshcheva G, Harms D, et al.. Intramyocardial Inflammation after COVID-19 vaccination: an endomyocardial biopsy-proven case series. Int J Mol Sci. 2022, 23:6940. 10.3390/ijms23136940",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.jbc.2022.101695",
"article-title": "The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling",
"author": "Robles JP",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Chem",
"key": "ref18",
"unstructured": "Robles JP, Zamora M, Adan-Castro E, Siqueiros-Marquez L, Martinez de la Escalera G, Clapp C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling. J Biol Chem. 2022, 298:101695. 10.1016/j.jbc.2022.101695",
"volume": "298",
"year": "2022"
},
{
"DOI": "10.1042/BSR20210611",
"article-title": "SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19",
"author": "Grobbelaar LM",
"doi-asserted-by": "publisher",
"journal-title": "Biosci Rep",
"key": "ref19",
"unstructured": "Grobbelaar LM, Venter C, Vlok M, et al.. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep. 2021, 41:BSR20210611. 10.1042/BSR20210611",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1016/j.ijbiomac.2021.10.112",
"article-title": "SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate",
"author": "Zheng Y",
"doi-asserted-by": "publisher",
"journal-title": "Int J Biol Macromol",
"key": "ref20",
"unstructured": "Zheng Y, Zhao J, Li J, et al.. SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate. Int J Biol Macromol. 2021, 193:1124-9. 10.1016/j.ijbiomac.2021.10.112",
"volume": "193",
"year": "2021"
},
{
"DOI": "10.1186/s13045-022-01329-w",
"article-title": "Evidence of SARS-CoV-2 spike protein on retrieved thrombi from COVID-19 patients",
"author": "De Michele M",
"doi-asserted-by": "publisher",
"journal-title": "J Hematol Oncol",
"key": "ref21",
"unstructured": "De Michele M, d'Amati G, Leopizzi M, et al.. Evidence of SARS-CoV-2 spike protein on retrieved thrombi from COVID-19 patients. J Hematol Oncol. 2022, 15:108. 10.1186/s13045-022-01329-w",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1007/s11481-021-10029-0",
"article-title": "SARS-CoV-2 spike protein disrupts blood-brain barrier integrity via RhoA activation",
"author": "DeOre BJ",
"doi-asserted-by": "publisher",
"journal-title": "J Neuroimmune Pharmacol",
"key": "ref22",
"unstructured": "DeOre BJ, Tran KA, Andrews AM, Ramirez SH, Galie PA. SARS-CoV-2 spike protein disrupts blood-brain barrier integrity via RhoA activation. J Neuroimmune Pharmacol. 2021, 16:722-8. 10.1007/s11481-021-10029-0",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-09410-7",
"article-title": "SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death",
"author": "Oh J",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "ref23",
"unstructured": "Oh J, Cho WH, Barcelon E, Kim KH, Hong J, Lee SJ. SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci Rep. 2022, 12:5496. 10.1038/s41598-022-09410-7",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/v13102021",
"article-title": "Spike proteins of SARS-CoV-2 induce pathological changes in molecular delivery and metabolic function in the brain endothelial cells",
"author": "Kim ES",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "ref24",
"unstructured": "Kim ES, Jeon MT, Kim KS, Lee S, Kim S, Kim DG. Spike proteins of SARS-CoV-2 induce pathological changes in molecular delivery and metabolic function in the brain endothelial cells. Viruses. 2021, 13:2021. 10.3390/v13102021",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.celrep.2023.112189",
"article-title": "SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice",
"author": "Fontes-Dantas FL",
"doi-asserted-by": "publisher",
"journal-title": "Cell Rep",
"key": "ref25",
"unstructured": "Fontes-Dantas FL, Fernandes GG, Gutman EG, et al.. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 2023, 42:112189. 10.1016/j.celrep.2023.112189",
"volume": "42",
"year": "2023"
},
{
"DOI": "10.3390/microorganisms10101996",
"article-title": "The SARS-CoV-2 S1 spike protein promotes MAPK and NF-kB activation in human lung cells and inflammatory cytokine production in human lung and intestinal epithelial cells",
"author": "Forsyth CB",
