The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2
et al., Viruses, doi:10.3390/v13030425, Mar 2021
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
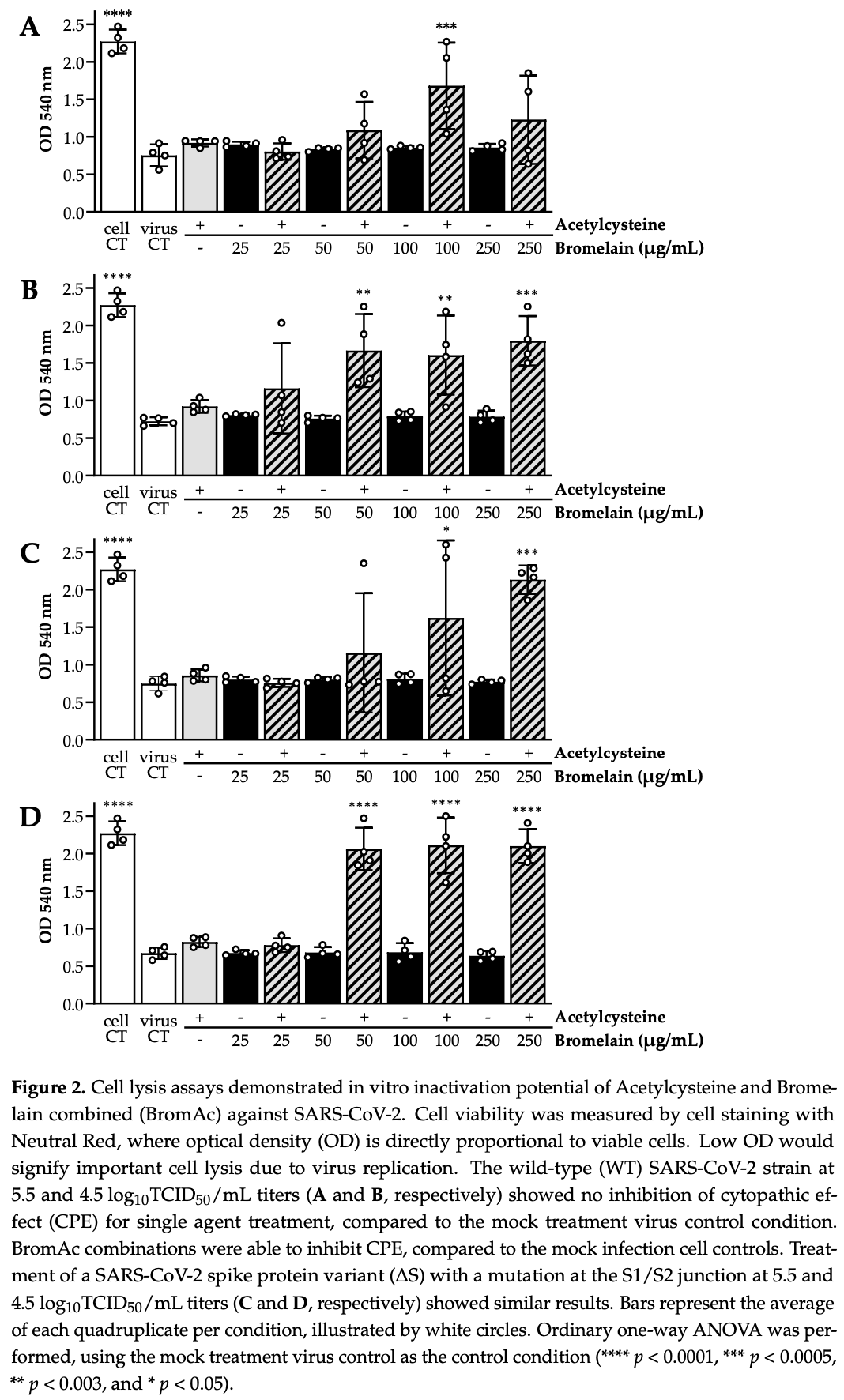
In vitro study showing dose dependent inactivation of SARS-CoV-2 with the combination of bromelain and acetylcysteine.
14 preclinical studies support the efficacy of N-acetylcysteine for COVID-19:
Severe COVID-19 is marked by endotheliopathy with elevated von Willebrand factor (VWF) levels and platelet/VWF-rich microthrombi; N-acetylcysteine can reduce VWF multimers and lyse VWF-dependent clots in vivo, potentially helping to alleviate thrombosis associated with COVID-1910-12.
