Melatonin effects on sleep quality and outcomes of COVID-19 patients: An open-label, randomized, controlled trial
et al., Journal of Medical Virology, doi:10.1002/jmv.27312, Aug 2021
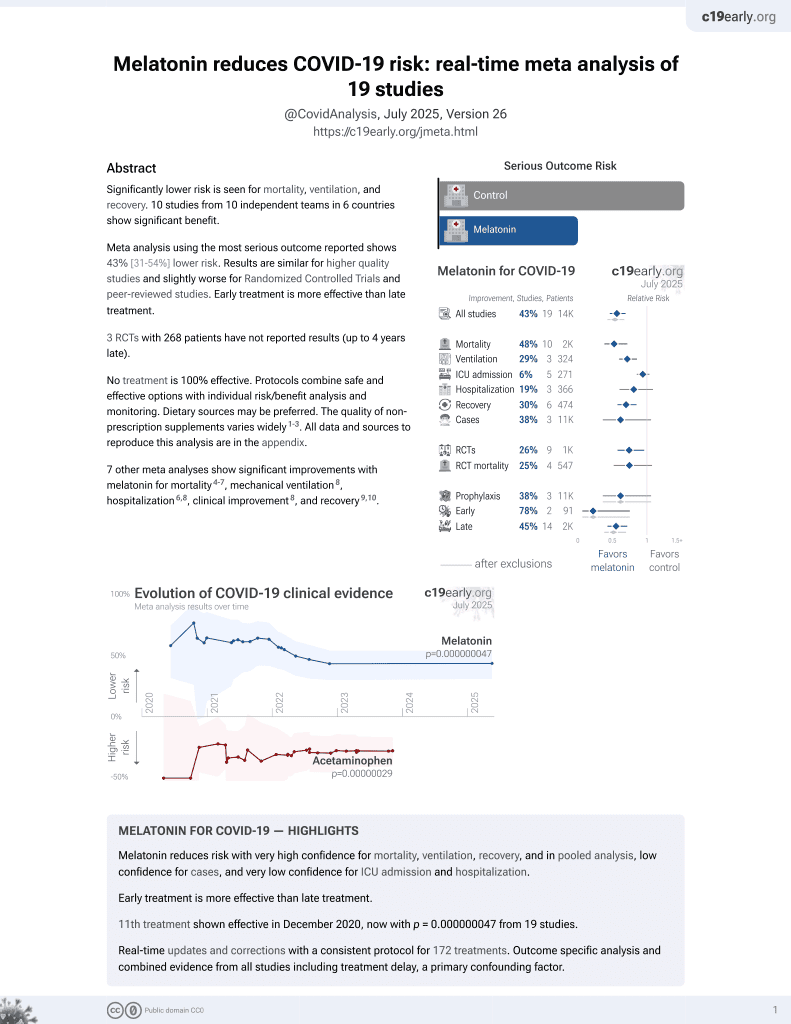
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
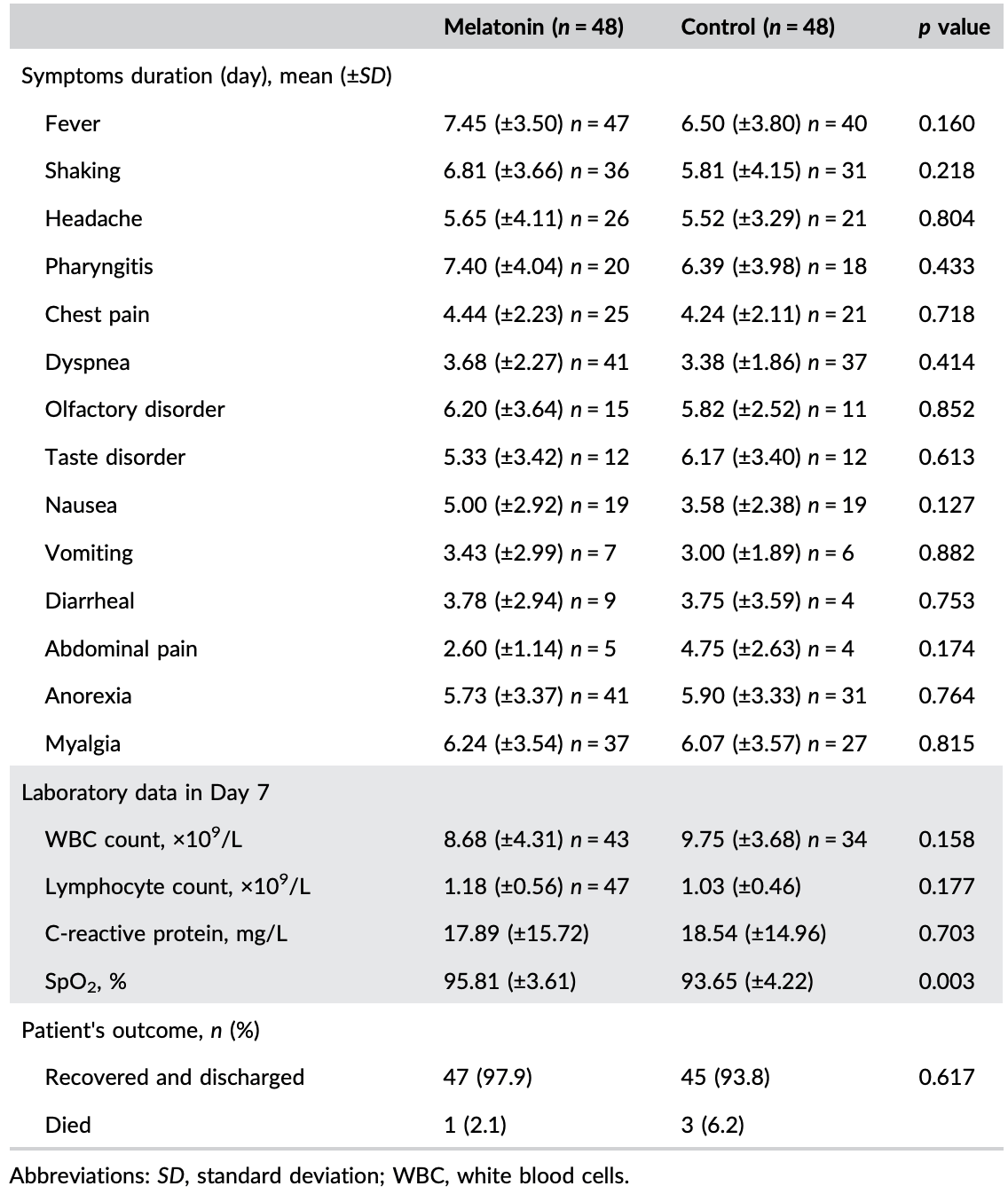
RCT 96 hospitalized patients in Iran, 48 treated with melatonin, showing improved sleep quality and SpO2 with treatment. 3mg oral melatonin daily. Authors recommend studies with a higher dose. IRCT20200411047030N1.
|
risk of death, 66.7% lower, RR 0.33, p = 0.62, treatment 1 of 48 (2.1%), control 3 of 48 (6.2%), NNT 24, day 10.
|
|
risk of ICU admission, 40.0% lower, RR 0.60, p = 0.41, treatment 6 of 48 (12.5%), control 10 of 48 (20.8%), NNT 12, day 10.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mousavi et al., 30 Aug 2021, Randomized Controlled Trial, Iran, peer-reviewed, 7 authors, study period 14 April, 2020 - 15 June, 2020.
