Antiviral effect of Bromelain combined with acetylcysteine against SARS-CoV-2 Omicron variant
et al., Scientific Reports, doi:10.1038/s41598-025-92242-y, Apr 2025
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
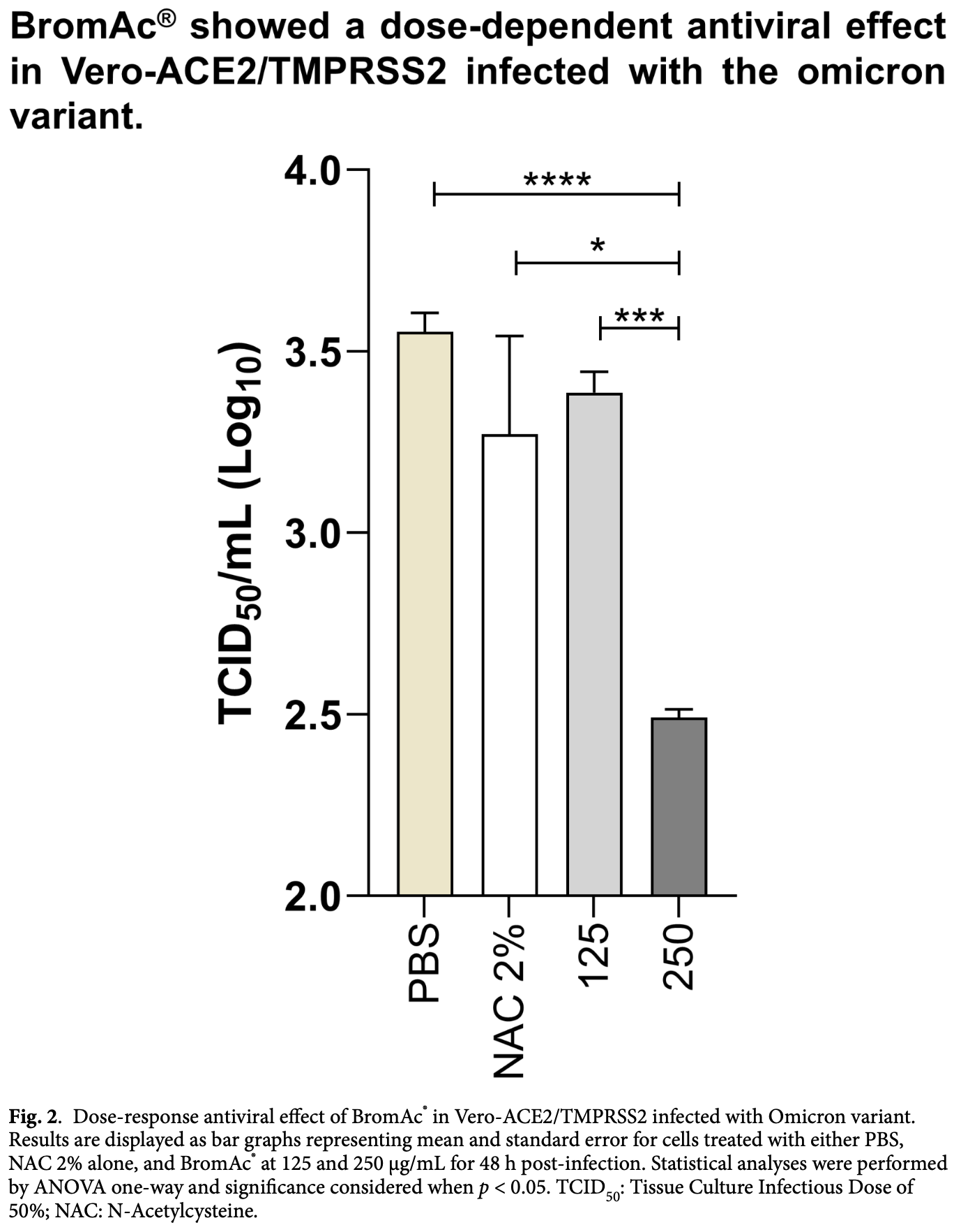
In vitro and ex vivo study showing that BromAc (bromelain and N-acetylcysteine) exhibits antiviral activity against SARS-CoV-2 Omicron variant. Authors demonstrate that BromAc at 250 μg/mL significantly reduces infectious viral particles by over 10-fold after 60 minutes of incubation and decreases viral yield by more than 1-log in Vero-ACE2/TMPRSS2 cells after 48 hours. Western blot analysis revealed that BromAc promotes cleavage of the S1 spike subunit in tracheal aspirate samples from critically ill COVID-19 patients. Flow cytometry confirmed decreased spike protein expression in epithelial cells. The compound showed no cytotoxicity at therapeutic concentrations and demonstrates both virucidal and anti-replication effects. BromAc also exhibited mucolytic properties, which could benefit patients with excess airway mucus.