N-acetylcysteine shows dose-dependent inhibition of SARS-CoV-24,7,9 , shows anti-inflammatory and immunomodulatory effects against SARS-CoV-2-induced immune responses in combination with bromelain6, suppressed virus-induced reactive oxygen species and blocked viral replication in a humanized mouse model and in human lung cells5, may limit COVID-19 induced cardiac damage by boosting cellular antioxidant defenses and potentially mitigating the oxidative stress caused by spike protein-induced ROS production in cardiac fibroblasts3, and reduces disulfide bonds in proteins and exhibits antioxidant properties that may inhibit viral replication and modulate inflammatory responses2.
NAC may be beneficial for COVID-19 by replenishing glutathione stores and reinforcing the glutathione peroxidase-4 pathway to inhibit ferroptosis, an oxidative stress-induced cell death pathway implicated in COVID-1913.
NAC reinforces glutathione levels, reduces ROS, and minimizes ferroptosis and cytokine storm14.
1.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
2.
Reis et al., Antiviral effect of Bromelain combined with acetylcysteine against SARS-CoV-2 Omicron variant, Scientific Reports, doi:10.1038/s41598-025-92242-y.
3.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
4.
Chaopreecha et al., Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC), Phytomedicine, doi:10.1016/j.phymed.2024.156279.
5.
Frasson et al., Identification of druggable host dependency factors shared by multiple SARS-CoV-2 variants of concern, Journal of Molecular Cell Biology. doi:10.1093/jmcb/mjae004, academic.oup.com/jmcb/advance-article/doi/10.1093/jmcb/mjae004/7596546.
6.
Ferreira et al., Taming the SARS-CoV-2-mediated proinflammatory response with BromAc®, Frontiers in Immunology, doi:10.3389/fimmu.2023.1308477.
7.
La Maestra et al., Inhibition of the Cell Uptake of Delta and Omicron SARS-CoV-2 Pseudoviruses by N-Acetylcysteine Irrespective of the Oxidoreductive Environment, Cells, doi:10.3390/cells11203313.
8.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
9.
Akhter et al., The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2, Viruses, doi:10.3390/v13030425.
10.
Martinez de Lizarrondo et al., Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi, Circulation, doi:10.1161/CIRCULATIONAHA.117.027290.
11.
Chen et al., N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice, Journal of Clinical Investigation, doi:10.1172/JCI41062.
12.
Goshua et al., Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study, The Lancet Haematology, doi:10.1016/S2352-3026(20)30216-7.
Akhter et al., 6 Mar 2021, USA, peer-reviewed, 9 authors.
Contact: david.morris@unsw.edu.au (corresponding author), javed.akhter@health.nsw.gov.au, vahan.kepenekian@chu-lyon.fr, samina.badar@unsw.edu.au, z3170073@ad.unsw.edu.au, sarah.valle@mucpharm.com, panthera6444@yahoo.com.au, gregory.queromes@univ-lyon1.fr, emilie.frobert@chu-lyon.fr.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2
Viruses, doi:10.3390/v13030425
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection is the cause of a worldwide pandemic, currently with limited therapeutic options. The spike glycoprotein and envelope protein of SARS-CoV-2, containing disulfide bridges for stabilization, represent an attractive target as they are essential for binding to the ACE2 receptor in host cells present in the nasal mucosa. Bromelain and Acetylcysteine (BromAc) has synergistic action against glycoproteins by breakage of glycosidic linkages and disulfide bonds. We sought to determine the effect of BromAc on the spike and envelope proteins and its potential to reduce infectivity in host cells. Recombinant spike and envelope SARS-CoV-2 proteins were disrupted by BromAc. Spike and envelope protein disulfide bonds were reduced by Acetylcysteine. In in vitro whole virus culture of both wild-type and spike mutants, SARS-CoV-2 demonstrated a concentration-dependent inactivation from BromAc treatment but not from single agents. Clinical testing through nasal administration in patients with early SARS-CoV-2 infection is imminent.
Author Contributions: Conceptualization, J.A., K.P., S.J.V., and D.L.M.; methodology, J.A., G.Q., K.P., S.B., and A.H.M.; validation, J.A., G.Q., K.P., V.K., S.B., and A.H.M.; investigation, J.A., G.Q., K.P., V.K., S.B., and A.H.M.; writing-original draft preparation, G.Q., K.P., V.K, A.H.M., E.F., and S.J.V.; supervision, D.L.M. and E.F.; project administration, S.J.V.; funding acquisition, S.J.V. and D.L.M. All authors have read and agreed to the published version of the manuscript.