Melatonin effects on sleep quality and outcomes of COVID‐19 patients: An open‐label, randomized, controlled trial
Journal of Medical Virology, doi:10.1002/jmv.27312
This trial aims to evaluate the effectiveness of adding melatonin to the treatment protocol of hospitalized coronavirus disease 2019 (COVID-19) patients. This was an open-label, randomized controlled clinical trial in hospitalized COVID-19 patients. Patients were randomized into a treatment arm receiving melatonin plus standard care or a control arm receiving standard care alone. The trial's primary endpoint was sleep quality examined by the Leeds Sleep Evaluation Questionnaire (LSEQ). The trial's secondary endpoints were symptoms alleviation by Day 7, intensive care unit admission, 10-day mortality, white blood cell count, lymphocyte count, C-reactive protein status, and peripheral capillary oxygen saturation. Ninety-six patients were recruited and allocated to either the melatonin arm (n = 48) or control arm (n = 48). Baseline characteristics were similar across treatment arms. There was no significant difference in symptoms on Day 7. The mean of the LSEQ scores was significantly higher in the melatonin group (p < 0.001). There was no significant difference in laboratory data, except for blood oxygen saturation, which has improved significantly in the melatonin group compared with the control group (95.81% vs. 93.65% respectively, p = 0.003). This clinical trial study showed that the combination of oral melatonin tablets and standard treatment could substantially improve sleep quality and blood oxygen saturation in hospitalized COVID-19 patients.
CONFLICT INTERESTS The authors declare that there are no conflict interests.
ETHICS STATEMENT The trial protocol was approved by the Ethics Committee of Mazandaran University of Medical Sciences (approval number IR.MAZUMS.REC.1399.056) on April 12, 2020, and registered with the Iranian Registry of Clinical Trials (IRCT20200411047030N1).
AUTHOR CONTRIBUTIONS Seyed Abbas Mousavi, Reza Alizadeh-Navaei, Majid Saeedi, and Amir Shamshirianh designed the study. Reza Alizadeh-Navaei worked on the statistical analysis. Hossein Mehravaran, Keyvan Heydari, and Amir Shamshirianh performed the research. Amir Shamshirian wrote the first draft of the manuscript. Akbar Hedayatizadeh-Omran, Seyed Abbas Mousavi, Reza Alizadeh-Navaei, Majid Saeedi, and Hossein Mehravaran helped with the preparation of the manuscript. All authors have read and approved the final manuscript.
References
Acuña-Castroviejo, Escames, Figueira, De La Oliva, Borobia et al., Clinical trial to test the efficacy of melatonin in COVID-19, J Pineal Res
Altena, Baglioni, Espie, Dealing with sleep problems during home confinement due to the COVID-19 outbreak: practical recommendations from a task force of the European CBT-I Academy, J Sleep Res
Anderson, Reiter, Melatonin: roles in influenza, COVID-19, and other viral infections, Rev Med Virol
Auld, Maschauer, Morrison, Skene, Riha, Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders, Sleep Med Rev
Cai, Yang, Liu, Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering
Cao, Wang, Wen, A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19, N Engl J Med
Castillo, Quizon, Juco, Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC-19 PRO): a case series, Melatonin Res
Chen, Zhang, Huang, Favipiravir versus arbidol for COVID-19: a randomized clinical trial
El-Missiry, El-Missiry, Othman, Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of COVID-19, Eur J Pharmacol
Frediansyah, Nainu, Dhama, Mudatsir, Harapan, Remdesivir and its antiviral activity against COVID-19: A systematic review, Clin Epidemiol Glob Health, doi:10.1016/j.cegh.2020.07.011
Glaus, Ruden, Remdesivir and COVID-19, Lancet
Grein, Ohmagari, Shin, Compassionate use of remdesivir for patients with severe COVID-19, N Engl J Med
Guan, Ni, -Y, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Heideman, Bhatnagar, Hilton, Bronson, Melatonin rhythms and pineal structure in a tropical bat, Anoura geoffroyi, that does not use photoperiod to regulate seasonal reproduction, J Pineal Res
Hlutkin, Zinchuk, Effect of melatonin on the blood oxygen transport during hypothermia and rewarming in rats, Adv Med Sci
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19: preliminary report, N Engl J Med
Huang, Cao, Liu, Shi, Wei, Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice, J Pineal Res
Iguchi, Kato, Ibayashi, Age-dependent reduction in serum melatonin concentrations in healthy human subjects, J Clin Endocrinol Metab
