Efficacy of Melatonin in the Treatment of Patients With COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
et al., Journal of Medical Virology, doi:10.1002/jmv.27595, Jan 2022
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
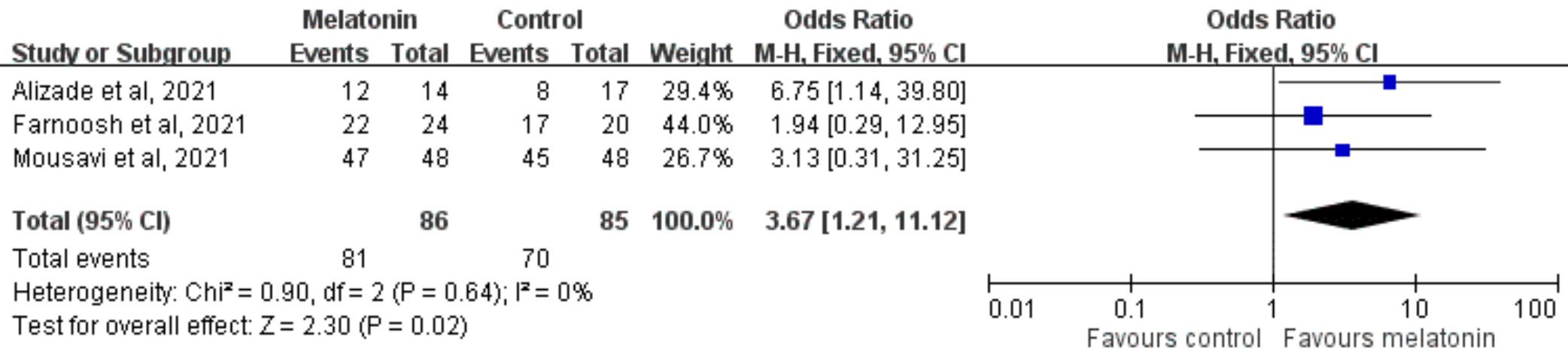
Systematic review and meta analysis including 3 of the 5 melatonin RCTs at the time, showing significantly higher recovery with treatment, and lower ICU admission and mortality without statistical significance. The analysis only includes trials before 9/11/21. Adding Hasan (October 2021) results in statistically significant lower mortality.
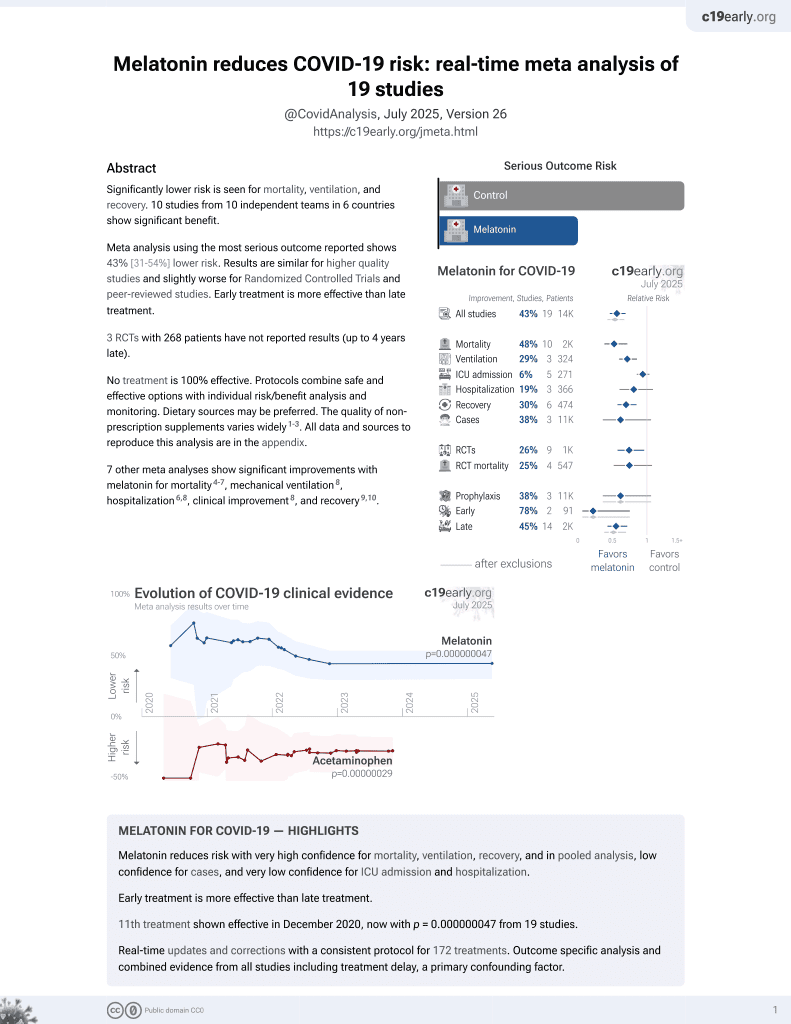
7 meta-analyses show significant improvements with melatonin for mortality1-4,
mechanical ventilation5,
hospitalization3,5,
improvement5, and
recovery6,7.