14 preclinical studies support the efficacy of N-acetylcysteine for COVID-19:
Severe COVID-19 is marked by endotheliopathy with elevated von Willebrand factor (VWF) levels and platelet/VWF-rich microthrombi; N-acetylcysteine can reduce VWF multimers and lyse VWF-dependent clots in vivo, potentially helping to alleviate thrombosis associated with COVID-1910-12.
N-acetylcysteine shows dose-dependent inhibition of SARS-CoV-24,7,9 , shows anti-inflammatory and immunomodulatory effects against SARS-CoV-2-induced immune responses in combination with bromelain6, suppressed virus-induced reactive oxygen species and blocked viral replication in a humanized mouse model and in human lung cells5, may limit COVID-19 induced cardiac damage by boosting cellular antioxidant defenses and potentially mitigating the oxidative stress caused by spike protein-induced ROS production in cardiac fibroblasts3, and reduces disulfide bonds in proteins and exhibits antioxidant properties that may inhibit viral replication and modulate inflammatory responses2.
NAC may be beneficial for COVID-19 by replenishing glutathione stores and reinforcing the glutathione peroxidase-4 pathway to inhibit ferroptosis, an oxidative stress-induced cell death pathway implicated in COVID-1913.
NAC reinforces glutathione levels, reduces ROS, and minimizes ferroptosis and cytokine storm14.
1.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
2.
Reis et al., Antiviral effect of Bromelain combined with acetylcysteine against SARS-CoV-2 Omicron variant, Scientific Reports, doi:10.1038/s41598-025-92242-y.
3.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
4.
Chaopreecha et al., Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC), Phytomedicine, doi:10.1016/j.phymed.2024.156279.
5.
Frasson et al., Identification of druggable host dependency factors shared by multiple SARS-CoV-2 variants of concern, Journal of Molecular Cell Biology. doi:10.1093/jmcb/mjae004, academic.oup.com/jmcb/advance-article/doi/10.1093/jmcb/mjae004/7596546.
6.
Ferreira et al., Taming the SARS-CoV-2-mediated proinflammatory response with BromAc®, Frontiers in Immunology, doi:10.3389/fimmu.2023.1308477.
7.
La Maestra et al., Inhibition of the Cell Uptake of Delta and Omicron SARS-CoV-2 Pseudoviruses by N-Acetylcysteine Irrespective of the Oxidoreductive Environment, Cells, doi:10.3390/cells11203313.
8.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
9.
Akhter et al., The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2, Viruses, doi:10.3390/v13030425.
10.
Martinez de Lizarrondo et al., Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi, Circulation, doi:10.1161/CIRCULATIONAHA.117.027290.
11.
Chen et al., N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice, Journal of Clinical Investigation, doi:10.1172/JCI41062.
12.
Goshua et al., Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study, The Lancet Haematology, doi:10.1016/S2352-3026(20)30216-7.
Reis et al., 7 Apr 2025, peer-reviewed, 14 authors.