Jehi, Ji, Milinovich, Individualizing risk prediction for positive coronavirus disease 2019 testing: results from 11,672 patients, Chest
Juybari, Pourhanifeh, Hosseinzadeh, Hemati, Mehrzadi, Melatonin potentials against viral infections including COVID-19: current evidence and new findings, Virus Res
Kleszczyński, Slominski, Steinbrink, Reiter, Clinical trials for use of melatonin to fight against COVID-19 are urgently needed, Nutrients
Liu, Chen, Wu, Lin, Wang et al., Effects of progressive muscle relaxation on anxiety and sleep quality in patients with COVID-19, Complement Ther Clin Pract
Lou, Liu, Yao, Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial, Eur J Pharm Sci
Mahase, Covid-19: what treatments are being investigated?, BMJ
Manchester, Coto-Montes, Boga, Melatonin: an ancient molecule that makes oxygen metabolically tolerable, J Pineal Res
Mistraletti, Umbrello, Sabbatini, Melatonin reduces the need for sedation in ICU patients: a randomized controlled trial, Minerva Anestesiol
Morin, Carrier, The acute effects of the COVID-19 pandemic on insomnia and psychological symptoms, Sleep Med
Mousavi, Sa, Heydari, Mehravaran, Melatonin effects on sleep quality and outcomes of COVID-19 patients: an open-label, randomized, controlled trial
Normand, The RECOVERY platform, N Engl J Med
Osborne, Davies, Lane, Lopinavir-ritonavir in the treatment of COVID-19: a dynamic systematic benefit-risk assessment, Drug Saf
Parrott, Hindmarch, The Leeds Sleep Evaluation Questionnaire in psychopharmacological investigations: a review, Psychopharmacology
Pathak, Salunke, Thivari, No benefit of hydroxychloroquine in COVID-19: results of systematic review and metaanalysis of randomized controlled trials, Diabetes Metab Syndrome
Ramlall, Zucker, Tatonetti, Melatonin is significantly associated with survival of intubated COVID-19 patients
Recovery, A randomised trial of treatments to prevent death in patients hospitalised with COVID-19
Reiter, Abreu-Gonzalez, Marik, Dominguez-Rodriguez, Therapeutic algorithm for use of melatonin in patients with COVID-19, Front Med
Reiter, Abreu-Gonzalez, Marik, Dominguez-Rodriguez, Therapeutic algorithm for use of melatonin in patients with COVID-19, Front Med
Rondanelli, Opizzi, Monteferrario, Antoniello, Manni et al., The effect of melatonin, magnesium, and zinc on primary insomnia in long-term care facility residents in Italy: a double-blind, placebo-controlled clinical trial, J Am Geriatr Soc
Seghi, Barbini, Franchini, Colombo, The challenge of mental health during COVID-19 outbreak: experience from metropolitan area of Milan, Eur Arch Psychiatry Clin Neurosci
Serfaty, Osborne, Buszewicz, Blizard, Raven, A randomized double-blind placebo-controlled trial of treatment as usual plus exogenous slow-release melatonin (6 mg) or placebo for sleep disturbance and depressed mood, Int Clin Psychopharmacol
Shneider, Kudriavtsev, Vakhrusheva, Can melatonin reduce the severity of COVID-19 pandemic?, Int Rev Immunol
Shneider, Kudriavtsev, Vakhrusheva, Can melatonin reduce the severity of COVID-19 pandemic?, Int Rev Immunol
Simpson, Manber, Treating Insomnia during the COVID-19 pandemic: observations and perspectives from a behavioral sleep medicine clinic, Behav Sleep Med
Sletten, Magee, Murray, Efficacy of melatonin with behavioural sleep-wake scheduling for delayed sleep-wake phase disorder: a double-blind, randomised clinical trial, PLoS Med
Tan, Hardeland, Estimated doses of melatonin for treating deadly virus infections: focus on COVID-19, Melatonin Res
Tarrasch, Laudon, Zisapel, Cross-cultural validation of the Leeds Sleep Evaluation Questionnaire (LSEQ) in insomnia patients, Hum Psychopharmacol
Tresguerres, Parks, Goss, V-H(+)-ATPase, Na(+)/K(+)-ATPase and NHE2 immunoreactivity in the gill epithelium of the Pacific hagfish (Epatretus stoutii), Comp Biochem Physiol A Mol Integr Physiol
Waldhauser, Weiszenbacher, Tatzer, Alterations in nocturnal serum melatonin levels in humans with growth and aging, J Clin Endocrinol Metab
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Weiss, Peters, Alhazzani, Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19), Intensive Care Med
Who, Coronavirus disease
Wu, Ji, Wang, Melatonin alleviates radiation-induced lung injury via regulation of miR-30e/NLRP3 Axis, Oxid Med Cell Longevity
Yip, Chang, Wallace, Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury, J Pineal Res
Zambrelli, Canevini, Gambini, Agostino, Delirium and sleep disturbances in COVID-19: a possible role for melatonin in hospitalized patients?, Sleep Med
Zhang, Wang, Ni, COVID-19: Melatonin as a potential adjuvant treatment, Life Sci