Conflicts of
References
Amini, Masoumi-Moghaddam, Morris, Utility of Bromelain and N-Acetylcysteine in Treatment of Peritoneal Dissemination of Gastrointestinal Mucin-Producing Malignancies
Andersen, Ianevski, Lysvand, Vitkauskiene, Oksenych et al., Discovery and development of safe-in-man broad-spectrum antiviral agents, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.02.018
Cai, Zhang, Xiao, Peng, Sterling et al., Distinct conformational states of SARS-CoV-2 spike protein, Science, doi:10.1126/science.abd4251
Calzetta, Rogliani, Facciolo, Rinaldi, Cazzola et al., N-Acetylcysteine protects human bronchi by modulating the release of neurokinin A in an ex vivo model of COPD exacerbation, Biomed Pharm, doi:10.1016/j.biopha.2018.04.011
Casalino, Gaieb, Goldsmith, Hjorth, Dommer et al., Beyond shielding: The roles of glycans in the SARS-CoV-2 spike protein, ACS Cent. Sci
Cazzola, Calzetta, Facciolo, Rogliani, Matera, Pharmacological investigation on the anti-oxidant and antiinflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation, Respir. Res, doi:10.1186/s12931-016-0500-y
Compans, Location of the glycoprotein in the membrane of Sindbis virus, Nat. New Biol, doi:10.1038/newbio229114a0
Coutard, Valle, De Lamballerie, Canard, Seidah et al., The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade, Antiviral Res, doi:10.1016/j.antiviral.2020.104742
De Flora, Balansky, La Maestra, Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19, FASEB J, doi:10.1096/fj.202001807
England, Homer, Knight, Ell, Nasal pH measurement: A reliable and repeatable parameter, Clin. Otolaryngol. Allied Sci, doi:10.1046/j.1365-2273.1999.00223.x
Folegatti, Ewer, Aley, Angus, Becker et al., Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial, Lancet, doi:10.1016/S0140-6736(20)31604-4
Frank, Brown, Capriotti, Westover, Pelletier et al., In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol. Head Neck Surg, doi:10.1001/jamaoto.2020.3053
Greig, Bouillant, Binding effects of concanavalin A on a coronavirus, Can. J. Comp. Med
Guerrero, Acosta, Inflammatory and oxidative stress in rotavirus infection, World J. Virol, doi:10.5501/wjv.v5.i2.38
Hati, Bhattacharyya, Impact of Thiol-Disulfide Balance on the Binding of Covid-19 Spike Protein with Angiotensin-Converting Enzyme 2 Receptor, ACS Omega, doi:10.1021/acsomega.0c02125
Hoffmann, Kleine-Weber, Pohlmann, A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells, Mol. Cell, doi:10.1016/j.molcel.2020.04.022
Hoffmann, Mosbauer, Hofmann-Winkler, Kaul, Kleine-Weber et al., Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature, doi:10.1038/s41586-020-2575-3
Hou, Okuda, Edwards, Martinez, Asakura et al., None
Iyer, Klee, Direct spectrophotometric measurement of the rate of reduction of disulfide bonds. The reactivity of the disulfide bonds of bovine -lactalbumin, J. Biol. Chem
Jaimes, Millet, Whittaker, Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site, iScience
Kato, Lee, Yount, Mascenik, SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract, Cell, doi:10.1016/j.cell.2020.05.042
Kennedy, The effect of enzymes on structural and biological properties of Semliki forest virus, J. Gen. Virol, doi:10.1099/0022-1317-23-2-129
Korber, Fischer, Gnanakaran, Yoon, Theiler et al., Tracking changes in SARS-CoV-2 Spike: Evidence that D614G increases infectivity of the COVID-19 virus, Cell, doi:10.1016/j.cell.2020.06.043
Lau, Wang, Mok, Zhang, Chu et al., Attenuated SARS-CoV-2 variants with deletions at the S1/S2 junction, Emerg Microbes Infect, doi:10.1080/22221751.2020.1756700
Lavillette, Barbouche, Yao, Boson, Cosset et al., Significant redox insensitivity of the functions of the SARS-CoV spike glycoprotein: Comparison with HIV envelope, J. Biol. Chem, doi:10.1074/jbc.M512529200
Li, Zhang, Sui, Kuhn, Moore et al., Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2, EMBO J, doi:10.1038/sj.emboj.7600640
Mathys, Balzarini, The role of cellular oxidoreductases in viral entry and virus infection-associated oxidative stress: Potential therapeutic applications, Expert. Opin. Ther. Targets, doi:10.1517/14728222.2015.1068760
Moreira, Guzman, Boopathi, Baker, Poma, Characterization of Structural and Energetic Differences between Conformations of the SARS-CoV-2 Spike Protein, Materials, doi:10.3390/ma13235362
Moreira, Guzman, Boopathi, Baker, Poma, Quantitative determination of mechanical stability in the novel coronavirus spike protein, Nanoscale, doi:10.1039/D0NR03969A
Morgan, Jaramillo, Shenoy, Raclawska, Emezienna et al., Disulfide disruption reverses mucus dysfunction in allergic airway disease, Nat. Commun, doi:10.1038/s41467-020-20499-0
Pillai, Akhter, Chua, Morris, A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei, Int. J. Cancer, doi:10.1002/ijc.28380
Pillai, Akhter, Morris, Assessment of a novel mucolytic solution for dissolving mucus in pseudomyxoma peritonei: An ex vivo and in vitro study, Pleura Peritoneum, doi:10.1515/pp-2017-0013
Pillai, Mekkawy, Akhter, Badar, Dong et al., Enhancing the potency of chemotherapeutic agents by combination with bromelain and N-acetylcysteine-An in vitro study with pancreatic and hepatic cancer cells, Am. J. Transl. Res