"doi-asserted-by": "publisher",
"journal-title": "Microorganisms",
"key": "ref26",
"unstructured": "Forsyth CB, Zhang L, Bhushan A, et al.. The SARS-CoV-2 S1 spike protein promotes MAPK and NF-kB activation in human lung cells and inflammatory cytokine production in human lung and intestinal epithelial cells. Microorganisms. 2022, 10:1996. 10.3390/microorganisms10101996",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.3390/ijms24033036",
"article-title": "SARS-CoV-2 spike protein activates human lung macrophages",
"author": "Palestra F",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "ref27",
"unstructured": "Palestra F, Poto R, Ciardi R, et al.. SARS-CoV-2 spike protein activates human lung macrophages. Int J Mol Sci. 2023, 24:3036. 10.3390/ijms24033036",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1038/s41598-023-38382-5",
"article-title": "SARS-CoV-2 spike protein induces lung endothelial cell dysfunction and thrombo-inflammation depending on the C3a/C3a receptor signalling",
"author": "Perico L",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "ref28",
"unstructured": "Perico L, Morigi M, Pezzotta A, et al.. SARS-CoV-2 spike protein induces lung endothelial cell dysfunction and thrombo-inflammation depending on the C3a/C3a receptor signalling. Sci Rep. 2023, 13:11392. 10.1038/s41598-023-38382-5",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.15252/emmm.202114844",
"article-title": "SARS-CoV-2 spike spurs intestinal inflammation via VEGF production in enterocytes",
"author": "Zeng FM",
"doi-asserted-by": "publisher",
"journal-title": "EMBO Mol Med",
"key": "ref29",
"unstructured": "Zeng FM, Li YW, Deng ZH, et al.. SARS-CoV-2 spike spurs intestinal inflammation via VEGF production in enterocytes. EMBO Mol Med. 2022, 14:e14844. 10.15252/emmm.202114844",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.jpeds.2021.11.058",
"article-title": "SARS-CoV-2 spike protein in intestinal cells of a patient with coronavirus disease 2019 multisystem inflammatory syndrome",
"author": "Mayordomo-Colunga J",
"doi-asserted-by": "publisher",
"journal-title": "J Pediatr",
"key": "ref30",
"unstructured": "Mayordomo-Colunga J, Vivanco-Allende A, López-Alonso I, López-Martínez C, Fernández-Vega I, Gil-Peña H, Rey C. SARS-CoV-2 spike protein in intestinal cells of a patient with coronavirus disease 2019 multisystem inflammatory syndrome. J Pediatr. 2022, 243:214-218.e5. 10.1016/j.jpeds.2021.11.058",
"volume": "243",
"year": "2022"
},
{
"article-title": "Histology, monocytes",
"author": "Espinoza VE",
"key": "ref31",
"unstructured": "Espinoza VE, Emmady PD. Histology, monocytes. StatPearls. StatPearls Publishing, Treasure Island, FL; 2023.",
"year": "2023"
},
{
"DOI": "10.3390/ijms24119373",
"article-title": "Effects of different types of recombinant SARS-CoV-2 spike protein on circulating monocytes’ structure",
"author": "Vettori M",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "ref32",
"unstructured": "Vettori M, Dima F, Henry BM, et al.. Effects of different types of recombinant SARS-CoV-2 spike protein on circulating monocytes’ structure. Int J Mol Sci. 2023, 24:9373. 10.3390/ijms24119373",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2022.831763",
"article-title": "The S1 subunit of the SARS-CoV-2 spike protein activates human monocytes to produce cytokines linked to COVID-19: relevance to galectin-3",
"author": "Schroeder JT",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "ref33",
"unstructured": "Schroeder JT, Bieneman AP. The S1 subunit of the SARS-CoV-2 spike protein activates human monocytes to produce cytokines linked to COVID-19: relevance to galectin-3. Front Immunol. 2022, 13:831763. 10.3389/fimmu.2022.831763",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-25259-2",
"article-title": "SARS-CoV-2 spike protein induces a differential monocyte activation that may contribute to age bias in COVID-19 severity",
"author": "Ait-Belkacem I",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "ref34",
"unstructured": "Ait-Belkacem I, Cartagena García C, Millet-Wallisky E, et al.. SARS-CoV-2 spike protein induces a differential monocyte activation that may contribute to age bias in COVID-19 severity. Sci Rep. 2022, 12:20824. 10.1038/s41598-022-25259-2",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2021.746021",