Zinchuk, Firago, Participation of melatonin in regulation of blood oxygen-transport function in oxidative stress induced by injection of lipopolisaccharide, Biomed Khim
Zinchuk, Poluyan, Hlutkin, Effects of melatonin on the oxygen transport in blood, gas transmitters, and prooxidant-antioxidant balance in the exercise, Hum Physiol
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.1002/jmv.27312",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.27312",
"abstract": "<jats:title>Abstract</jats:title><jats:p>This trial aims to evaluate the effectiveness of adding melatonin to the treatment protocol of hospitalized coronavirus disease 2019 (COVID‐19) patients. This was an open‐label, randomized controlled clinical trial in hospitalized COVID‐19 patients. Patients were randomized into a treatment arm receiving melatonin plus standard care or a control arm receiving standard care alone. The trial's primary endpoint was sleep quality examined by the Leeds Sleep Evaluation Questionnaire (LSEQ). The trial's secondary endpoints were symptoms alleviation by Day 7, intensive care unit admission, 10‐day mortality, white blood cell count, lymphocyte count, C‐reactive protein status, and peripheral capillary oxygen saturation. Ninety‐six patients were recruited and allocated to either the melatonin arm (<jats:italic>n</jats:italic> = 48) or control arm (<jats:italic>n</jats:italic> = 48). Baseline characteristics were similar across treatment arms. There was no significant difference in symptoms on Day 7. The mean of the LSEQ scores was significantly higher in the melatonin group (<jats:italic>p </jats:italic>< 0.001). There was no significant difference in laboratory data, except for blood oxygen saturation, which has improved significantly in the melatonin group compared with the control group (95.81% vs. 93.65% respectively, <jats:italic>p </jats:italic>= 0.003). This clinical trial study showed that the combination of oral melatonin tablets and standard treatment could substantially improve sleep quality and blood oxygen saturation in hospitalized COVID‐19 patients.</jats:p>",
"alternative-id": [
"10.1002/jmv.27312"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-08-05"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-08-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-09-08"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0953-0705",
"affiliation": [
{
"name": "Department of Psychiatry, Psychiatry and Behavioral Sciences Research Center, Addiction Institute Mazandaran University of Medical Sciences Sari Iran"
}
],
"authenticated-orcid": false,
"family": "Mousavi",
"given": "Seyed Abbas",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-2843-7832",
"affiliation": [
{
"name": "Gastrointestinal Cancer Research Center, Non‐Communicable Diseases Institute Mazandaran University of Medical Sciences Sari Iran"
},
{
"name": "Student Research Committee, School of Medicine Mazandaran University of Medical Sciences Ramsar Iran"
}
],
"authenticated-orcid": false,
"family": "Heydari",
"given": "Keyvan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8409-1736",
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care, Department of Internal Medicine, School of Medicine Mazandaran University of Medical Sciences Sari Iran"
}
],
"authenticated-orcid": false,
"family": "Mehravaran",
"given": "Hossein",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2617-9961",
"affiliation": [
{
"name": "Pharmaceutical Sciences Research Center Mazandaran University of Medical Sciences Sari Iran"
}
],
"authenticated-orcid": false,
"family": "Saeedi",
"given": "Majid",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0580-000X",
"affiliation": [
{
"name": "Gastrointestinal Cancer Research Center, Non‐Communicable Diseases Institute Mazandaran University of Medical Sciences Sari Iran"
}
],
"authenticated-orcid": false,
"family": "Alizadeh‐Navaei",
"given": "Reza",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9219-883X",
"affiliation": [
{
"name": "Gastrointestinal Cancer Research Center, Non‐Communicable Diseases Institute Mazandaran University of Medical Sciences Sari Iran"
}
],
"authenticated-orcid": false,
"family": "Hedayatizadeh‐Omran",
"given": "Akbar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2735-0209",
"affiliation": [
{
"name": "Gastrointestinal Cancer Research Center, Non‐Communicable Diseases Institute Mazandaran University of Medical Sciences Sari Iran"
},
{
"name": "Department of Medical Laboratory Sciences, Student Research Committee, School of Allied Medical Science Mazandaran University of Medical Sciences Sari Iran"