Contact: sarah@mucpharm.com, david.morris@unsw.edu.au, reisjordana@gmail.com, jreis@icb.ufmg.br.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Antiviral effect of Bromelain combined with acetylcysteine against SARS-CoV-2 Omicron variant
Scientific Reports, doi:10.1038/s41598-025-92242-y
The recent pandemic represented one of the biggest challenges of modern civilization. SARS-CoV-2 remains an imminent public health threat and currently, there is no effective and greatly affordable treatment for severe COVID-19. Although standard management with dexamethasone, and physical management including physiotherapy, prone positioning and mechanical ventilation are used, severe disease patients may still succumb to infection. In this regard, BromAc ® is a combination therapy of a refined protein derived from Bromelain and acetylcysteine, that shows significant mucolytic and antiinflammatory properties. In the present study, we performed in vitro, and ex vivo analyses to assess the effect of BromAc ® in inhibiting Omicron variant of SARS-CoV-2 at different levels. Here, we provide evidence of the in vitro virucidal activity of BromAc ® in Vero-ACE2/TMPRSS2 cell line infected with the Omicron variant. BromAc ® can also abrogate SARS-CoV-2 RNA genomic copies in tracheal aspirate (TA) samples from critically ill COVID-19 patients after long term exposure. These results were confirmed by lower spike expression observed in EpCAM + PanCK neg epithelial cells from tracheal aspirate samples after BromAc ® treatment. Furthermore, atomized BromAc ® promoted cleavage of the S1 Spike subunit in TA samples, demonstrating the mechanism of the antiviral activity displayed by BromAc ® in human samples. These results bring novel evidence of antiviral activity in cell lines in vitro as well as in tracheal aspirate samples from critically ill COVID-19 patients, which support its potential use as an adjunct to COVID-19 management in future waves of Omicron subvariants.
Author contributions Designing research study: DLM, SJV, JGCdR. Conducting experiments, acquiring data: EVSR, LLF, FAC, GMF, LCO, TFSM. Analyzing data: EVSR, LDC, TFSM, JGCdR. Advisory Committee: FGF, APS, MSE, MMT. Provided reagents and funding: FGF, APS, MMT, SJV, DLM, JGCdR. Supervised research project and acquired funding: SJV, DLM, JGCdR. Writing the manuscript: EVSR, MMT, LLF, TFSM, SJV, MSE, DLM, TFSM, JGCdR. All authors reviewed and approved the final version.
Declarations
Competing interests The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. DLM, SJV and MSE are employed by MucPharm. All the remaining authors declare no conflict of interest.
Ethics approval and consent to participate Research was approved by Institutional Research Board Committee. This research had approval from the Brazilian Institutional Ethics Committee (Approval number: 45919121.6.0000.5526). There was written consent from the next of kin on behalf of the patients for participation in the research. This study followed the principles of the Helsinki Declaration and resolution #466/2012 of the Brazilian Ministry of Health for research involving human subjects.
Consent for publication Informed consent was obtained by next of kin of all participants enrolled in the study.
Additional information
Supplementary Information The online version contains supplementary..
References
Abuzinadah, Ahmad, Al-Thawdi, Zakai, Jamal, Exploring the binding interaction of active compound of pineapple against foodborne bacteria and novel coronavirus (SARS-CoV-2) based on molecular docking and simulation studies, Nutrients
Ahamad, Ali, Secco, Giacca, Gupta, Anti-fungal drug Anidulafungin inhibits SARS-CoV-2 spike-induced syncytia formation by targeting ACE2-spike protein interaction, Front. Genet
Akhter, The combination of bromelain and acetylcysteine (Bromac) synergistically inactivates SARS-CoV-2, Viruses
Alexpandi, De Mesquita, Pandian, Ravi, Quinolines-based SARS-CoV-2 3CLpro and RdRp inhibitors and spike-RBD-ACE2 inhibitor for drug-repurposing against COVID-19: An in Silico analysis, Front. Microbiol
Benton, The effect of the D614G substitution on the structure of the Spike glycoprotein of SARS-CoV-2, Proc. Natl. Acad. Sci
Cai, Distinct conformational states of SARS-CoV-2 spike protein, Science
Castro, Promotion of neutralizing antibody-independent immunity to wild-type and SARS-CoV-2 variants of concern using an RBD-Nucleocapsid fusion protein, Nat. Commun
Chen, SARS-CoV-2 activates lung epithelial cell proinflammatory signaling and leads to immune dysregulation in COVID-19 patients, eBioMedicine