"article-title": "Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection",
"author": "Patterson BK",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "ref35",
"unstructured": "Patterson BK, Francisco EB, Yogendra R, et al.. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front Immunol. 2021, 12:746021. 10.3389/fimmu.2021.746021",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41375-021-01332-z",
"article-title": "An evidence that SARS-CoV-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner",
"author": "Kucia M",
"doi-asserted-by": "publisher",
"journal-title": "Leukemia",
"key": "ref36",
"unstructured": "Kucia M, Ratajczak J, Bujko K, et al.. An evidence that SARS-CoV-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner. Leukemia. 2021, 35:3026-9. 10.1038/s41375-021-01332-z",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2622-0",
"article-title": "SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness",
"author": "Corbett KS",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "ref37",
"unstructured": "Corbett KS, Edwards DK, Leist SR, et al.. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020, 586:567-71. 10.1038/s41586-020-2622-0",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1126/science.abb2507",
"article-title": "Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation",
"author": "Wrapp D",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "ref38",
"unstructured": "Wrapp D, Wang N, Corbett KS, et al.. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020, 367:1260-3. 10.1126/science.abb2507",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1002/prca.202300048",
"article-title": "Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: possible molecular mechanisms",
"author": "Brogna C",
"doi-asserted-by": "publisher",
"journal-title": "Proteomics Clin Appl",
"key": "ref39",
"unstructured": "Brogna C, Cristoni S, Marino G, et al.. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: possible molecular mechanisms. Proteomics Clin Appl. 2023, e2300048. 10.1002/prca.202300048",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28568",
"article-title": "Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19",
"author": "Craddock V",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "ref40",
"unstructured": "Craddock V, Mahajan A, Spikes L, et al.. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J Med Virol. 2023, 95:e28568. 10.1002/jmv.28568",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.5281/zenodo.8120771",
"article-title": "A systematic review of autopsy findings in deaths after COVID-19 vaccination [PREPRINT]",
"author": "Hulscher N",
"doi-asserted-by": "publisher",
"journal-title": "Zenodo",
"key": "ref41",
"unstructured": "Hulscher N, Alexander P, Amerling R, et al.. A systematic review of autopsy findings in deaths after COVID-19 vaccination [PREPRINT]. Zenodo. 2023, 10.5281/zenodo.8120771",
"year": "2023"
},
{
"DOI": "10.1016/j.ajem.2020.09.065",
"article-title": "Thrombotic complications of COVID-19",
"author": "Avila J",
"doi-asserted-by": "publisher",
"journal-title": "Am J Emerg Med",
"key": "ref42",
"unstructured": "Avila J, Long B, Holladay D, Gottlieb M. Thrombotic complications of COVID-19. Am J Emerg Med. 2021, 39:213-8. 10.1016/j.ajem.2020.09.065",
"volume": "39",
"year": "2021"
},
{
"article-title": "Clinical rationale for SARS-CoV-2 base spike protein detoxification in post COVID-19 and vaccine injury syndromes",
"author": "McCullough PA",
"journal-title": "J Am Physicians Surg",
"key": "ref43",
"unstructured": "McCullough PA, Wynn C, Procter BC. Clinical rationale for SARS-CoV-2 base spike protein detoxification in post COVID-19 and vaccine injury syndromes. J Am Physicians Surg. 2023, 28:90-3.",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.1039/d2fo03389e",
"article-title": "Microbial nattokinase: from synthesis to potential application",
"author": "Sheng Y",
"doi-asserted-by": "publisher",
"journal-title": "Food Funct",
"key": "ref44",