}
],
"authenticated-orcid": false,
"family": "Shamshirian",
"given": "Amir",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
30
]
],
"date-time": "2021-08-30T14:42:01Z",
"timestamp": 1630334521000
},
"deposited": {
"date-parts": [
[
2023,
8,
25
]
],
"date-time": "2023-08-25T17:41:25Z",
"timestamp": 1692985285000
},
"funder": [
{
"DOI": "10.13039/501100004160",
"award": [
"IR.MAZUMS.REC.1399.056"
],
"doi-asserted-by": "publisher",
"name": "Mazandaran University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T19:46:17Z",
"timestamp": 1711827977110
},
"is-referenced-by-count": 41,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
9,
8
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
8
]
],
"date-time": "2021-09-08T00:00:00Z",
"timestamp": 1631059200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.27312",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/jmv.27312",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.27312",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "263-271",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
9,
8
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
8
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "e_1_2_11_2_1",
"unstructured": "WHO.Coronavirus disease (COVID‐19).2020."
},
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1007/s00134-019-05877-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1136/bmj.m1252",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"key": "e_1_2_11_7_1",
"unstructured": "World Health Organization (WHO).Solidarity” clinical trial for COVID‐19 treatments. World Health Organization (WHO) Situation reports Geneva: WHO. Accessed April 5 2020.https://www.whoint/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments2020"
},
{
"key": "e_1_2_11_8_1",
"unstructured": "RECOVERY.A randomised trial of treatments to prevent death in patients hospitalised with COVID‐19 (coronavirus).2020.http://www.isrctn.com/ISRCTN50189673"
},
{
"DOI": "10.1056/NEJMe2025674",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.1016/j.smrv.2016.06.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.1016/j.lfs.2020.117583",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1016/j.sleep.2020.04.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"article-title": "Melatonin reduces the need for sedation in ICU patients: a randomized controlled trial",
"author": "Mistraletti G",
"first-page": "1298",
"issue": "12",
"journal-title": "Minerva Anestesiol",
"key": "e_1_2_11_13_1",
"volume": "81",
"year": "2015"
},
{
"DOI": "10.1111/jpi.12020",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"article-title": "Melatonin alleviates radiation‐induced lung injury via regulation of miR‐30e/NLRP3 Axis",
"author": "Wu X",
"first-page": "4087298",
"journal-title": "Oxid Med Cell Longevity",
"key": "e_1_2_11_15_1",
"volume": "2019",
"year": "2019"
},
{
"DOI": "10.1111/j.1600-079X.2009.00733.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"DOI": "10.1080/08830185.2020.1756284",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.3389/fmed.2020.00226",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1016/j.ejphar.2020.173329",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.1111/jpi.12683",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1016/j.ctcp.2020.101132",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1007/BF00434408",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"DOI": "10.1002/hup.534",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_1"
},
{
"DOI": "10.1016/j.dsx.2020.08.033",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1007/s40264-020-00966-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_1"
},
{
"DOI": "10.1016/j.ejps.2020.105631",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_27_1"
},
{
"article-title": "Favipiravir versus arbidol for COVID‐19: a randomized clinical trial",
"author": "Chen C",
"journal-title": "MedRxiv",
"key": "e_1_2_11_28_1",
"year": "2020"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_29_1"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_1"
},
{
"DOI": "10.1056/NEJMoa2007016",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_31_1"
},
{
"DOI": "10.1016/S0140-6736(20)32021-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_32_1"
},
{
"DOI": "10.1016/j.cegh.2020.07.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_33_1"
},
{
"key": "e_1_2_11_34_1",
"unstructured": "US Food and Drug Administration.Emergency Use Authorization (EUA) information and list of all current EUAs. 2020."
