Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00222-8, COLCORONA, NCT04322682, Jan 2021 (preprint)
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
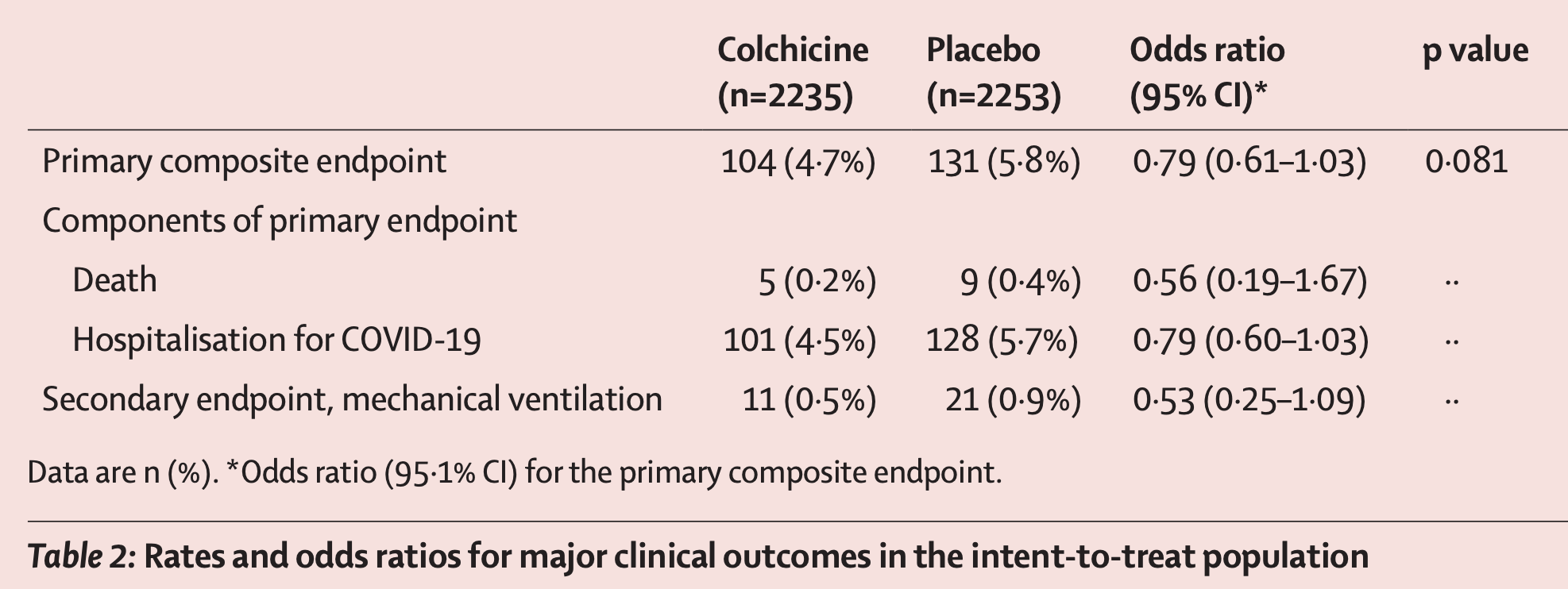
RCT for relatively low risk outpatients, 2235 treated with colchicine a mean of 5.3 days after the onset of symptoms, and 2253 controls, showing lower mortality, ventilation, and hospitalization with treatment.
This study was submitted to NEJM which delayed for ~6 months and then said they were not interested, then to JAMA which delayed for ~6 months and then said they were not interested, and then to the Lancet which delayed for ~6 months and then said they were not interested, and finally was published in Lancet Respiratory Medicine1.
|
risk of death, 43.9% lower, RR 0.56, p = 0.30, treatment 5 of 2,235 (0.2%), control 9 of 2,253 (0.4%), NNT 569, odds ratio converted to relative risk.
|
|
risk of death/hospitalization, 20.0% lower, RR 0.80, p = 0.08, treatment 104 of 2,235 (4.7%), control 131 of 2,253 (5.8%), NNT 86, odds ratio converted to relative risk, primary outcome.
