Melatonin as adjuvant treatment in COVID-19 patients. A meta-analysis of randomized and propensity matched studies
et al., Signa Vitae, doi:10.22514/sv.2023.076, Aug 2023
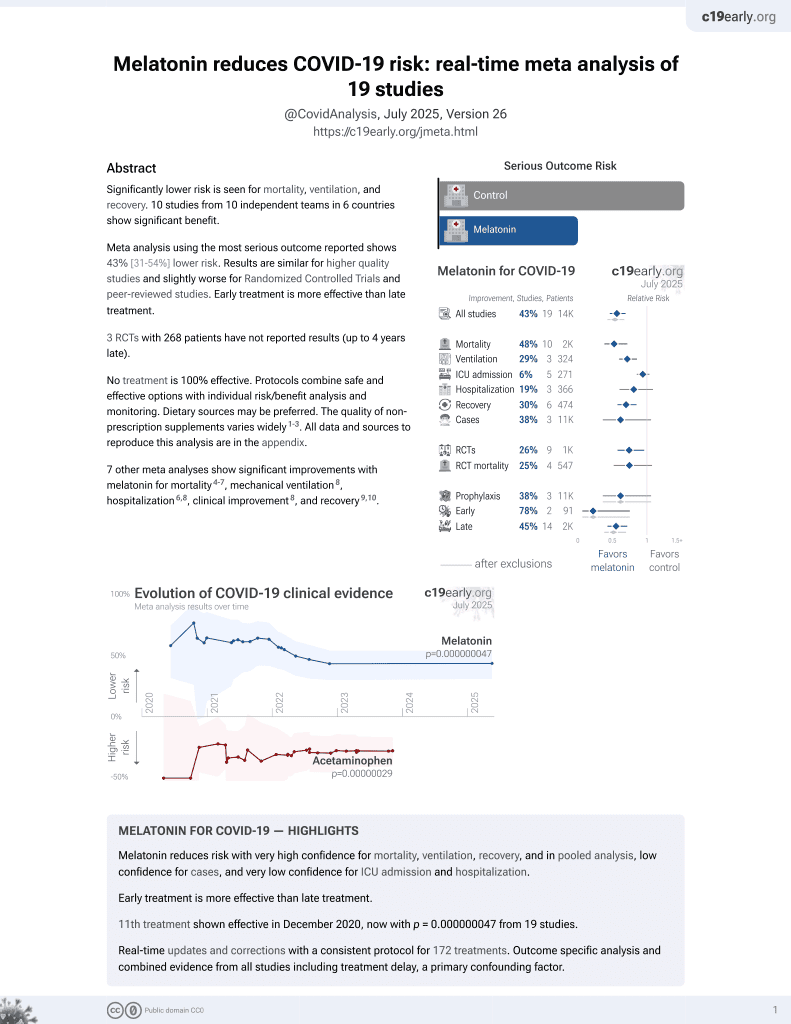
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
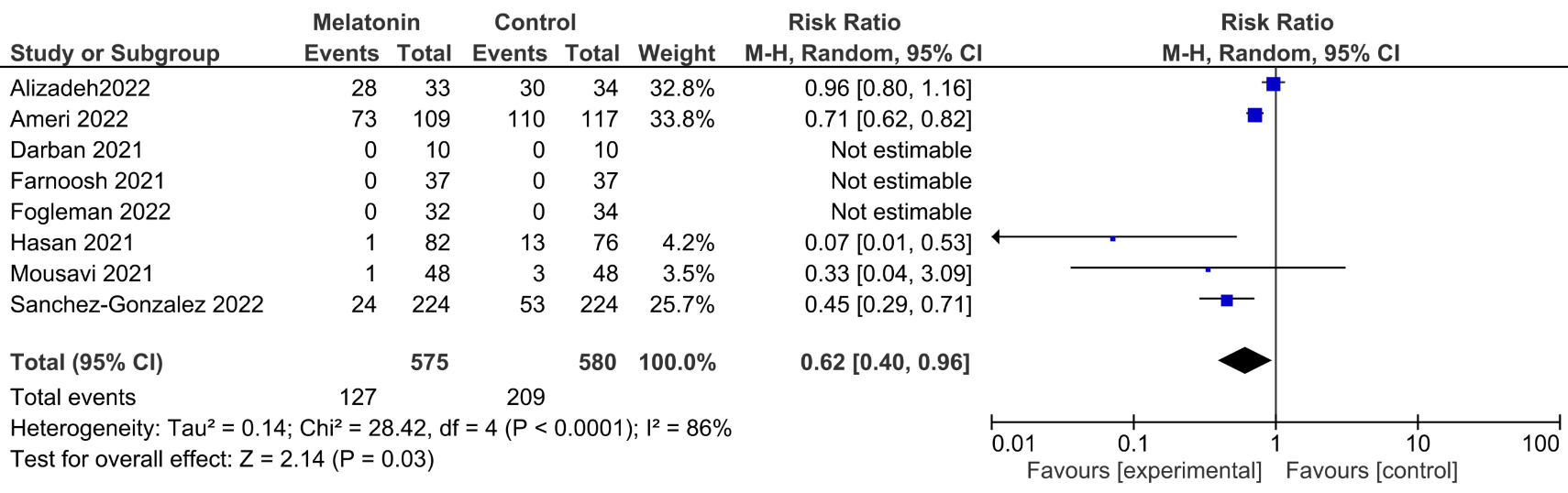
Meta analysis of seven melatonin RCTs and one propensity matched study for hospitalized COVID-19 patients, showing significantly lower mortality with treatment.