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_35_1"
},
{
"DOI": "10.3390/nu12092561",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"DOI": "10.1016/j.virusres.2020.198108",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_37_1"
},
{
"DOI": "10.1002/rmv.2109",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_38_1"
},
{
"DOI": "10.1080/08830185.2020.1756284",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_39_1"
},
{
"DOI": "10.1016/j.cbpa.2006.06.045",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_40_1"
},
{
"DOI": "10.1111/j.1600-079X.1996.tb00245.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_41_1"
},
{
"DOI": "10.1210/jcem-66-3-648",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_42_1"
},
{
"DOI": "10.1210/jcem-55-1-27",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_43_1"
},
{
"DOI": "10.15585/mmwr.mm6914e4",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_44_1"
},
{
"DOI": "10.1101/2020.10.15.20213546",
"doi-asserted-by": "crossref",
"key": "e_1_2_11_45_1",
"unstructured": "RamlallV ZuckerJ TatonettiN.Melatonin is significantly associated with survival of intubated COVID‐19 patients.medRxiv[Preprint]. Published online October 18 2020."
},
{
"DOI": "10.1016/j.chest.2020.05.580",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_46_1"
},
{
"DOI": "10.1371/journal.pmed.1002587",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_47_1"
},
{
"DOI": "10.1097/YIC.0b013e32832c260b",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_48_1"
},
{
"DOI": "10.1111/j.1532-5415.2010.03232.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_49_1"
},
{
"DOI": "10.1080/15402002.2020.1765781",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_50_1"
},
{
"article-title": "The challenge of mental health during COVID‐19 outbreak: experience from metropolitan area of Milan",
"author": "Seghi F",
"first-page": "1",
"journal-title": "Eur Arch Psychiatry Clin Neurosci",
"key": "e_1_2_11_51_1",
"volume": "271",
"year": "2020"
},
{
"DOI": "10.1016/j.sleep.2020.06.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_52_1"
},
{
"DOI": "10.1111/jsr.13052",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_53_1"
},
{
"DOI": "10.32794/mr11250063",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_54_1"
},
{
"DOI": "10.1134/S0362119719050219",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_55_1"
},
{
"DOI": "10.18097/PBMC20176306520",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_56_1"
},
{
"DOI": "10.1111/jpi.12267",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_57_1"
},
{
"DOI": "10.2478/v10039-008-0035-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_58_1"
},
{
"DOI": "10.3389/fmed.2020.00226",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_59_1"
},
{
"DOI": "10.32794/mr11250062",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_60_1"
},
{
"key": "e_1_2_11_61_1",
"unstructured": "Centers for Disease Control and Prevention.Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19).2020.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html"
}
],
"reference-count": 60,
"references-count": 60,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.27312"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Melatonin effects on sleep quality and outcomes of COVID‐19 patients: An open‐label, randomized, controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "94"
}