Ryser, Levy, Mandel, Disciullo, Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction, Proc. Natl. Acad. Sci, doi:10.1073/pnas.91.10.4559
Sagar, Rathinavel, Lutz, Struble, Khurana et al., Bromelain inhibits SARS-CoV-2 infection via targeting ACE-2, TMPRSS2, and spike protein, Clin. Transl. Med, doi:10.1002/ctm2.281
Schlegel, Omar, Jentsch, Morell, Kempf, Semliki Forest virus envelope proteins function as proton channels, Biosci. Rep, doi:10.1007/BF01127500
Schlegel, Schaller, Jentsch, Kempf, Semliki Forest virus core protein fragmentation: Its possible role in nucleocapsid disassembly, Biosci. Rep, doi:10.1007/BF01150478
Schoeman, Fielding, Coronavirus envelope protein: Current knowledge, Virol. J, doi:10.1186/s12985-019-1182-0
Song, Zhang, Yin, Wang, Zhou et al., COVID-19 treatment: Close to a cure?-a rapid review of pharmacotherapies for the novel coronavirus, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106080
Suhail, Zajac, Fossum, Lowater, Mccracken et al., Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review, Protein J, doi:10.1007/s10930-020-09935-8
Suk, Boylan, Trehan, Tang, Schneider et al., N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles, Mol. Ther, doi:10.1038/mt.2011.160
Valle, Akhter, Mekkawy, Lodh, Pillai et al., A novel treatment of bromelain and acetylcysteine (BromAc) in patients with peritoneal mucinous tumours: A phase I first in man study, Eur. J. Surg. Oncol, doi:10.1016/j.ejso.2019.10.033
Vankadari, Wilce, Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26, Emerg. Microbes Infect, doi:10.1080/22221751.2020.1739565
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein, Cell, doi:10.1016/j.cell.2020.02.058
Walsh, Frenck, Jr, Falsey, Kitchin et al., Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates, N. Engl. J. Med, doi:10.1056/NEJMoa2027906
Watanabe, Allen, Wrapp, Mclellan, Crispin, Site-specific glycan analysis of the SARS-CoV-2 spike, Science, doi:10.1126/science.abb9983
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Zhang, Ju, Ma, Wang, N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: A randomized controlled trial, Medicine, doi:10.1097/MD.0000000000013087
Zhou, Chen, Hu, Li, Song et al., A Novel Bat Coronavirus Closely Related to SARS-CoV-2 Contains Natural Insertions at the S1/S2 Cleavage Site of the Spike Protein, Curr. Biol, doi:10.1016/j.cub.2020.05.023
Zhu, Guan, Li, Huang, Jiang et al., Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial, Lancet, doi:10.1016/S0140-6736(20)31605-6
DOI record:
{
"DOI": "10.3390/v13030425",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v13030425",
"abstract": "<jats:p>Severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection is the cause of a worldwide pandemic, currently with limited therapeutic options. The spike glycoprotein and envelope protein of SARS-CoV-2, containing disulfide bridges for stabilization, represent an attractive target as they are essential for binding to the ACE2 receptor in host cells present in the nasal mucosa. Bromelain and Acetylcysteine (BromAc) has synergistic action against glycoproteins by breakage of glycosidic linkages and disulfide bonds. We sought to determine the effect of BromAc on the spike and envelope proteins and its potential to reduce infectivity in host cells. Recombinant spike and envelope SARS-CoV-2 proteins were disrupted by BromAc. Spike and envelope protein disulfide bonds were reduced by Acetylcysteine. In in vitro whole virus culture of both wild-type and spike mutants, SARS-CoV-2 demonstrated a concentration-dependent inactivation from BromAc treatment but not from single agents. Clinical testing through nasal administration in patients with early SARS-CoV-2 infection is imminent.</jats:p>",
"alternative-id": [
"v13030425"
],
"author": [
{
"affiliation": [],
"family": "Akhter",
"given": "Javed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Quéromès",
"given": "Grégory",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pillai",
"given": "Krishna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kepenekian",
"given": "Vahan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badar",
"given": "Samina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mekkawy",
"given": "Ahmed H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frobert",
"given": "Emilie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valle",
"given": "Sarah J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morris",
"given": "David L.",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
8
]
],
"date-time": "2021-03-08T02:52:15Z",
"timestamp": 1615171935000
},
"deposited": {
"date-parts": [
[
2021,
3,
8
]
],
"date-time": "2021-03-08T09:50:50Z",
"timestamp": 1615197050000
},
"funder": [
{
"award": [
"COVID research"
],
"name": "Mucpharm pty ltd"
}
],
"indexed": {
"date-parts": [
[
2023,
1,
15
]
],
"date-time": "2023-01-15T05:24:56Z",
"timestamp": 1673760296125
},
"is-referenced-by-count": 18,
"issue": "3",
"issued": {
"date-parts": [
[
2021,
3,
6
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2021,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
6
]
],