"unstructured": "Sheng Y, Yang J, Wang C, Sun X, Yan L. Microbial nattokinase: from synthesis to potential application. Food Funct. 2023, 14:2568-85. 10.1039/d2fo03389e",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1007/s00253-014-6135-3",
"article-title": "Nattokinase: production and application",
"author": "Dabbagh F",
"doi-asserted-by": "publisher",
"journal-title": "Appl Microbiol Biotechnol",
"key": "ref45",
"unstructured": "Dabbagh F, Negahdaripour M, Berenjian A, et al.. Nattokinase: production and application. Appl Microbiol Biotechnol. 2014, 98:9199-206. 10.1007/s00253-014-6135-3",
"volume": "98",
"year": "2014"
},
{
"DOI": "10.1016/j.bbrc.2021.07.034",
"article-title": "Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro",
"author": "Oba M",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Biophys Res Commun",
"key": "ref46",
"unstructured": "Oba M, Rongduo W, Saito A, et al.. Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro. Biochem Biophys Res Commun. 2021, 570:21-5. 10.1016/j.bbrc.2021.07.034",
"volume": "570",
"year": "2021"
},
{
"DOI": "10.3390/molecules27175405",
"article-title": "Degradative effect of nattokinase on spike protein of SARS-CoV-2",
"author": "Tanikawa T",
"doi-asserted-by": "publisher",
"journal-title": "Molecules",
"key": "ref47",
"unstructured": "Tanikawa T, Kiba Y, Yu J, et al.. Degradative effect of nattokinase on spike protein of SARS-CoV-2. Molecules. 2022, 27:5405. 10.3390/molecules27175405",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1038/srep11601",
"article-title": "A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles",
"author": "Kurosawa Y",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "ref48",
"unstructured": "Kurosawa Y, Nirengi S, Homma T, Esaki K, Ohta M, Clark JF, Hamaoka T. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci Rep. 2015, 5:11601. 10.1038/srep11601",
"volume": "5",
"year": "2015"
},
{
"article-title": "The cysteine proteinases of the pineapple plant",
"author": "Rowan AD",
"journal-title": "Biochem J",
"key": "ref49",
"unstructured": "Rowan AD, Buttle DJ, Barrett AJ. The cysteine proteinases of the pineapple plant. Biochem J. 1990, 266:869-75.",
"volume": "266",
"year": "1990"
},
{
"DOI": "10.1055/s-2006-960949",
"article-title": "On the pharmacology of bromelain: an update with special regard to animal studies on dose-dependent effects",
"author": "Lotz-Winter H",
"doi-asserted-by": "publisher",
"journal-title": "Planta Med",
"key": "ref50",
"unstructured": "Lotz-Winter H. On the pharmacology of bromelain: an update with special regard to animal studies on dose-dependent effects. Planta Med. 1990, 56:249-53. 10.1055/s-2006-960949",
"volume": "56",
"year": "1990"
},
{
"DOI": "10.1016/j.metop.2020.100066",
"article-title": "The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19",
"author": "Kritis P",
"doi-asserted-by": "publisher",
"journal-title": "Metabol Open",
"key": "ref51",
"unstructured": "Kritis P, Karampela I, Kokoris S, Dalamaga M. The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metabol Open. 2020, 8:100066. 10.1016/j.metop.2020.100066",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/v13030425",
"article-title": "The combination of bromelain and acetylcysteine (Bromac) synergistically inactivates SARS-CoV-2",
"author": "Akhter J",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "ref52",
"unstructured": "Akhter J, Quéromès G, Pillai K, et al.. The combination of bromelain and acetylcysteine (Bromac) synergistically inactivates SARS-CoV-2. Viruses. 2021, 13:425. 10.3390/v13030425",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1007/PL00000936",
"article-title": "Bromelain: biochemistry, pharmacology and medical use",
"author": "Maurer HR",
"doi-asserted-by": "publisher",
"journal-title": "Cell Mol Life Sci",
"key": "ref53",
"unstructured": "Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell Mol Life Sci. 2001, 58:1234-45. 10.1007/PL00000936",
"volume": "58",
"year": "2001"
},
{
"DOI": "10.3390/life11040317",
"article-title": "Bromelain a potential bioactive compound: a comprehensive overview from a pharmacological perspective",
"author": "Chakraborty AJ",
"doi-asserted-by": "publisher",