Currently there are 19 melatonin for COVID-19 studies, showing 33% lower mortality [19‑44%], 32% lower ventilation [19‑43%], 14% lower ICU admission [-1‑28%], 18% lower hospitalization [3‑30%], and 38% fewer cases [-6‑64%].
1.
Pilia et al., Does melatonin reduce mortality in COVID-19?, Annals of Medicine and Surgery, doi:10.1016/j.amsu.2022.103817.
2.
Tóth et al., Melatonin as adjuvant treatment in COVID-19 patients. A meta-analysis of randomized and propensity matched studies, Signa Vitae, doi:10.22514/sv.2023.076.
3.
Amin et al., Role of Melatonin in Management of COVID-19: A Systematic Review, Microbes, Infection and Chemotherapy, doi:10.54034/mic.e1982.
4.
Qin et al., Benefits of melatonin on mortality in severe-to-critical COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials, Clinics, doi:10.1016/j.clinsp.2025.100638.
5.
Taha et al., Safety and efficacy of melatonin as an adjuvant therapy in COVID-19 patients: Systematic review and meta-analysis, Advances in Medical Sciences, doi:10.1016/j.advms.2023.09.007.
Lan et al., 14 Jan 2022, peer-reviewed, 6 authors.
Efficacy of melatonin in the treatment of patients with COVID‐19: A systematic review and meta‐analysis of randomized controlled trials
Journal of Medical Virology, doi:10.1002/jmv.27595
This study investigated the effect of melatonin on clinical outcomes in patients with COVID-19. We searched PubMed, the Web of Science, the Cochrane Library, Ovid MEDLINE, and Clinicaltrials.gov for randomized controlled trials (RCTs) published before September 11, 2021. Only RCTs that compared the clinical efficacy of melatonin with a placebo in the treatment of patients with COVID-19 was included. The primary outcome measure was the clinical recovery rate. We included 3 RCTs in this meta-analysis. Melatonin 3 mg thrice daily was administered in one RCT, and 3 or 6 mg daily before bedtime in other two trials. Treatment duration was 14 days in two RCTs and 7 days in one trial. The clinical recovery rates were 94.2% (81/86) and 82.4% (70/85) in the melatonin and control groups, respectively. Overall, patients receiving melatonin had a higher clinical recovery rate than did the controls (odds ratio [OR], 3.67; 95% CI, 1.21-11.12; I 2 = 0%, P = .02). The risk of intensive care unit admission was numerically lower in the melatonin group than in the control group (8.3% [6/72] vs 17.6% [12/68], OR, 0.45; 95% CI, 0.16-1.25; I 2 = 0%, P = .13), and the risk of mortality was numerically lower in the melatonin group than in the control group (1.4% [1/72] vs 4.4% [3/68], OR, 0.32; 95% CI, 0.03-3.18; I 2 = 0%, P = .33). In conclusion, melatonin may help improve the clinical outcomes of patients with COVID-19.
Accepted
Article in the treatment of COVID-19. Finally, because the definition of the rate of clinical recovery was not comprehensively described in the original studies, we were unable to make imprecise statement regarding this outcome. In conclusion, this meta-analysis revealed that melatonin may help improve the clinical outcomes of patients with COVID-19 and suggested its potential role. However, further large-scale research is required to confirm our findings.
