Does melatonin reduce mortality in COVID-19?
et al., Annals of Medicine and Surgery, doi:10.1016/j.amsu.2022.103817, May 2022
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
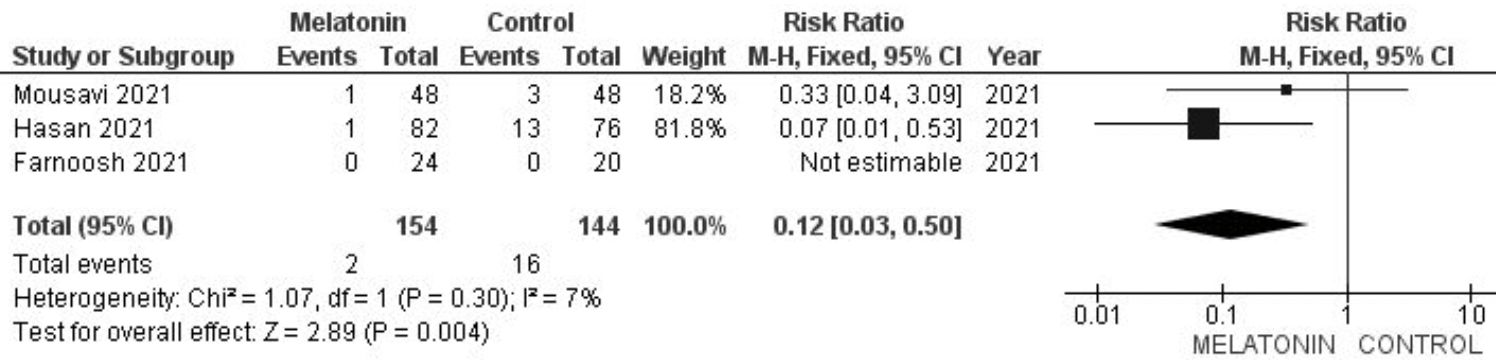
Meta analysis of 3 melatonin RCTs showing significantly lower mortality with treatment. Authors note the small sample size in RCTs reporting mortality results to date.
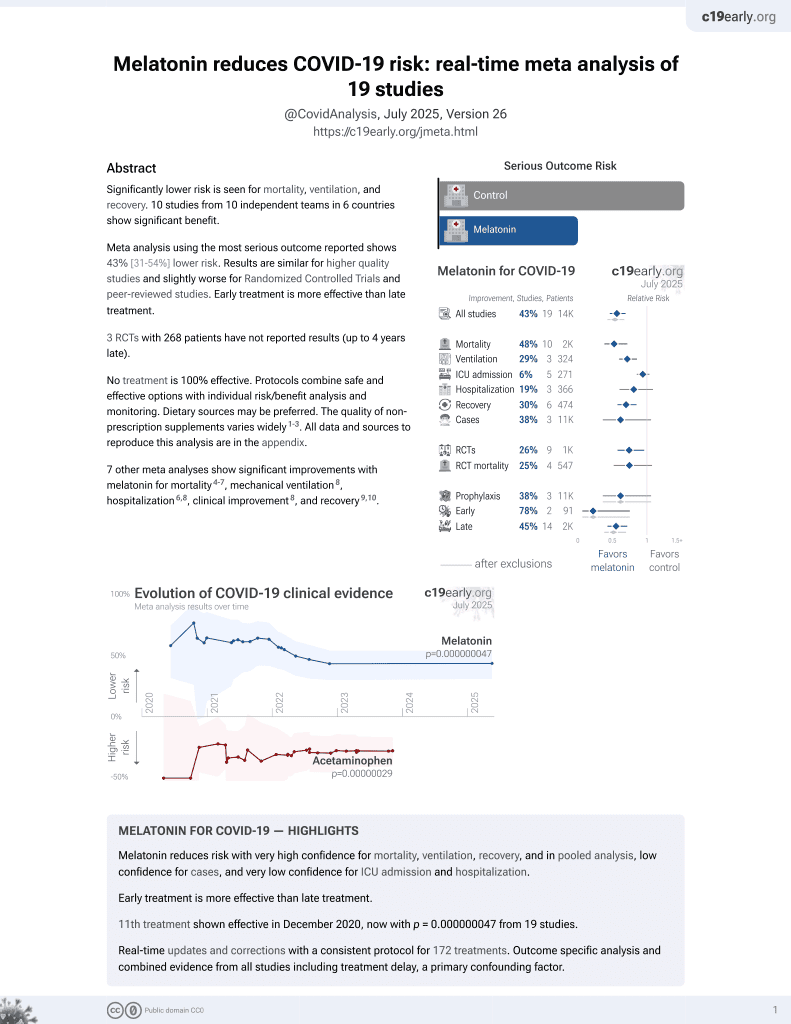
7 meta-analyses show significant improvements with melatonin for mortality1-4,
mechanical ventilation5,
hospitalization3,5,
improvement5, and
recovery6,7.