"journal-title": "Life (Basel)",
"key": "ref54",
"unstructured": "Chakraborty AJ, Mitra S, Tallei TE, et al.. Bromelain a potential bioactive compound: a comprehensive overview from a pharmacological perspective. Life (Basel). 2021, 11:317. 10.3390/life11040317",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3389/fmicb.2019.00912",
"article-title": "Anti-infective properties of the golden spice curcumin",
"author": "Praditya D",
"doi-asserted-by": "publisher",
"journal-title": "Front Microbiol",
"key": "ref55",
"unstructured": "Praditya D, Kirchhoff L, Brüning J, Rachmawati H, Steinmann J, Steinmann E. Anti-infective properties of the golden spice curcumin. Front Microbiol. 2019, 10:912. 10.3389/fmicb.2019.00912",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.ejphar.2020.173551",
"article-title": "Curcumin, a traditional spice component, can hold the promise against COVID-19?",
"author": "Soni VK",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Pharmacol",
"key": "ref56",
"unstructured": "Soni VK, Mehta A, Ratre YK, et al.. Curcumin, a traditional spice component, can hold the promise against COVID-19?. Eur J Pharmacol. 2020, 886:173551. 10.1016/j.ejphar.2020.173551",
"volume": "886",
"year": "2020"
},
{
"DOI": "10.1016/j.compbiomed.2022.105552",
"article-title": "Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study",
"author": "Nag A",
"doi-asserted-by": "publisher",
"journal-title": "Comput Biol Med",
"key": "ref57",
"unstructured": "Nag A, Banerjee R, Paul S, Kundu R. Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study. Comput Biol Med. 2022, 146:105552. 10.1016/j.compbiomed.2022.105552",
"volume": "146",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7844",
"article-title": "Promising clinical outcomes of nano-curcumin treatment as an adjunct therapy in hospitalized COVID-19 patients: a randomized, double-blinded, placebo-controlled trial",
"author": "Sadeghizadeh M",
"doi-asserted-by": "publisher",
"journal-title": "Phytother Res",
"key": "ref58",
"unstructured": "Sadeghizadeh M, Asadollahi E, Jahangiri B, et al.. Promising clinical outcomes of nano-curcumin treatment as an adjunct therapy in hospitalized COVID-19 patients: a randomized, double-blinded, placebo-controlled trial. Phytother Res. 2023, 37:3631-44. 10.1002/ptr.7844",
"volume": "37",
"year": "2023"
},
{
"DOI": "10.1002/ptr.7294",
"article-title": "A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients",
"author": "Hassaniazad M",
"doi-asserted-by": "publisher",
"journal-title": "Phytother Res",
"key": "ref59",
"unstructured": "Hassaniazad M, Eftekhar E, Inchehsablagh BR, et al.. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res. 2021, 35:6417-27. 10.1002/ptr.7294",
"volume": "35",
"year": "2021"
},
{
"article-title": "Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions",
"author": "Cheng AL",
"journal-title": "Anticancer Res",
"key": "ref60",
"unstructured": "Cheng AL, Hsu CH, Lin JK, et al.. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21:2895-900.",
"volume": "21",
"year": "2001"
},
{
"DOI": "10.1021/acsomega.2c07326",
"article-title": "Curcumin formulations for better bioavailability: what we learned from clinical trials thus far?",
"author": "Hegde M",
"doi-asserted-by": "publisher",
"journal-title": "ACS Omega",
"key": "ref61",
"unstructured": "Hegde M, Girisa S, BharathwajChetty B, Vishwa R, Kunnumakkara AB. Curcumin formulations for better bioavailability: what we learned from clinical trials thus far?. ACS Omega. 2023, 8:10713-46. 10.1021/acsomega.2c07326",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1038/s42003-022-03841-8",
"article-title": "Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture",
"author": "Yuan Z",
"doi-asserted-by": "publisher",
"journal-title": "Commun Biol",
"key": "ref62",
"unstructured": "Yuan Z, Pavel MA, Wang H, et al.. Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture. Commun Biol. 2022, 5:958. 10.1038/s42003-022-03841-8",
"volume": "5",
"year": "2022"
},
{
"key": "ref63",
"unstructured": "HCQ for COVID-19. real-time meta analysis of 413 studies. (2023). Accessed: November 4, 2023: https://c19hcq.org/meta.html."
