Melatonin does not reduce mortality in adult hospitalized patients with COVID-19: a multicenter retrospective observational study
et al., Journal of Travel Medicine, doi:10.1093/jtm/taab195, Feb 2022
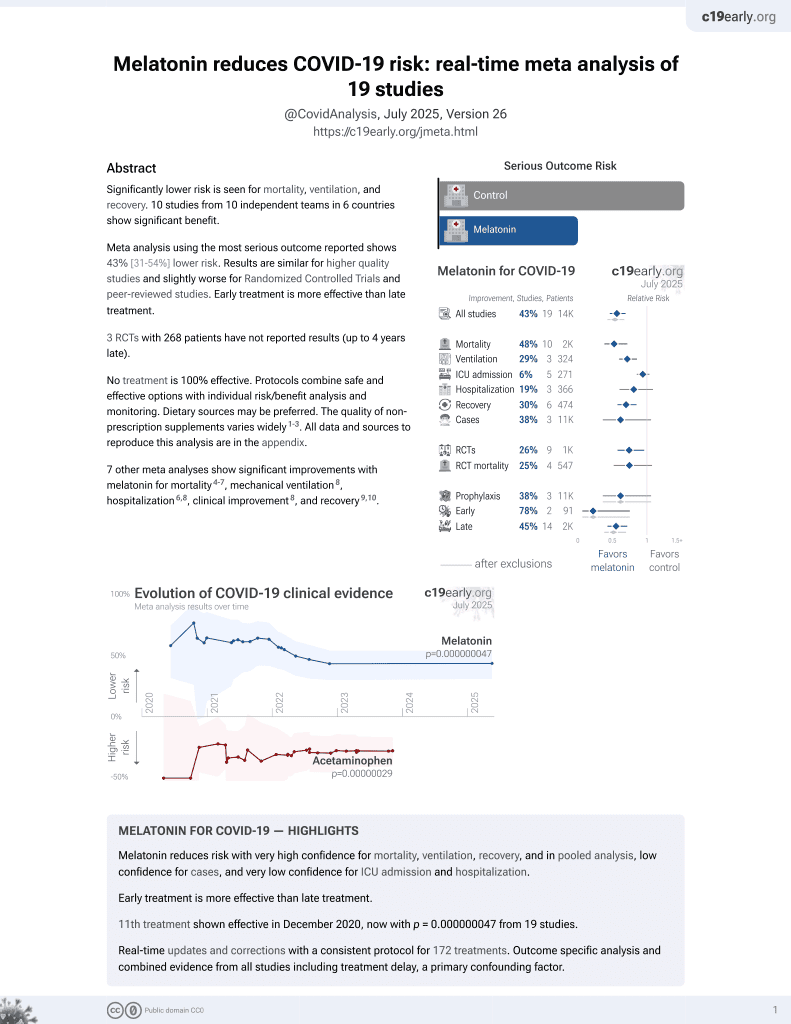
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
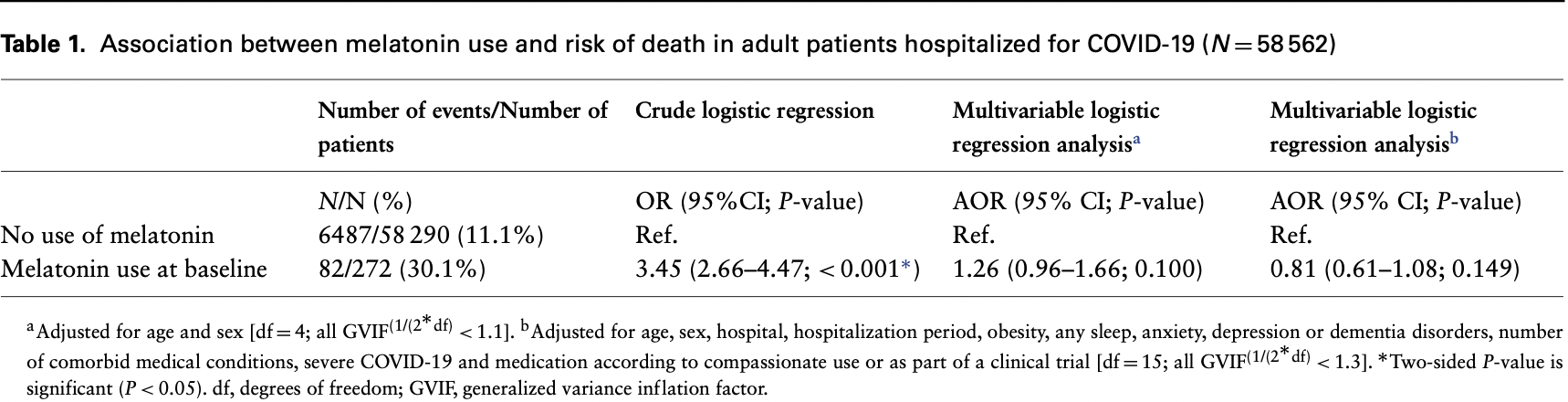
Retrospective database analysis in France with 272 patients treated with melatonin, showing 19% lower mortality after adjustments, without statistical significance. Risk was lower for higher dosage (not statistically significant). Age was only in three age ranges and severe COVID was binary, likely leading to substantial residual confounding. Unadjusted differences were extreme with 60% >80 years old for melatonin compared to 15% for control. Mean daily dose 2.61mg. The title of the paper is incorrect, the most adjusted results show melatonin did reduce mortality (without reaching statistical significance).
|
risk of death, 19.0% lower, RR 0.81, p = 0.15, treatment 82 of 272 (30.1%), control 6,487 of 58,290 (11.1%), adjusted per study, model b.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sánchez-Rico et al., 5 Feb 2022, retrospective, France, peer-reviewed, 6 authors, study period 24 January, 2020 - 31 October, 2021.