|
|
risk of mechanical ventilation, 46.8% lower, RR 0.53, p = 0.09, treatment 11 of 2,235 (0.5%), control 21 of 2,253 (0.9%), NNT 227, odds ratio converted to relative risk.
|
|
risk of hospitalization, 20.0% lower, RR 0.80, p = 0.09, treatment 101 of 2,235 (4.5%), control 128 of 2,253 (5.7%), NNT 86, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tardif et al., 27 Jan 2021, Double Blind Randomized Controlled Trial, multiple countries, peer-reviewed, 44 authors, study period 23 March, 2020 - 21 January, 2021, average treatment delay 5.3 days, dosage 1mg days 1-3, 0.5mg days 4-30, trial NCT04322682 (history) (COLCORONA).
Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(21)00222-8
Background Evidence suggests a role for excessive inflammation in COVID-19 complications. Colchicine is an oral anti-inflammatory medication beneficial in gout, pericarditis, and coronary disease. We aimed to investigate the effect of colchicine on the composite of COVID-19-related death or hospital admission.
Methods The present study is a phase 3, randomised, double-blind, adaptive, placebo-controlled, multicentre trial. The study was done in Brazil, Canada, Greece, South Africa, Spain, and the USA, and was led by the Montreal Heart Institute. Patients with COVID-19 diagnosed by PCR testing or clinical criteria who were not being treated in hospital were eligible if they were at least 40 years old and had at least one high-risk characteristic. The randomisation list was computer-generated by an unmasked biostatistician, and masked randomisation was centralised and done electronically through an automated interactive web-response system. The allocation sequence was unstratified and used a 1:1 ratio with a blocking schema and block sizes of six. Patients were randomly assigned to receive orally administered colchicine (0•5 mg twice per day for 3 days and then once per day for 27 days thereafter) or matching placebo. The primary efficacy endpoint was the composite of death or hospital admission for COVID-19. Vital status at the end of the study was available for 97•9% of patients. The analyses were done according to the intention-to-treat principle. The COLCORONA trial is registered with ClinicalTrials.gov (NCT04322682) and is now closed to new participants. Findings Trial enrolment began in March 23, 2020, and was completed in Dec 22, 2020. A total of 4488 patients (53•9% women; median age 54•0 years, IQR 47•0-61•0) were enrolled and 2235 patients were randomly assigned to colchicine and 2253 to placebo. The primary endpoint occurred in 104 (4•7%) of 2235 patients in the colchicine group and 131 (5•8%) of 2253 patients in the placebo group (odds ratio [OR] 0•79, 95•1% CI 0•61-1•03; p=0•081). Among the 4159 patients with PCR-confirmed COVID-19, the primary endpoint occurred in 96 (4•6%) of 2075 patients in the colchicine group and 126 (6•0%) of 2084 patients in the placebo group (OR 0•75, 0•57-0•99; p=0•042). Serious adverse events were reported in 108 (4•9%) of 2195 patients in the colchicine group and 139 (6•3%) of 2217 patients in the placebo group (p=0•051); pneumonia occurred in 63 (2•9%) of 2195 patients in the colchicine group and 92 (4•1%) of 2217 patients in the placebo group (p=0•021). Diarrhoea was reported in 300 (13•7%) of 2195 patients in the colchicine group and 161 (7•3%) of 2217 patients in the placebo group (p<0•0001). Interpretation In community-treated patients including those without a mandatory diagnostic test, the effect of colchicine on COVID-19-related clinical events was not statistically significant. Among patients with PCR-confirmed COVID-19, colchicine led to a lower rate of the composite of death or hospital..