7 meta-analyses show significant improvements with melatonin for mortality1-4,
mechanical ventilation5,
hospitalization3,5,
improvement5, and
recovery6,7.
Currently there are 19 melatonin for COVID-19 studies, showing 33% lower mortality [19‑44%], 32% lower ventilation [19‑43%], 14% lower ICU admission [-1‑28%], 18% lower hospitalization [3‑30%], and 38% fewer cases [-6‑64%].
|
risk of death, 38.0% lower, RR 0.62, p = 0.03.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Pilia et al., Does melatonin reduce mortality in COVID-19?, Annals of Medicine and Surgery, doi:10.1016/j.amsu.2022.103817.
2.
Tóth et al., Melatonin as adjuvant treatment in COVID-19 patients. A meta-analysis of randomized and propensity matched studies, Signa Vitae, doi:10.22514/sv.2023.076.
3.
Amin et al., Role of Melatonin in Management of COVID-19: A Systematic Review, Microbes, Infection and Chemotherapy, doi:10.54034/mic.e1982.
4.
Qin et al., Benefits of melatonin on mortality in severe-to-critical COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials, Clinics, doi:10.1016/j.clinsp.2025.100638.
5.
Taha et al., Safety and efficacy of melatonin as an adjuvant therapy in COVID-19 patients: Systematic review and meta-analysis, Advances in Medical Sciences, doi:10.1016/j.advms.2023.09.007.
Tóth et al., 16 Aug 2023, peer-reviewed, 12 authors.
Contact: landoni.giovanni@hsr.it.
Melatonin as adjuvant treatment in COVID-19 patients. A meta-analysis of randomized and propensity matched studies
Signa Vitae, doi:10.22514/sv.2023.076
Melatonin is a neurohormone well-known as sleep disorder treatment. A few clinical trials have recently pointed out the biological plausibility of utilising melatonin in the treatment of coronavirus disease 2019 (COVID-19, SARS-CoV-2) patients. Melatonin wide range of activities include anti-inflammatory, antiviral and antioxidant effects. Our meta-analysis aimed to investigate the effect of melatonin on mortality in COVID-19 patients with different disease severity. We searched PubMed, EMBASE, Web of Science with no language restrictions updated on February 2023 for randomized and propensity matched studies, comparing melatonin plus standard COVID-19 therapy vs. standard COVID-19 therapy alone. Patients had to be hospitalised with a confirmed diagnosis of SARS-CoV-2 infection. Primary outcome was mortality at the longest follow-up available. We included 7 randomized and 1 propensity matched studies enrolling 1155 overall patients with a mean age of 61 ± 19.5 years. We found a reduced mortality rate in the overall population (127/575 (22%) vs. 209/580 (36%) Relative Risk: 0.62 (confidence interval (CI): 0.40, 0.96), I 2 = 86% p = 0.03, with the results confirmed when pooling the 5 studies which administered melatonin in non-intensivecare-unit patients (26/423 (6.1%) vs. 69/419 (16%) Relative Risk 0.30 (CI: 0.10, 0.86), I 2 = 40% p = 0.02). According to recent randomized and propensity matched evidence, melatonin might be a life-saving adjuvant therapy in COVID-19 patients. This effect was mainly driven by non-intensive care unit patients.
AC K NOW LED G ME N T Not applicable.
F U ND ING This research received no external funding.
CO NFL ICT OF IN T ERE ST The authors declare no conflict of interest. Giovanni Landoni is serving as one of the Editorial Board members of this journal. We declare that Giovanni Landoni had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to OK.
S U PPL EM ENTAR Y MAT ERIA L Supplementary material associated with this article can be found, in the online version, at https://oss.signavitae. com/mre-signavitae/article/1691722790965526528/ attachment/Supplementary%20material.docx.