},
{
"DOI": "10.1016/j.ajem.2020.12.014",
"article-title": "Safety and efficacy of hydroxychloroquine in 152 outpatients with confirmed COVID-19: a pilot observational study",
"author": "Sogut O",
"doi-asserted-by": "publisher",
"journal-title": "Am J Emerg Med",
"key": "ref64",
"unstructured": "Sogut O, Can MM, Guven R, et al.. Safety and efficacy of hydroxychloroquine in 152 outpatients with confirmed COVID-19: a pilot observational study. Am J Emerg Med. 2021, 40:41-6. 10.1016/j.ajem.2020.12.014",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1080/00498254.2021.1909782",
"article-title": "Colchicine for the treatment of COVID-19 patients: efficacy, safety, and model informed dosage regimens",
"author": "Karatza E",
"doi-asserted-by": "publisher",
"journal-title": "Xenobiotica",
"key": "ref65",
"unstructured": "Karatza E, Ismailos G, Karalis V. Colchicine for the treatment of COVID-19 patients: efficacy, safety, and model informed dosage regimens. Xenobiotica. 2021, 51:643-56. 10.1080/00498254.2021.1909782",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1093/eurheartj/ehab115",
"article-title": "Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials",
"author": "Fiolet AT",
"doi-asserted-by": "publisher",
"journal-title": "Eur Heart J",
"key": "ref66",
"unstructured": "Fiolet AT, Opstal TS, Mosterd A, et al.. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2021, 42:2765-75. 10.1093/eurheartj/ehab115",
"volume": "42",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13556",
"article-title": "Colchicine for the treatment of myocardial injury in patients with coronavirus disease 2019 (COVID-19)—an old drug with new life?",
"author": "Rabbani AB",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw Open",
"key": "ref67",
"unstructured": "Rabbani AB, Parikh RV, Rafique AM. Colchicine for the treatment of myocardial injury in patients with coronavirus disease 2019 (COVID-19)—an old drug with new life?. JAMA Netw Open. 2020, 3:e2013556. 10.1001/jamanetworkopen.2020.13556",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2022.08.2056",
"article-title": "A rare case of pleurodynia after the COVID-19 vaccine",
"author": "Ajala O",
"doi-asserted-by": "publisher",
"journal-title": "Chest",
"key": "ref68",
"unstructured": "Ajala O, Azhar A, Kalaji W, et al.. A rare case of pleurodynia after the COVID-19 vaccine. Chest. 2022, 162:A2508-9. 10.1016/j.chest.2022.08.2056",
"volume": "162",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"author": "Tardif JC",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "ref69",
"unstructured": "Tardif JC, Bouabdallaoui N, L'Allier PL, et al.. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021, 9:924-32. 10.1016/S2213-2600(21)00222-8",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0266245",
"article-title": "Safety and efficacy of colchicine in COVID-19 patients: a systematic review and meta-analysis of randomized control trials",
"author": "Yasmin F",
"doi-asserted-by": "publisher",
"journal-title": "PLoS One",
"key": "ref70",
"unstructured": "Yasmin F, Najeeb H, Moeed A, et al.. Safety and efficacy of colchicine in COVID-19 patients: a systematic review and meta-analysis of randomized control trials. PLoS One. 2022, 17:e0266245. 10.1371/journal.pone.0266245",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.26434/chemrxiv.12687923.v1",
"article-title": "Conformational perturbation of SARS-CoV-2 spike protein using N-acetyl cysteine, a molecular scissor: a probable strategy to combat COVID-19 [PREPRINT]",
"author": "Debnath U",
"doi-asserted-by": "publisher",
"journal-title": "ChemRxiv",
"key": "ref71",
"unstructured": "Debnath U, Dewaker V, Prabhakar YS, Bhattacharyya P, Mandal A. Conformational perturbation of SARS-CoV-2 spike protein using N-acetyl cysteine, a molecular scissor: a probable strategy to combat COVID-19 [PREPRINT]. ChemRxiv. 2021, 10.26434/chemrxiv.12687923.v1",
"year": "2021"
},
{
"DOI": "10.1016/j.bbrc.2022.01.106",
"article-title": "The function of SARS-CoV-2 spike protein is impaired by disulfide-bond disruption with mutation at cysteine-488 and by thiol-reactive N-acetyl-cysteine and glutathione",
"author": "Murae M",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Biophys Res Commun",
"key": "ref72",
"unstructured": "Murae M, Shimizu Y, Yamamoto Y, et al.. The function of SARS-CoV-2 spike protein is impaired by disulfide-bond disruption with mutation at cysteine-488 and by thiol-reactive N-acetyl-cysteine and glutathione. Biochem Biophys Res Commun. 2022, 597:30-6. 10.1016/j.bbrc.2022.01.106",
"volume": "597",
"year": "2022"
},
{
"DOI": "10.1177/15353702221099579",
"article-title": "Potential therapeutic effects of ivermectin in COVID-19",
"author": "Barati N",
"doi-asserted-by": "publisher",
"journal-title": "Exp Biol Med (Maywood)",
"key": "ref73",