References
Abu-Fanne, Stepanova, Litvinov, Neutrophil defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability, Blood
Bmi, ) 30•0 (6•3) Smoking 217 (9•7%) 212 (9•4%, Hypertension
Bompard, Monnier, Saab, Pulmonary embolism in patients with COVID-19 pneumonia, Eur Respir J
Cavalli, Luca, Campochiaro, Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study, Lancet Rheumatol
Cerquaglia, Diaco, Nucera, Regina, Montalto et al., Pharmacological and clinical basis of treatment of familial Mediterranean fever (FMF) with colchicine or analogues: an update, Curr Drug Targ Inflamm Allergy
Coomes, Haghbayan, Interleukin-6 in Covid-19: A systematic review and meta-analysis, Rev Med Virol
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA Network Open
Dupuis, Sirois, Rhéaume, Colchicine reduces lung injury in experimental acute respiratory distress syndrome, PLoS One
Faigenbaum, June, Cytokine storm, N Engl J Med
Fedson, Treating the host response: an alternative way to manage Ebola in Africa and the next influenza pandemic, J Glob Health
Imazio, Bobbio, Cecchia, Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial, Circulation
Leichman, Hadassah researchers pinpoint source of corona blood clots
Lopes, Bonjomo, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, doubleblinded, placebo-controlled clinical trial, RMD Open
Nidorf, Fiolet, Mosterd, Colchicine in patients with chronic coronary disease, N Engl J Med
Nieto-Torres, Verdiá-Báguena, Jimenez-Guardeno, Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome, Virology
Perico, Ostermann, Bontempeill, Colchicine interferes with L-selectin and leukocyte function associated antigen-1 expression on human T lymphocytes and inhibits T cell activation, J Am Soc Nephrol
Pope, Tschopp, The role of interleukin-1 and the inflammasome in gout: implications for therapy, Arthritis Rheumat
Ramakrishnan, Nicolau, Jr, Langford, Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00160-0
Ravelli, Gigant, Curmi, Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain, Nature
Rodrigues, De Sa, Ishimoto, Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J Exp Med
Salama, Han, Yau, Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Samuel, Tardif, Bouabdallaoui, Colchicine for secondary prevention of cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials, Can J Cardiol, doi:10.1016/j.cjca.2020.10.006
Scarsi, Piantoni, Colombo, Association between treatment with colchicine and improved survival in a single-center cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis
Shah, Allen, Harchandani, Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects, Inflammation
Takahashi, Ellingson, Wong, Sex differences in immune responses that underlie COVID-19 disease outcomes, Nature
Tardif, Kouz, Waters, Efficacy and safety of low-dose colchicine after myocardial infarction, N Engl J Med
Thwaites, Uruchurtu, Siggins, Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19, Sci Immunol
Wichmann, Sperhake, Lutgehetmann, Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study, Ann Intern Med
Zavaleta, Corzo, Silva, Characteristics and risk factors for mortality in patients hospitalized by COVID-19 in a public hospital in Tacna, SciFlo, doi:10.1590/SciELOPreprints.1764
DOI record:
{
"DOI": "10.1016/s2213-2600(21)00222-8",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(21)00222-8",
"alternative-id": [
"S2213260021002228"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(21)00222-8"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(21)00225-3"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Tardif",
"given": "Jean-Claude",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bouabdallaoui",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "L'Allier",
"given": "Philippe L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaudet",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Binita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pillinger",
"given": "Michael H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez-Sendon",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Luz",
"given": "Protasio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verret",
"given": "Lucie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Audet",
"given": "Sylvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupuis",
"given": "Jocelyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Denault",
"given": "André",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pelletier",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tessier",
"given": "Philippe A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samson",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fortin",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tardif",
"given": "Jean-Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Busseuil",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goulet",
"given": "Elisabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lacoste",
"given": "Chantal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubois",
"given": "Anick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joshi",
"given": "Avni Y",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Waters",
"given": "David D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hsue",
"given": "Priscilla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lepor",
"given": "Norman E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lesage",
"given": "Frédéric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sainturet",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roy-Clavel",