Conflict of
References
Acuña-Castroviejo, Escames, Figueira, De La Oliva, Borobia et al., Clinical trial to test the efficacy of melatonin in COVID-19, J Pineal Res
Alizadeh, Keyhanian, Ghaderkhani, Dashti-Khavidaki, Shoormasti et al., A pilot study on controlling coronavirus disease 2019 (COVID-19) inflammation using melatonin supplement, Iran J Allergy Asthma Immunol
Artigas, Coma, Matos-Filipe, In-silico drug repurposing study predicts the combination of pirfenidone and melatonin as a promising candidate therapy to reduce SARS-CoV-2 infection progression and respiratory distress caused by cytokine storm, PLoS One
Farnoosh, Akbariqomi, Badri, Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: A randomized, double-blind clinical trial, Arch Med Res, doi:10.1016/j.arcmed.2021.06.006
Ferlazzo, Andolina, Cannata, Is Melatonin the Cornucopia of the 21st Century? Antioxidants
Higgins, Altman, Gøtzsche, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, Accepted Article Trials
Kreuzberger, Hirsch, Chai, SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19, Cochrane Database Syst Rev
Lai, Chao, Hsueh, Clinical efficacy of antiviral agents against coronavirus disease 2019: A systematic review of randomized controlled trials, J Microbiol Immunol Infect
Lai, Chen, Wang, Chen, Wang et al., Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials, J Antimicrob Chemother
Mousavi, Heydari, Mehravaran, Melatonin effects on sleep quality and outcomes of COVID-19 patients: An open-label, randomized, controlled trial, J Med Virol
Page, Mckenzie, Bossuyt, Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement, J Clin Epidemiol
Ramlall, Zucker, Tatonetti, Melatonin is significantly associated with survival of intubated COVID-19 patients
Ramos, López-Muñoz, Gil-Martín, The coronavirus disease 2019 (COVID-19): Key emphasis on melatonin safety and therapeutic Efficacy, Antioxidants, doi:10.3390/antiox10071152
Reiter, Abreu-Gonzalez, Marik, Dominguez-Rodriguez, Therapeutic algorithm for use of melatonin in patients with COVID-19, Front Med
Rios, Cardoso, Morra, Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews, Syst Rev
Rodríguez-Rubio, Figueira, Acuña-Castroviejo, Borobia, Escames et al., A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial, Trials
Shankar-Hari, Vale, Godolphin, Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A meta-analysis, JAMA
Sterne, Murthy, Diaz, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis, JAMA
Who, None
Zhou, Hou, Shen, Huang, Martin et al., Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2, Cell Discov
Ziaei, Davoodian, Dadvand, Evaluation of the efficacy and safety of Melatonin in moderately ill patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial, Trials
DOI record:
{
"DOI": "10.1002/jmv.27595",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.27595",
"alternative-id": [
"10.1002/jmv.27595"
],
"archive": [
"Portico"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-01-14"
}
],
"author": [
{
"affiliation": [
{
"name": "School of Pharmaceutical Sciences and Medical Technology, Putian University Putian China"
}
],
"family": "Lan",
"given": "Shao‐Huan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "School of Pharmacy, China Medical University Taichung"
}
],
"family": "Lee",
"given": "Hong‐Zin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Medicine, Chi Mei Medical Center, Liouying Taiwan"
}
],
"family": "Chao",
"given": "Chien‐Ming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Yijia Pharmacy Tainan 70846 Taiwan"
}
],
"family": "Chang",
"given": "Shen‐Peng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Management, Putian University Putian China"
}
],
"family": "Lu",
"given": "Li‐Chin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6334-2388",
"affiliation": [
{
"name": "Department of Internal Medicine, Kaohsiung Veterans General Hospital, Tainan Branch Tainan Taiwan"
}
],
"authenticated-orcid": false,
"family": "Lai",
"given": "Chih‐Cheng",
"sequence": "additional"
}
],
"container-title": [
"Journal of Medical Virology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
1,
15
]
],
"date-time": "2022-01-15T01:58:05Z",
"timestamp": 1642211885000
},
"deposited": {
"date-parts": [
[
2022,
1,
15
]
],
"date-time": "2022-01-15T01:58:06Z",
"timestamp": 1642211886000
},
"indexed": {
"date-parts": [
[
2022,
1,
15
]
],
"date-time": "2022-01-15T06:40:31Z",
"timestamp": 1642228831106
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0146-6615"
},
{
"type": "electronic",
"value": "1096-9071"
}
],
"issued": {
"date-parts": [
[
2022,
1,
14
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
14
]
],
"date-time": "2022-01-14T00:00:00Z",
"timestamp": 1642118400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
14
]
],
"date-time": "2022-01-14T00:00:00Z",
"timestamp": 1642118400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.27595",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.27595",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
1,
14
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
14
]
]
},
"publisher": "Wiley",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"Journal of Medical Virology"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": [
"Efficacy of Melatonin in the Treatment of Patients With COVID‐19: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}