Melatonin does not reduce mortality in adult hospitalized patients with COVID-19: a multicenter retrospective observational study
Journal of Travel Medicine, doi:10.1093/jtm/taab195
Melatonin is an hormone secreted from the pineal gland indicated in the treatment of insomnia and circadian sleep disturbances. 1 In the needed search for an effective treatment for patients with COVID-19, 2 this molecule has been previously proposed as a potential useful treatment against COVID-19 thanks to its antioxidant, immunomodulatory, anti-inflammatory and potential SARS-CoV-2 main protease inhibition activities. [3] [4] [5] [6] However, while a recent randomized clinical trial 7 involving patients hospitalized with mild to moderate COVID-19 suggests that oral melatonin administration as an adjuvant therapy added to the standard of care may improve respiratory symptoms and time of patient discharge vs standard of care alone, another randomized trial found no substantial improvement in patients hospitalized for severe In this report, we used data from a multicenter retrospective study involving patients hospitalized for laboratory-confirmed COVID-19 in Greater Paris University hospitals, as detailed elsewhere, 9 and sought to examine the association between melatonin use and mortality in this population. Observational studies examining the potential usefulness of existing treatments against COVID-19 can be crucial to help prioritize molecules in clinical trials.
Supplementary data Supplementary data are available at JTM online.
Author contributions M.S.R. contributed to the study design, performed statistical analyses and wrote the first draft of the manuscript. P.d.l.M. and J.J.H.M. performed statistical analyses and contributed to the writing of the manuscript. N.H. designed the study and critically revised the manuscript. F.L. and P.A.G. contributed to study design and critically revised the manuscript for scientific content.
Conflict of interest None declared.
Ethical statement This observational study using routinely collected data received approval from the Institutional Review Board of the AP-HP Clinical Data Warehouse (decision CSE-20-20_COVID19, IRB00011591, 8 April 2020). AP-HP Clinical Data Warehouse initiatives ensure patient information and informed consent regarding the different approved studies through a transparency portal in accordance with European Regulation on data protection and authorization n • 1 980 120 from National Commission for Information Technology and Civil Liberties (CNIL).
References
Darban, Malek, Memarian, Gohari, Efficacy of high dose vitamin C, melatonin and zinc in Iranian patients with acute respiratory syndrome due to coronavirus infection: a pilot randomized trial, J Cell Mol Anesth
Farnoosh, Akbariqomi, Badri, Bagheri, Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: a randomized, double-blind clinical trial, Arch Med Res
Feitosa, Júnior, Neto, Matos, COVID-19: rational discovery of the therapeutic potential of melatonin as a SARS-CoV-2 main protease inhibitor, Int J Med Sci
Hoertel, Blachier, Blanco, Olfson, A stochastic agentbased model of the SARS-CoV-2 epidemic in France, Nat Med
Hoertel, Sánchez-Rico, Gulbins, Kornhuber, Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational Multicenter study, Clin Pharmacol Ther
Hoertel, Sánchez-Rico, Vernet, Beeker, Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study, Mol Psychiatry
Juybari, Pourhanifeh, Hosseinzadeh, Melatonin potentials against viral infections including COVID-19: current evidence and new findings, Virus Res
Palagini, Manni, Aguglia, Amore, International expert opinions and recommendations on the use of melatonin in the treatment of insomnia and circadian sleep disturbances in adult neuropsychiatric disorders, Front Psych
Ramlall, Zucker, Tatonetti, Melatonin is significantly associated with survival of intubated COVID-19 patients, doi:10.1101/2020.10.15.20213546
Zhang, Wang, Ni, Di, COVID-19: melatonin as a potential adjuvant treatment, Life Sci
DOI record:
{
"DOI": "10.1093/jtm/taab195",
"ISSN": [
"1195-1982",
"1708-8305"
],
"URL": "http://dx.doi.org/10.1093/jtm/taab195",
"container-title": [
"Journal of Travel Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
27
]
],
"date-time": "2022-01-27T20:11:03Z",
"timestamp": 1643314263000
},
"deposited": {
"date-parts": [
[
2022,
1,
28
]
],
"date-time": "2022-01-28T12:08:34Z",
"timestamp": 1643371714000
},
"indexed": {
"date-parts": [
[
2022,
1,
29
]
],
"date-time": "2022-01-29T06:52:39Z",
"timestamp": 1643439159009
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1195-1982"
},
{
"type": "electronic",
"value": "1708-8305"
}
],
"issued": {
"date-parts": [
[
2022
]
]
},
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022
]
]
},
"published-online": {
"date-parts": [
[
2022
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"Journal of Travel Medicine"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"OUP accepted manuscript"
],
"type": "journal-article"
}