"unstructured": "Barati N, Motavallihaghi S, Nikfar B, Chaichian S, Momtazi-Borojeni AA. Potential therapeutic effects of ivermectin in COVID-19. Exp Biol Med (Maywood). 2022, 247:1388-96. 10.1177/15353702221099579",
"volume": "247",
"year": "2022"
},
{
"DOI": "10.18006/2022.10(1).144.149",
"article-title": "Molecular docking studies of apigenin, kaempferol, and quercetin as potential target against spike receptor protein of SARS CoV",
"author": "Tuli H",
"doi-asserted-by": "publisher",
"journal-title": "J Exp Biol Agric Sci",
"key": "ref74",
"unstructured": "Tuli H, Sood S, Pundir A, et al.. Molecular docking studies of apigenin, kaempferol, and quercetin as potential target against spike receptor protein of SARS CoV. J Exp Biol Agric Sci. 2022, 10:144-9. 10.18006/2022.10(1).144.149",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.etap.2020.103411",
"article-title": "Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking",
"author": "Engin AB",
"doi-asserted-by": "publisher",
"journal-title": "Environ Toxicol Pharmacol",
"key": "ref75",
"unstructured": "Engin AB, Engin ED, Engin A. Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking. Environ Toxicol Pharmacol. 2020, 78:103411. 10.1016/j.etap.2020.103411",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2021.110741",
"article-title": "Nicotinic receptors as SARS-CoV-2 spike co-receptors?",
"author": "Dormoy V",
"doi-asserted-by": "publisher",
"journal-title": "Med Hypotheses",
"key": "ref76",
"unstructured": "Dormoy V, Perotin JM, Gosset P, Maskos U, Polette M, Deslée G. Nicotinic receptors as SARS-CoV-2 spike co-receptors?. Med Hypotheses. 2022, 158:110741. 10.1016/j.mehy.2021.110741",
"volume": "158",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2006.04.014",
"article-title": "Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction",
"author": "Ho TY",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "ref77",
"unstructured": "Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007, 74:92-101. 10.1016/j.antiviral.2006.04.014",
"volume": "74",
"year": "2007"
},
{
"DOI": "10.1080/07391102.2020.1796811",
"article-title": "Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an in silico study for drug development",
"author": "Pandey P",
"doi-asserted-by": "publisher",
"journal-title": "J Biomol Struct Dyn",
"key": "ref78",
"unstructured": "Pandey P, Rane JS, Chatterjee A, Kumar A, Khan R, Prakash A, Ray S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an in silico study for drug development. J Biomol Struct Dyn. 2021, 39:6306-16. 10.1080/07391102.2020.1796811",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.33263/BRIAC116.1497414985",
"article-title": "SARS-CoV-2 spike glycoprotein as inhibitory target for Insilico screening of natural compounds",
"author": "Omoboyowa DA",
"doi-asserted-by": "publisher",
"journal-title": "Biointerface Res Appl Chem",
"key": "ref79",
"unstructured": "Omoboyowa DA, Balogun TA, Chukwudozie O, et al.. SARS-CoV-2 spike glycoprotein as inhibitory target for Insilico screening of natural compounds. Biointerface Res Appl Chem. 2021, 11:14974-85. 10.33263/BRIAC116.1497414985",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7107",
"article-title": "Silibinin as potential tool against SARS-CoV-2: in silico spike receptor-binding domain and main protease molecular docking analysis, and in vitro endothelial protective effects",
"author": "Speciale A",
"doi-asserted-by": "publisher",
"journal-title": "Phytother Res",
"key": "ref80",
"unstructured": "Speciale A, Muscarà C, Molonia MS, Cimino F, Saija A, Giofrè SV. Silibinin as potential tool against SARS-CoV-2: in silico spike receptor-binding domain and main protease molecular docking analysis, and in vitro endothelial protective effects. Phytother Res. 2021, 35:4616-25. 10.1002/ptr.7107",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.3390/antiox10060967",
"article-title": "N-acetylcysteine (NAC): impacts on human health",
"author": "Tenório MC",
"doi-asserted-by": "publisher",
"journal-title": "Antioxidants (Basel)",
"key": "ref81",
"unstructured": "Tenório MC, Graciliano NG, Moura FA, Oliveira AC, Goulart MO. N-acetylcysteine (NAC): impacts on human health. Antioxidants (Basel). 2021, 10:967. 10.3390/antiox10060967",
"volume": "10",
"year": "2021"
},
{
"key": "ref82",
"unstructured": "The effect of micellized food supplements on health-related quality of life in patients with post-acute COVID-19 syndrome. (2022). Accessed. November 15, 2023: https://clinicaltrials.gov/study/NCT05150782?cond=Long%20COVID&term=Post%20Acute%20Sequelae%20of%20COVID-19&intr=Curc...."
}
],
"reference-count": 82,
"references-count": 82,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/207654-clinical-approach-to-post-acute-sequelae-after-covid-19-infection-and-vaccination"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": "Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination",
"type": "journal-article"
}