Coelho-Dos-Reis, Ex-vivo mucolytic and anti-inflammatory activity of bromac in tracheal aspirates from COVID-19, Biomed. Pharmacother
Corman, Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Eurosurveillance
Douglas, Tracing the international arrivals of SARS-CoV-2 Omicron variants after aotearoa new Zealand reopened its border, Nat. Commun
Ferreira, Taming the SARS-CoV-2-mediated proinflammatory response with BromAc ®, Front. Immunol
Gonçalves, Timeline kinetics of systemic and airway immune mediator storm for comprehensive analysis of disease outcome in critically ill covid-19 patients, Front. Immunol
Hikisz, Bernasinska-Slomczewska, Beneficial properties of bromelain, Nutrients
Lee, Hong, Jang, N-acetylcysteine decreases airway inflammation and responsiveness in asthma by modulating Claudin 18 expression, Korean J. Intern. Med
Liu, SARS-CoV-2 cell tropism and multiorgan infection, Cell. Discov
Pillai, Ehteda, Akhter, Chua, Morris, Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells, Anticancer Drugs
Poppe, The NF-κB-dependent and -independent transcriptome and chromatin landscapes of human coronavirus 229E-infected cells, PLoS Pathog
Sagar, Bromelain inhibits SARS-CoV-2 infection via targeting ACE-2, TMPRSS2, and Spike protein, Clin. Transl. Med
Shntaif, Alrazzak, Bader, Almarzoqi, Determination the binding ability of n-acetyl cysteine and its derivatives with sars-cov-2 main protease using molecular docking and molecular dynamics studies, Ukr. Biochem. J
Tallei, An analysis based on molecular docking and molecular dynamics simulation study of bromelain as anti-SARS-CoV-2 variants, Front. Pharmacol
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus biology and replication: Implications for SARS-CoV-2, Nat. Rev. Microbiol
Van Eijk, COVID-19: Immunopathology, pathophysiological mechanisms, and treatment options, J. Pathol
DOI record:
{
"DOI": "10.1038/s41598-025-92242-y",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-025-92242-y",
"alternative-id": [
"92242"
],
"article-number": "11882",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "1 October 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "26 February 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "7 April 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. DLM, SJV and MSE are employed by MucPharm. All the remaining authors declare no conflict of interest."
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Research was approved by Institutional Research Board Committee. This research had approval from the Brazilian Institutional Ethics Committee (Approval number: 45919121.6.0000.5526). There was written consent from the next of kin on behalf of the patients for participation in the research. This study followed the principles of the Helsinki Declaration and resolution #466/2012 of the Brazilian Ministry of Health for research involving human subjects."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Informed consent was obtained by next of kin of all participants enrolled in the study."
}
],
"author": [
{
"affiliation": [],
"family": "Reis",
"given": "Erik Vinicius de Sousa",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ferreira",
"given": "Linziane Lopes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clarindo",
"given": "Felipe Alves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marques-Ferreira",
"given": "Geovane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Oliveira",
"given": "Leonardo Camilo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moraes",
"given": "Thaís de Fátima Silva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Carvalho",
"given": "Luciana Debortoli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Fonseca",
"given": "Flávio Guimarães",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sabino",
"given": "Adriano de Paula",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eapen",
"given": "Mathew Suji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teixeira",
"given": "Mauro Martins",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valle",
"given": "Sarah J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morris",
"given": "David L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coelho-dos-Reis",
"given": "Jordana Grazziela Alves",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
4,
7
]
],
"date-time": "2025-04-07T18:07:23Z",
"timestamp": 1744049243000
},
"deposited": {
"date-parts": [
[
2025,
4,
7
]
],
"date-time": "2025-04-07T18:07:40Z",
"timestamp": 1744049260000
},
"indexed": {
"date-parts": [
[
2025,
4,
7
]
],
"date-time": "2025-04-07T18:40:06Z",
"timestamp": 1744051206717,
"version": "3.40.3"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