R E F ER ENCE S
References
Ahmed, Soomro, Salih, Samantasinghar, Asif et al., A comprehensive review of artificial intelligence and network based approaches to drug repurposing in Covid-19
Alizadeh, Dianatkhah, Alimohamadi, Moradi, Akbarpour et al., High dose melatonin as an adjuvant therapy in intubated patients with COVID-19: a randomized clinical trial, Journal of Taibah University Medical Sciences
Alizadeh, Keyhanian, Ghaderkhani, Dashti-Khavidaki, Shoormasti et al., A pilot study on controlling coronavirus disease 2019 (COVID-19) inflammation using melatonin supplement, Iranian Journal of Allergy, Asthma and Immunology
Ameri, Asadi, Ziaei, Vatankhah, Safa et al., Efficacy and safety of oral melatonin in patients with severe COVID-19: a randomized controlled trial, Inflammopharmacology
Angelo, Marseglia, Reiter, Buonocore, Gitto, Melatonin and neonatal sepsis: a promising antioxidant adjuvant agent, American Journal of Perinatology
Benedict, Cedernaes, Could a good night's sleep improve COVID-19 vaccine efficacy? The Lancet Respiratory Medicine
Cardinali, Melatonin and healthy aging. Vitamins and Hormones
Cecon, Fernandois, Renault, Coelho, Wenzel et al., Melatonin drugs inhibit SARS-CoV-2 entry into the brain and virusinduced damage of cerebral small vessels, Cellular and Molecular Life Sciences
Covello, Pasin, Fresilli, Tóth, Damiani et al., Meta-analysis of glucocorticoids for Covid-19 patients not receiving oxygen. NEJM Evidence
Cuesta, Cerezuela, Esteban, Meseguer, In vivo actions of melatonin on the innate immune parameters in the teleost fish gilthead seabream, Journal of Pineal Research
Darban, Malek, Memarian, Gohari, Kiani et al., Efficacy of high dose vitamin c, melatonin and zinc in iranian patients with acute respiratory syndrome due to coronavirus infection: a pilot randomized trial, Journal of Cellular and Molecular Anesthesia
El-Missiry, El-Missiry, Othman, Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of Covid-19, European Journal of Pharmacology
Farnoosh, Akbariqomi, Badri, Bagheri, Izadi et al., Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: a randomized, doubleblind clinical trial. Archives of Medical Research
Fogleman, Cohen, Mercier, Farrell, Rutz et al., A pilot of a randomized control trial of melatonin and vitamin C for mild-to-moderate COVID-19, Journal of the American Board of Family Medicine
García, Rodriguez-Rubio, Mariblanca, De Soto, García et al., A randomized multicenter clinical trial to evaluate the efficacy of melatonin in the prophylaxis of SARS-CoV-2 infection in high-risk contacts (MeCOVID Trial): a structured summary of a study protocol for a randomised controlled trial
Hasan, Atrakji, Mehuaiden, The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients, International Journal of Infectious Diseases
Hosseini, Badri, Gouvarchin Ghaleh, Hassanpour, Alishiri et al., Melatonin as a complementary and prophylactic agent against COVID-19 in high-risk populations: a narrative review of recent findings from clinical and preclinical studies, Fundamental & Clinical Pharmacology
Kato, Nishiyama, Nishimura, Noda, Okabe et al., Drug repurposing for the treatment of COVID-19, Journal of Pharmacological Sciences
Kow, Ramachandram, Hasan, Melatonin: revisited role as vaccine adjuvant during outbreaks of COVID-19 caused by the delta variant, Journal of Neuroimmune Pharmacology
Lan, Lee, Chao, Chang, Lu et al., Efficacy of melatonin in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials, Journal of Medical Virology
Liu, Kang, Zhao, Zhuang, Li et al., Comprehensive narrative review of real-world COVID-19 vaccines: viewpoints and opportunities, Medical Review
Marzolini, Kuritzkes, Marra, Boyle, Gibbons et al., Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (paxlovid) and comedications, Clinical Pharmacology & Therapeutics
Mayo, Sainz, Antoli, Herrera, Martin et al., Melatonin regulation of antioxidant enzyme gene expression, Cellular and Molecular Life Sciences
Mayo, Sainz, Tan, Hardeland, Leon et al., Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages, Journal of Neuroimmunology
Mousavi, Heydari, Mehravaran, Saeedi, Alizadeh-Navaei et al., Melatonin effects on sleep quality and outcomes of COVID-19 patients: an open-label, randomized, controlled trial, Journal of Medical Virology
Pilia, Alborino, Covello, Does melatonin reduce mortality in COVID-19? Annals of Medicine and Surgery
Ramlall, Zucker, Tatonetti, Melatonin is significantly associated with survival of intubated COVID-19 patients. medRχiv