Currently there are 19 melatonin for COVID-19 studies, showing 33% lower mortality [19‑44%], 32% lower ventilation [19‑43%], 14% lower ICU admission [-1‑28%], 18% lower hospitalization [3‑30%], and 38% fewer cases [-6‑64%].
1.
Pilia et al., Does melatonin reduce mortality in COVID-19?, Annals of Medicine and Surgery, doi:10.1016/j.amsu.2022.103817.
2.
Tóth et al., Melatonin as adjuvant treatment in COVID-19 patients. A meta-analysis of randomized and propensity matched studies, Signa Vitae, doi:10.22514/sv.2023.076.
3.
Amin et al., Role of Melatonin in Management of COVID-19: A Systematic Review, Microbes, Infection and Chemotherapy, doi:10.54034/mic.e1982.
4.
Qin et al., Benefits of melatonin on mortality in severe-to-critical COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials, Clinics, doi:10.1016/j.clinsp.2025.100638.
5.
Taha et al., Safety and efficacy of melatonin as an adjuvant therapy in COVID-19 patients: Systematic review and meta-analysis, Advances in Medical Sciences, doi:10.1016/j.advms.2023.09.007.
Pilia et al., 18 May 2022, Italy, peer-reviewed, 3 authors.
Contact: dotterospilia@gmail.com.
Does melatonin reduce mortality in COVID-19?
Annals of Medicine and Surgery, doi:10.1016/j.amsu.2022.103817
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Annals of Medicine and Surgery The following information is required for submission. Please note that failure to respond to these questions/statements will mean your submission will be returned. If you have nothing to declare in any of these categories then this should be stated.
Please state any conflicts of interest All authors must disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding. None to declare.
Please state any sources of funding for your research All sources of funding should be declared as an acknowledgement at the end of the text. Authors should declare the role of study sponsors, if any, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. If the study sponsors had no such involvement, the authors should so state. None to declare.
Ethical Approval Research studies involving patients require ethical approval. Please state whether approval has been given, name the relevant ethics committee and the state the reference number for their judgement. None to declare.
J o u r n a l P r e -p r o o f Consent Studies on patients or volunteers require ethics committee approval and fully..