4,
7
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
7
]
],
"date-time": "2025-04-07T00:00:00Z",
"timestamp": 1743984000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
7
]
],
"date-time": "2025-04-07T00:00:00Z",
"timestamp": 1743984000000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-025-92242-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-92242-y",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-92242-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2025,
4,
7
]
]
},
"published-online": {
"date-parts": [
[
2025,
4,
7
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1002/path.5642",
"author": "LE van Eijk",
"doi-asserted-by": "publisher",
"first-page": "307",
"journal-title": "J. Pathol.",
"key": "92242_CR1",
"unstructured": "van Eijk, L. E. et al. COVID-19: Immunopathology, pathophysiological mechanisms, and treatment options. J. Pathol. 254, 307–331 (2021).",
"volume": "254",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-34186-9",
"author": "J Douglas",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nat. Commun.",
"key": "92242_CR2",
"unstructured": "Douglas, J. et al. Tracing the international arrivals of SARS-CoV-2 Omicron variants after aotearoa new Zealand reopened its border. Nat. Commun. 13, 1–10 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1126/science.abd4251",
"author": "Y Cai",
"doi-asserted-by": "publisher",
"first-page": "1586",
"journal-title": "Science",
"key": "92242_CR3",
"unstructured": "Cai, Y. et al. Distinct conformational states of SARS-CoV-2 spike protein. Science 369, 1586–1592 (2020).",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.3390/v13030425",
"author": "J Akhter",
"doi-asserted-by": "publisher",
"first-page": "425",
"journal-title": "Viruses",
"key": "92242_CR4",
"unstructured": "Akhter, J. et al. The combination of bromelain and acetylcysteine (Bromac) synergistically inactivates SARS-CoV-2. Viruses 13, 425 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2022.112753",
"author": "JGA Coelho-dos-Reis",
"doi-asserted-by": "publisher",
"first-page": "112753",
"journal-title": "Biomed. Pharmacother.",
"key": "92242_CR5",
"unstructured": "Coelho-dos-Reis, J. G. A. et al. Ex-vivo mucolytic and anti-inflammatory activity of bromac in tracheal aspirates from COVID-19. Biomed. Pharmacother. 148, 112753 (2022).",
"volume": "148",
"year": "2022"
},
{
"DOI": "10.1097/CAD.0000000000000039",
"doi-asserted-by": "crossref",
"key": "92242_CR6",
"unstructured": "Pillai, K., Ehteda, A., Akhter, J., Chua, T. C. & Morris, D. L. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs 2,150 – 60 (2014)."
},
{
"DOI": "10.1038/s41467-022-32547-y",
"author": "JT Castro",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nat. Commun.",
"key": "92242_CR7",
"unstructured": "Castro, J. T. et al. Promotion of neutralizing antibody-independent immunity to wild-type and SARS-CoV-2 variants of concern using an RBD-Nucleocapsid fusion protein. Nat. Commun. 13, 1–16 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"author": "VM Corman",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Eurosurveillance",
"key": "92242_CR8",
"unstructured": "Corman, V. M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 25, 1 (2020).",
"volume": "25",
"year": "2020"
},
{
"key": "92242_CR9",
"unstructured": "Therapeutic Goods Association. Australian product information-lagevrio® (molnupiravir) Capsules 1 NAME OF THE MEDICINE (2023)."
},
{
"DOI": "10.1073/pnas.2022586118",
"author": "DJ Benton",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "Proc. Natl. Acad. Sci.",
"key": "92242_CR10",
"unstructured": "Benton, D. J. et al. The effect of the D614G substitution on the structure of the Spike glycoprotein of SARS-CoV-2. Proc. Natl. Acad. Sci. 118, 9 (2021).",
"volume": "118",
"year": "2021"
},
{
"key": "92242_CR11",
"unstructured": "World Health Organization (WHO). https://www.who.int/activities/tracking-SARS-CoV-2-variants (2024)."
},
{
"DOI": "10.3904/kjim.2019.105",
"author": "PH Lee",
"doi-asserted-by": "publisher",
"first-page": "1229",
"journal-title": "Korean J. Intern. Med.",
"key": "92242_CR12",
"unstructured": "Lee, P. H., Hong, J. & Jang, A. S. N-acetylcysteine decreases airway inflammation and responsiveness in asthma by modulating Claudin 18 expression. Korean J. Intern. Med. 35, 1229–1237 (2020).",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1006286",
"doi-asserted-by": "crossref",
"key": "92242_CR13",
"unstructured": "Poppe, M. et al. The NF-κB-dependent and -independent transcriptome and chromatin landscapes of human coronavirus 229E-infected cells. PLoS Pathog. 13 (2017)."