"date-time": "2021-03-06T00:00:00Z",
"timestamp": 1614988800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/13/3/425/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "425",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
3,
6
]
]
},
"published-online": {
"date-parts": [
[
2021,
3,
6
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)https://coronavirus.jhu.edu/map.html"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106080",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/S0140-6736(20)31605-6",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1016/S0140-6736(20)31604-4",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1126/science.abd4251",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/j.antiviral.2020.104742",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1080/22221751.2020.1739565",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1021/acsomega.0c02125",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1074/jbc.M512529200",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1517/14728222.2015.1068760",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1126/science.abb2507",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1039/D0NR03969A",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.3390/ma13235362",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"author": "Amini",
"key": "ref14",
"series-title": "Utility of Bromelain and N-Acetylcysteine in Treatment of Peritoneal Dissemination of Gastrointestinal Mucin-Producing Malignancies",
"year": "2016"
},
{
"DOI": "10.1007/BF01150478",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"article-title": "Binding effects of concanavalin A on a coronavirus",
"author": "Greig",
"first-page": "122",
"journal-title": "Can. J. Comp. Med.",
"key": "ref16",
"volume": "41",
"year": "1977"
},
{
"DOI": "10.1002/ijc.28380",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1186/s12985-019-1182-0",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1515/pp-2017-0013",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.ejso.2019.10.033",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"article-title": "Enhancing the potency of chemotherapeutic agents by combination with bromelain and N-acetylcysteine—An in vitro study with pancreatic and hepatic cancer cells",
"author": "Pillai",
"first-page": "7404",
"journal-title": "Am. J. Transl. Res.",
"key": "ref21",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/S0021-9258(19)44430-X",
"article-title": "Direct spectrophotometric measurement of the rate of reduction of disulfide bonds. The reactivity of the disulfide bonds of bovine -lactalbumin",
"author": "Iyer",
"doi-asserted-by": "crossref",
"first-page": "707",
"journal-title": "J. Biol. Chem.",
"key": "ref22",
"volume": "248",
"year": "1973"
},
{
"DOI": "10.1097/MD.0000000000013087",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1038/s41467-020-20499-0",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.biopha.2018.04.011",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1186/s12931-016-0500-y",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1038/mt.2011.160",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1007/s10930-020-09935-8",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1096/fj.202001807",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.5501/wjv.v5.i2.38",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1038/sj.emboj.7600640",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1126/science.abb9983",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1021/acscentsci.0c01056",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1073/pnas.91.10.4559",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1099/0022-1317-23-2-129",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1007/BF01127500",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1038/newbio229114a0",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1002/ctm2.281",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1016/j.cell.2020.06.043",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1016/j.cub.2020.05.023",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.isci.2020.101212",
"article-title": "Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site",
"author": "Jaimes",
"doi-asserted-by": "crossref",
"first-page": "101212",
"journal-title": "iScience",
"key": "ref42",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1756700",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1016/j.molcel.2020.04.022",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1056/NEJMoa2027906",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.ijid.2020.02.018",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1001/jamaoto.2020.3053",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1046/j.1365-2273.1999.00223.x",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"doi-asserted-by": "publisher",
"key": "ref50"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/13/3/425"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": "The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2",
"type": "journal-article",
"volume": "13"
}