"given": "Eve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bassevitch",
"given": "Zohar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orfanos",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stamatescu",
"given": "Gabriela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grégoire",
"given": "Jean C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Busque",
"given": "Lambert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lavallée",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hétu",
"given": "Pierre-Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paquette",
"given": "Jean-Sébastien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deftereos",
"given": "Spyridon G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Levesque",
"given": "Sylvie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cossette",
"given": "Mariève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nozza",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chabot-Blanchet",
"given": "Malorie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubé",
"given": "Marie-Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guertin",
"given": "Marie-Claude",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boivin",
"given": "Guy",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
5,
27
]
],
"date-time": "2021-05-27T22:37:47Z",
"timestamp": 1622155067000
},
"deposited": {
"date-parts": [
[
2021,
11,
18
]
],
"date-time": "2021-11-18T06:38:14Z",
"timestamp": 1637217494000
},
"funder": [
{
"DOI": "10.13039/100000050",
"doi-asserted-by": "publisher",
"name": "National Heart Lung and Blood Institute"
},
{
"DOI": "10.13039/100000865",
"doi-asserted-by": "publisher",
"name": "Bill and Melinda Gates Foundation"
},
{
"DOI": "10.13039/100007109",
"doi-asserted-by": "publisher",
"name": "May and Samuel Rudin Family Foundation"
},
{
"DOI": "10.13039/501100012651",
"doi-asserted-by": "publisher",
"name": "Montreal Heart Institute Foundation"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T09:16:01Z",
"timestamp": 1712654161485
},
"is-referenced-by-count": 199,
"issue": "8",
"issued": {
"date-parts": [
[
2021,
8
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2021,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
1
]
],
"date-time": "2021-08-01T00:00:00Z",
"timestamp": 1627776000000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
3
]
],
"date-time": "2021-05-03T00:00:00Z",
"timestamp": 1620000000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021002228?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021002228?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "924-932",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
8
]
]
},
"published-print": {
"date-parts": [
[
2021,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMra2026131",
"article-title": "Cytokine storm",
"author": "Faigenbaum",
"doi-asserted-by": "crossref",
"first-page": "2255",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00222-8_bib1",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.7189/jogh.09.010322",
"article-title": "Treating the host response: an alternative way to manage Ebola in Africa and the next influenza pandemic",
"author": "Fedson",
"doi-asserted-by": "crossref",
"journal-title": "J Glob Health",
"key": "10.1016/S2213-2600(21)00222-8_bib2",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19 - Preliminary report",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00222-8_bib3",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"article-title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial",
"author": "Ramakrishnan",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00222-8_bib4",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2030340",
"article-title": "Tocilizumab in patients hospitalized with Covid-19 pneumonia",
"author": "Salama",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00222-8_bib5",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(20)30127-2",
"article-title": "Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study",
"author": "Cavalli",
"doi-asserted-by": "crossref",
"first-page": "e325",
"journal-title": "Lancet Rheumatol",
"key": "10.1016/S2213-2600(21)00222-8_bib6",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.virol.2015.08.010",
"article-title": "Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome",
"author": "Nieto-Torres",
"doi-asserted-by": "crossref",
"first-page": "330",
"journal-title": "Virology",
"key": "10.1016/S2213-2600(21)00222-8_bib7",
"volume": "485",
"year": "2015"
},
{
"article-title": "Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients",
"author": "Rodrigues",
"journal-title": "J Exp Med",
"key": "10.1016/S2213-2600(21)00222-8_bib8",
"volume": "218",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2141",
"article-title": "Interleukin-6 in Covid-19: A systematic review and meta-analysis",
"author": "Coomes",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Rev Med Virol",
"key": "10.1016/S2213-2600(21)00222-8_bib9",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1126/sciimmunol.abg9873",
"article-title": "Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19",
"author": "Thwaites",
"doi-asserted-by": "crossref",
"journal-title": "Sci Immunol",
"key": "10.1016/S2213-2600(21)00222-8_bib10",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1161/CIRCULATIONAHA.105.542738",
"article-title": "Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial",