},
{
"DOI": "10.15407/ubj93.05.043",
"author": "AH Shntaif",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "Ukr. Biochem. J.",
"key": "92242_CR14",
"unstructured": "Shntaif, A. H., Alrazzak, N. A., Bader, A. & Almarzoqi, A. M. Determination the binding ability of n-acetyl cysteine and its derivatives with sars-cov-2 main protease using molecular docking and molecular dynamics studies. Ukr. Biochem. J. 93, 43–51 (2021).",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.717757",
"author": "TE Tallei",
"doi-asserted-by": "publisher",
"first-page": "717757",
"journal-title": "Front. Pharmacol.",
"key": "92242_CR15",
"unstructured": "Tallei, T. E. et al. An analysis based on molecular docking and molecular dynamics simulation study of bromelain as anti-SARS-CoV-2 variants. Front. Pharmacol. 12, 717757 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3390/nu14153045",
"author": "MF Abuzinadah",
"doi-asserted-by": "publisher",
"first-page": "3045",
"journal-title": "Nutrients",
"key": "92242_CR16",
"unstructured": "Abuzinadah, M. F., Ahmad, V., Al-Thawdi, S., Zakai, S. A. & Jamal, Q. M. Exploring the binding interaction of active compound of pineapple against foodborne bacteria and novel coronavirus (SARS-CoV-2) based on molecular docking and simulation studies. Nutrients 14, 3045 (2022).",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41579-020-00468-6",
"author": "P V’kovski",
"doi-asserted-by": "publisher",
"first-page": "155",
"journal-title": "Nat. Rev. Microbiol.",
"key": "92242_CR17",
"unstructured": "V’kovski, P., Kratzel, A., Steiner, S., Stalder, H. & Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 19, 155–170 (2020).",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.3390/nu13124313",
"author": "P Hikisz",
"doi-asserted-by": "publisher",
"first-page": "4313",
"journal-title": "Nutrients",
"key": "92242_CR18",
"unstructured": "Hikisz, P. & Bernasinska-Slomczewska, J. Beneficial properties of bromelain. Nutrients 13, 4313 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1002/ctm2.281",
"author": "S Sagar",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Clin. Transl. Med.",
"key": "92242_CR19",
"unstructured": "Sagar, S. et al. Bromelain inhibits SARS-CoV-2 infection via targeting ACE-2, TMPRSS2, and Spike protein. Clin. Transl. Med. 11, 281 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3389/fmicb.2020.01796",
"author": "R Alexpandi",
"doi-asserted-by": "publisher",
"first-page": "1796",
"journal-title": "Front. Microbiol.",
"key": "92242_CR20",
"unstructured": "Alexpandi, R., de Mesquita, J. F., Pandian, S. K. & Ravi, A. V. Quinolines-based SARS-CoV-2 3CLpro and RdRp inhibitors and spike-RBD-ACE2 inhibitor for drug-repurposing against COVID-19: An in Silico analysis. Front. Microbiol. 11, 1796 (2020).",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fgene.2022.866474",
"author": "S Ahamad",
"doi-asserted-by": "publisher",
"first-page": "866474",
"journal-title": "Front. Genet.",
"key": "92242_CR21",
"unstructured": "Ahamad, S., Ali, H., Secco, I., Giacca, M. & Gupta, D. Anti-fungal drug Anidulafungin inhibits SARS-CoV-2 spike-induced syncytia formation by targeting ACE2-spike protein interaction. Front. Genet. 13, 866474 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41421-021-00249-2",
"author": "J Liu",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Cell. Discov.",
"key": "92242_CR22",
"unstructured": "Liu, J. et al. SARS-CoV-2 cell tropism and multiorgan infection. Cell. Discov. 7, 1–4 (2021).",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103500",
"doi-asserted-by": "crossref",
"key": "92242_CR23",
"unstructured": "Chen, H. et al. SARS-CoV-2 activates lung epithelial cell proinflammatory signaling and leads to immune dysregulation in COVID-19 patients. eBioMedicine 70, 103500 (2021)."
},
{
"author": "JJ Gonçalves",
"first-page": "13",
"journal-title": "Front. Immunol.",
"key": "92242_CR24",
"unstructured": "Gonçalves, J. J. Timeline kinetics of systemic and airway immune mediator storm for comprehensive analysis of disease outcome in critically ill covid-19 patients. Front. Immunol. 3, 13 (2022).",
"volume": "3",
"year": "2022"
},
{
"author": "GM Ferreira",
"first-page": "14",
"journal-title": "Front. Immunol.",
"key": "92242_CR25",
"unstructured": "Ferreira, G. M. et al. Taming the SARS-CoV-2-mediated proinflammatory response with BromAc®. Front. Immunol. 13, 14 (2023).",
"volume": "13",
"year": "2023"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-025-92242-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Antiviral effect of Bromelain combined with acetylcysteine against SARS-CoV-2 Omicron variant",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "15"
}
reis14