Ramos, Míguez, Morgado, Sanchez-Correa, Gordillo et al., Melatonin enhances responsiveness to Dichelobacter nodosus vaccine in sheep and increases peripheral blood CD4 T lymphocytes and IgG-expressing B lymphocytes. Veterinary Immunology and Immunopathology
Recovery Collaborative, Horby, Lim, Emberson, Mafham et al., Dexamethasone in hospitalized patients with Covid-19, The New England Journal of Medicine
Rodrigues, Cunha, Vassilevskaia, Viveiros, Cunha, Drug repurposing for COVID-19: a review and a novel strategy to identify new targets and potential drug candidates, Molecules
Rodríguez-Rubio, Figueira, Acuña-Castroviejo, Borobia, Escames et al., A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial
Sterne, Hernán, Reeves, Savović, Berkman et al., ROBINS-I: a tool for assessing risk of bias in nonrandomised studies of interventions, BMJ
Sterne, Savović, Page, Elbers, Blencowe et al., RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ
Sánchez-González, Mahíllo-Fernández, Villar-Álvarez, Llanos, What if melatonin could help patients with severe COVID-19, Journal of Clinical Sleep Medicine
Tordjman, Chokron, Delorme, Charrier, Bellissant et al., Melatonin: pharmacology, functions and therapeutic benefits, Current Neuropharmacology
Wan, Wang, Liu, Tong, Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range, BMC Medical Research Methodology
Wang, Wu, Cai, Zhang, The safety and efficacy of melatonin in the treatment of COVID-19: a systematic review and metaanalysis
Zhou, Hou, Shen, Mehra, Kallianpur et al., A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19, PLOS Biology
Ziaei, Davoodian, Dadvand, Safa, Hassanipour et al., Evaluation of the efficacy and safety of melatonin in moderately ill patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial
DOI record:
{
"DOI": "10.22514/sv.2023.076",
"URL": "http://dx.doi.org/10.22514/sv.2023.076",
"abstract": "<jats:p>Melatonin is a neurohormone well-known as sleep disorder treatment. A few clinical trials have recently pointed out the biological plausibility of utilising melatonin in the treatment of coronavirus disease 2019 (COVID-19, SARS-CoV-2) patients. Melatonin wide range of activities include anti-inflammatory, antiviral and antioxidant effects. Our meta-analysis aimed to investigate the effect of melatonin on mortality in COVID-19 patients with different disease severity. We searched PubMed, EMBASE, Web of Science with no language restrictions updated on February 2023 for randomized and propensity matched studies, comparing melatonin plus standard COVID-19 therapy vs. standard COVID-19 therapy alone. Patients had to be hospitalised with a confirmed diagnosis of SARS-CoV-2 infection. Primary outcome was mortality at the longest follow-up available. We included 7 randomized and 1 propensity matched studies enrolling 1155 overall patients with a mean age of 61 ± 19.5 years. We found a reduced mortality rate in the overall population (127/575 (22%) vs. 209/580 (36%) Relative Risk: 0.62 (confidence interval (CI): 0.40, 0.96), I2 = 86% p = 0.03, with the results confirmed when pooling the 5 studies which administered melatonin in non-intensive-care-unit patients (26/423 (6.1%) vs. 69/419 (16%) Relative Risk 0.30 (CI: 0.10, 0.86), I2 = 40% p = 0.02). According to recent randomized and propensity matched evidence, melatonin might be a life-saving adjuvant therapy in COVID-19 patients. This effect was mainly driven by non-intensive care unit patients.</jats:p>",
"container-title": "Signa Vitae",
"container-title-short": "SV",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
16
]
],
"date-time": "2023-08-16T08:09:24Z",
"timestamp": 1692173364000
},
"deposited": {
"date-parts": [
[
2023,
8,
16
]
],
"date-time": "2023-08-16T08:09:25Z",
"timestamp": 1692173365000
},
"indexed": {
"date-parts": [
[
2023,
8,
17
]
],
"date-time": "2023-08-17T05:05:02Z",
"timestamp": 1692248702560
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023
]
]
},
"member": "26027",
"original-title": [],
"prefix": "10.22514",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "MRE Press",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.signavitae.com/articles/10.22514/sv.2023.076"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Microbiology (medical)",
"Immunology",
"Immunology and Allergy",
"General Agricultural and Biological Sciences",
"General Earth and Planetary Sciences",
"General Environmental Science",
"Automotive Engineering",
"Industrial and Manufacturing Engineering",
"General Medicine",
"General Medicine",
"General Medicine",
"General Medicine"
],
"subtitle": [],
"title": "Melatonin as adjuvant treatment in COVID-19 patients. A meta-analysis of randomized and propensity matched studies",
"type": "journal-article"
}