"author": "Imazio",
"doi-asserted-by": "crossref",
"first-page": "2012",
"journal-title": "Circulation",
"key": "10.1016/S2213-2600(21)00222-8_bib11",
"volume": "112",
"year": "2005"
},
{
"DOI": "10.1056/NEJMoa1912388",
"article-title": "Efficacy and safety of low-dose colchicine after myocardial infarction",
"author": "Tardif",
"doi-asserted-by": "crossref",
"first-page": "2497",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00222-8_bib12",
"volume": "381",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2021372",
"article-title": "Colchicine in patients with chronic coronary disease",
"author": "Nidorf",
"doi-asserted-by": "crossref",
"first-page": "1831",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00222-8_bib13",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.2174/1568010053622984",
"article-title": "Pharmacological and clinical basis of treatment of familial Mediterranean fever (FMF) with colchicine or analogues: an update",
"author": "Cerquaglia",
"doi-asserted-by": "crossref",
"first-page": "117",
"journal-title": "Curr Drug Targ Inflamm Allergy",
"key": "10.1016/S2213-2600(21)00222-8_bib14",
"volume": "4",
"year": "2005"
},
{
"DOI": "10.1038/nature02393",
"article-title": "Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain",
"author": "Ravelli",
"doi-asserted-by": "crossref",
"first-page": "198",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(21)00222-8_bib15",
"volume": "428",
"year": "2004"
},
{
"DOI": "10.1002/art.22938",
"article-title": "The role of interleukin-1 and the inflammasome in gout: implications for therapy",
"author": "Pope",
"doi-asserted-by": "crossref",
"first-page": "3183",
"journal-title": "Arthritis Rheumat",
"key": "10.1016/S2213-2600(21)00222-8_bib16",
"volume": "56",
"year": "2007"
},
{
"DOI": "10.1681/ASN.V74594",
"article-title": "Colchicine interferes with L-selectin and leukocyte function associated antigen-1 expression on human T lymphocytes and inhibits T cell activation",
"author": "Perico",
"doi-asserted-by": "crossref",
"first-page": "594",
"journal-title": "J Am Soc Nephrol",
"key": "10.1016/S2213-2600(21)00222-8_bib17",
"volume": "7",
"year": "1996"
},
{
"DOI": "10.1371/journal.pone.0242318",
"article-title": "Colchicine reduces lung injury in experimental acute respiratory distress syndrome",
"author": "Dupuis",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S2213-2600(21)00222-8_bib18",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"article-title": "Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial",
"author": "Deftereos",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Network Open",
"key": "10.1016/S2213-2600(21)00222-8_bib19",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"article-title": "Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial",
"author": "Lopes",
"doi-asserted-by": "crossref",
"journal-title": "RMD Open",
"key": "10.1016/S2213-2600(21)00222-8_bib20",
"volume": "7",
"year": "2021"
},
{
"article-title": "Characteristics and risk factors for mortality in patients hospitalized by COVID-19 in a public hospital in Tacna",
"author": "Zavaleta",
"journal-title": "SciFlo",
"key": "10.1016/S2213-2600(21)00222-8_bib21",
"year": "2021"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"article-title": "Association between treatment with colchicine and improved survival in a single-center cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome",
"author": "Scarsi",
"doi-asserted-by": "crossref",
"first-page": "1286",
"journal-title": "Ann Rheum Dis",
"key": "10.1016/S2213-2600(21)00222-8_bib22",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2700-3",
"article-title": "Sex differences in immune responses that underlie COVID-19 disease outcomes",
"author": "Takahashi",
"doi-asserted-by": "crossref",
"first-page": "315",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(21)00222-8_bib24",
"volume": "588",
"year": "2020"
},
{
"article-title": "Colchicine for secondary prevention of cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials",
"author": "Samuel",
"journal-title": "Can J Cardiol",
"key": "10.1016/S2213-2600(21)00222-8_bib25",
"year": "2020"
},
{
"DOI": "10.1182/blood-2018-07-861237",
"article-title": "Neutrophil defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability",
"author": "Abu-Fanne",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Blood",
"key": "10.1016/S2213-2600(21)00222-8_bib26",
"volume": "133",
"year": "2019"
},
{
"author": "Leichman",
"key": "10.1016/S2213-2600(21)00222-8_bib27"
},
{
"DOI": "10.1007/s10753-015-0237-7",
"article-title": "Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects",
"author": "Shah",
"doi-asserted-by": "crossref",
"first-page": "182",
"journal-title": "Inflammation",
"key": "10.1016/S2213-2600(21)00222-8_bib28",
"volume": "39",
"year": "2016"
},
{
"DOI": "10.1183/13993003.01365-2020",
"article-title": "Pulmonary embolism in patients with COVID-19 pneumonia",
"author": "Bompard",
"doi-asserted-by": "crossref",
"journal-title": "Eur Respir J",
"key": "10.1016/S2213-2600(21)00222-8_bib29",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.7326/M20-2003",
"article-title": "Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study",
"author": "Wichmann",
"doi-asserted-by": "crossref",
"first-page": "268",
"journal-title": "Ann Intern Med",
"key": "10.1016/S2213-2600(21)00222-8_bib30",
"volume": "173",
"year": "2020"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260021002228"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "9"
}