References
Farnoosh, Akbariqomi, Badri, Bagheri, Izadi et al., Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial, Arch Med Res, doi:10.1016/j.arcmed.2021.06.006
Hasan, Atrakji, Mehuaiden, The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients, Int J Infect Dis, doi:10.1016/j.ijid.2021.10.012
Mousavi, Heydari, Mehravaran, Saeedi, Alizadeh-Navaei et al., Melatonin effects on sleep quality and outcomes of COVID-19 patients: An open-label, randomized, controlled trial, J Med Virol, doi:10.1002/jmv.27312
Shneider, Kudriavtsev, Vakhrusheva, Can melatonin reduce the severity of COVID-19 pandemic?, Int Rev Immunol, doi:10.1080/08830185.2020.1756284
Sánchez-Rico, De La Muela, Herrera-Morueco, Geoffroy, Limosin et al., Université de Paris/INSERM COVID-19 Research Collaboration/AP-HP COVID CDR Initiative/Entrepôt de Données de Santé AP-HP Consortium. Melatonin does not reduce mortality in adult hospitalized patients with COVID-19: a multicenter retrospective observational study, J Travel Med, doi:10.1093/jtm/taab195
Zhou, Hou, Shen, Huang, Martin et al., Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2, Cell Discov, doi:10.1038/s41421-020-0153-3
DOI record:
{
"DOI": "10.1016/j.amsu.2022.103817",
"ISSN": [
"2049-0801"
],
"URL": "http://dx.doi.org/10.1016/j.amsu.2022.103817",
"alternative-id": [
"S2049080122005775"
],
"article-number": "103817",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4831-9526",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pilia",
"given": "Eros",
"sequence": "first"
},
{
"affiliation": [],
"family": "Alborino",
"given": "Ettore",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6696-5361",
"affiliation": [],
"authenticated-orcid": false,
"family": "Covello",
"given": "Remo Daniel",
"sequence": "additional"
}
],
"container-title": "Annals of Medicine and Surgery",
"container-title-short": "Annals of Medicine and Surgery",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
18
]
],
"date-time": "2022-05-18T01:22:25Z",
"timestamp": 1652836945000
},
"deposited": {
"date-parts": [
[
2022,
5,
18
]
],
"date-time": "2022-05-18T01:22:34Z",
"timestamp": 1652836954000
},
"indexed": {
"date-parts": [
[
2022,
5,
18
]
],
"date-time": "2022-05-18T01:42:39Z",
"timestamp": 1652838159782
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
1
]
],
"date-time": "2022-05-01T00:00:00Z",
"timestamp": 1651363200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 13,
"start": {
"date-parts": [
[
2022,
5,
14
]
],
"date-time": "2022-05-14T00:00:00Z",
"timestamp": 1652486400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2049080122005775?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2049080122005775?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "103817",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
5
]
]
},
"published-print": {
"date-parts": [
[
2022,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.amsu.2022.103817_bib1",
"unstructured": "World Health Organization (WHO), Weekly Epidemiological Update on COVID-19 - 20 April 2022 Edition 88, Emergency Situational Updates]."
},
{
"author": "Sánchez-Rico",
"journal-title": "J. Trav. Med.",
"key": "10.1016/j.amsu.2022.103817_bib2",
"year": "2022"
},
{
"DOI": "10.1080/08830185.2020.1756284",
"article-title": "Can melatonin reduce the severity of COVID-19 pandemic?",
"author": "Shneider",
"doi-asserted-by": "crossref",
"first-page": "153",
"issue": "4",
"journal-title": "Int. Rev. Immunol.",
"key": "10.1016/j.amsu.2022.103817_bib4",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1038/s41421-020-0153-3",
"article-title": "Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Cell Discov.",
"key": "10.1016/j.amsu.2022.103817_bib5",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2021.10.012",
"article-title": "The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients",
"author": "Hasan",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Int. J. Infect. Dis.",
"key": "10.1016/j.amsu.2022.103817_bib6",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27312",
"article-title": "Melatonin effects on sleep quality and outcomes of COVID-19 patients: an open-label, randomized, controlled trial",
"author": "Mousavi",
"doi-asserted-by": "crossref",
"first-page": "263",
"issue": "1",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.amsu.2022.103817_bib7",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1016/j.arcmed.2021.06.006",
"article-title": "Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: a randomized, double-blind clinical trial",
"author": "Farnoosh",
"doi-asserted-by": "crossref",
"first-page": "79",
"issue": "1",
"journal-title": "Arch. Med. Res.",
"key": "10.1016/j.amsu.2022.103817_bib8",
"volume": "53",
"year": "2022"
},
{
"key": "10.1016/j.amsu.2022.103817_bib9",
"series-title": "[Computer Program]. Version 5.4",
"year": "2020"
}
],
"reference-count": 8,
"references-count": 8,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2049080122005775"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Surgery"
],
"subtitle": [],
"title": "Does melatonin reduce mortality in COVID-19?",
"type": "journal-article"
}