Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial
et al., Inflammopharmacology, doi:10.1007/s10787-022-00939-7, NCT04487964, Mar 2022
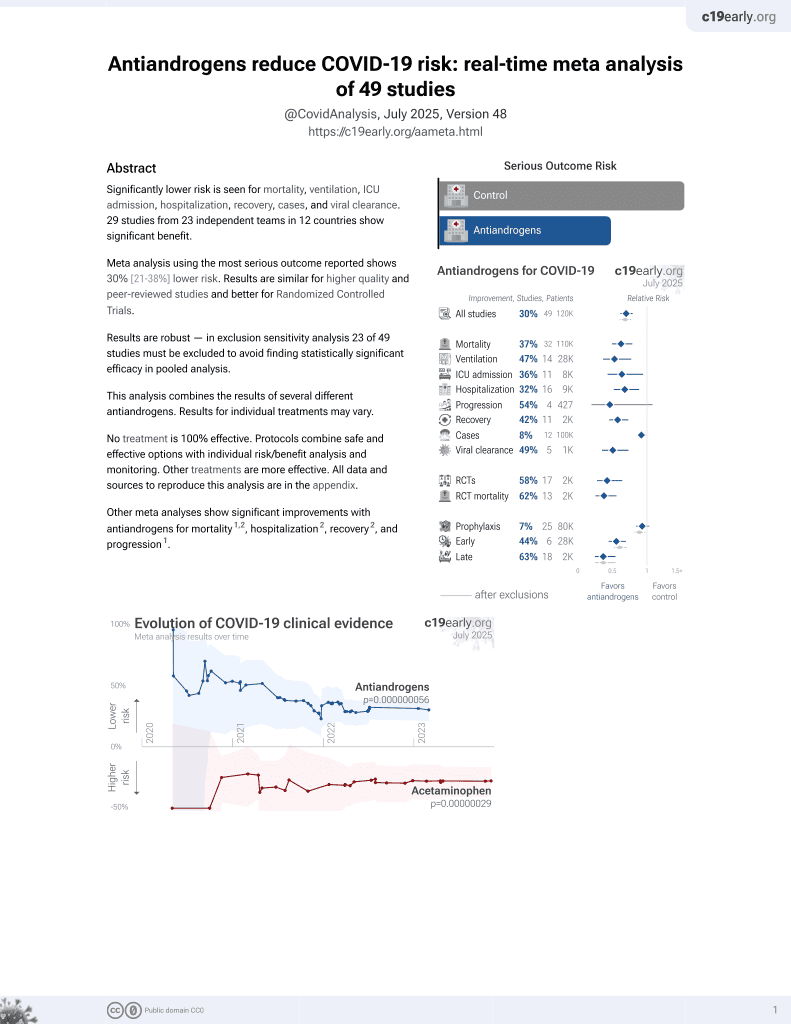
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
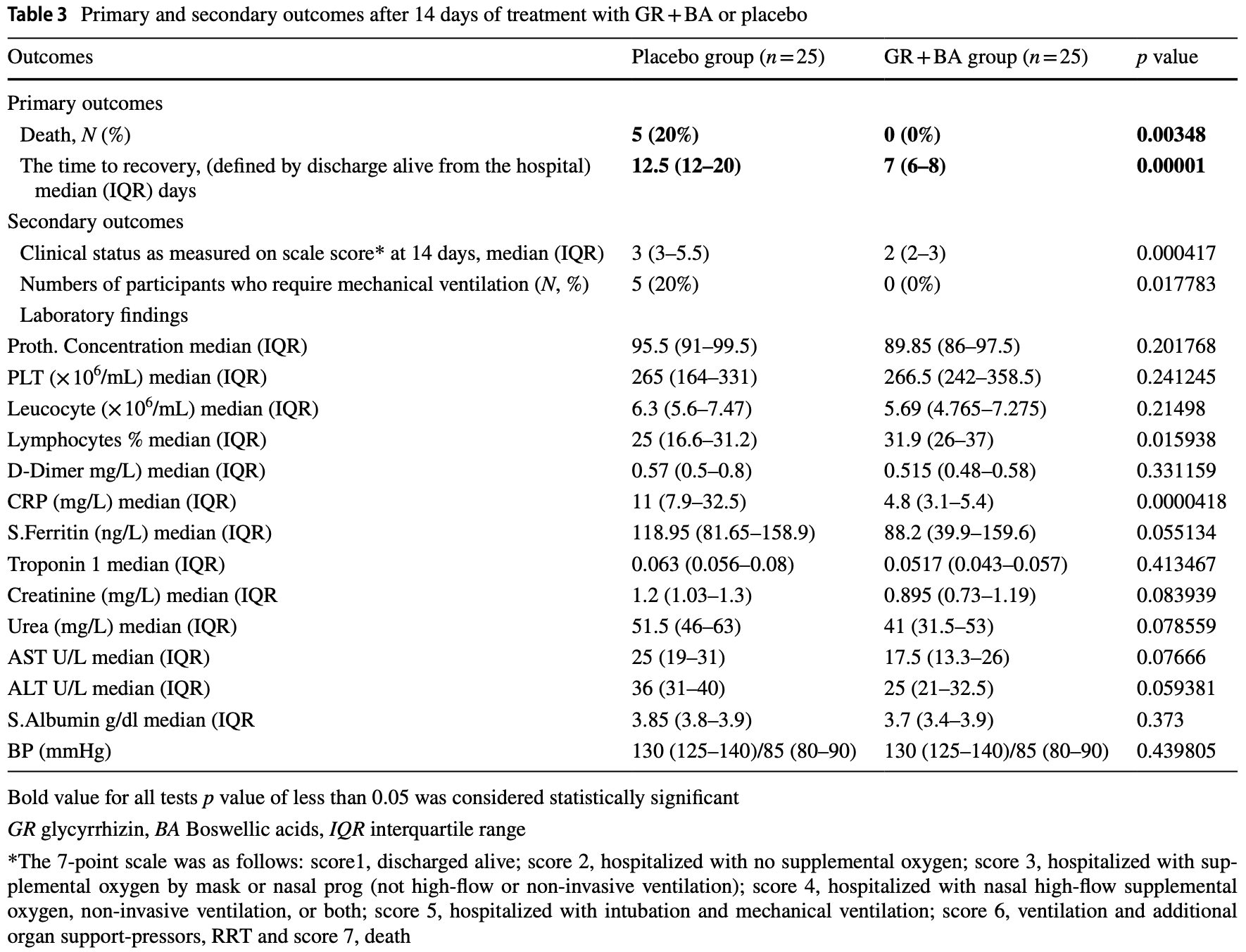
RCT with 50 hospitalized COVID+ patients in Egypt, 25 treated with glycyrrhizin and boswellic acid, showing improved recovery with treatment. Glycyrrhizin 60mg and boswellic acid 200mg bid for 2 weeks. NCT04487964 (history).
|
risk of death, 90.9% lower, RR 0.09, p = 0.05, treatment 0 of 25 (0.0%), control 5 of 25 (20.0%), NNT 5.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14.
|
|
risk of mechanical ventilation, 90.9% lower, RR 0.09, p = 0.05, treatment 0 of 25 (0.0%), control 5 of 25 (20.0%), NNT 5.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14.
|
|
recovery time, 44.0% lower, relative time 0.56, p < 0.001, treatment 25, control 25.
|
|
risk of no recovery, 33.3% lower, RR 0.67, p < 0.001, treatment 25, control 25, relative clinical status, day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gomaa et al., 1 Mar 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Egypt, peer-reviewed, median age 60.0, 5 authors, study period June 2021 - November 2021, average treatment delay 6.0 days, this trial uses multiple treatments in the treatment arm (combined with boswellic acid) - results of individual treatments may vary, trial NCT04487964 (history).
Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial
Inflammopharmacology, doi:10.1007/s10787-022-00939-7
Recent evidence points to a potential therapeutic role for glycyrrhizin(GR) and boswellic acids (BA) in the treatment of COVID-19 but conclusive evidence is lacking. Our aim is to investigate the efficacy of GR + BA versus placebo for the treatment of hospitalized patients with moderate SARS-CoV-2 or COVID-19 variants infection. The current study is a randomized, double-blind, placebo-controlled, single-center trial. Patients with SARS-CoV-2 or COVID-19 variants diagnosed by PCR test who were admitted to Sohag University hospital were eligible if they were at least 18 years of age and had moderate symptoms. Patients were randomly assigned to receive oral GR capsule (60 mg) and BA (200 mg) twice daily for 14 days or a matching placebo. All patients also received treatment with the institutional protocol for COVID-19. The primary outcome was mortality and time to recovery. Secondary outcome was clinical status score, 14 days after receiving study drugs. Adverse events from use of study drugs have been evaluated for up to 14 days. The trial is registered at ClinicalTrials.gov (Identifier NCT04487964). During the 6-month enrollment period (June-November, 2021) only 50 patients (54% women; median age 60 years, IQR 54-65) met eligibility and were randomly assigned. Evaluation of the primary outcome at 14 days showed that there were five deaths in the placebo group and no deaths in the GR + BA group. With regard to recovery time, it was significantly shorter (p = 0.0001) in the group receiving GR + BA capsule compared to the placebo group (median 7.0; IQR 6.0-8.0 days vs. median 12.5; IQR 12-20 days). Clinical status on the ordinal score scale as a secondary outcome showed a significant difference between the GR + BA group (median (IQR) score, 2 [2-3]) and placebo groups (mean (IQR) score, 3 [3-5.5]). There was a significant decrease in CRB (p = 0.000041) in GR + BA compared with the placebo group. In conclusion, this safe, inexpensive, antiviral, immunomodulating and anti-inflammatory combination may be considered for use in mild to moderate infections of SARS-CoV-2 or COVID-19 variants. The study is limited by the small sample size; therefore, larger randomized trials are required.
Keywords COVID-19 • Glycyrrhizin • Boswellic aids • Mortality rate • Time to recovery • Clinical status score Abbreviations BA Boswellic acids ACE2 Angiotensin-converting enzyme 2 COVID-19 Coronavirus disease 2019 CRB C-reactive protein GR Glycyrrhizin HMGB1 High-mobility group box 1 protein HsGAPDH Human GAPDH IQR Interquartile rang NLRP3 Nod-like receptor protein3 NF-κB3 Nuclear factor kappa B3 SARS-CoV-2 Severe acute respiratory syndrome coronavirus-2 Inflammopharmacology * Adel A. Gomaa
Author contributors Concept and design was done by AAG; acquisition, analysis, or interpretation of data was done by AAG, HSM, RBA, MAG, and DSH; drafting of the manuscript was done by AAG and MAG; critical revision of the manuscript for important intellectual content was done by AAG, HSM and RBA; statistical analysis was done by AAG, MAG and DSH; supervision was provided by AAG, HSM and RBA.
Declarations Conflict of interest All authors declare no competing interests.
Ethical approval The study was approved by the local ethical committee of the Faculty of Medicine, Assiut University, Egypt, complying with international standards of clinical trials (IRB No: 17101070; 6/5/2020) and was registered on ClinicalTrials.gov (Identifier NCT04487964). Consent to participate Informed consent to participate in the study has been obtained from participants and a form of consent is included as supplement. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative..
References
Ahmad, Waheed, Abro, Abbasi, Ismail, Molecular screening of glycyrrhizin-based inhibitors against ACE2 host receptor of SARS-CoV-2, J Mol Model, doi:10.1007/s00894-021-04816-y
Alam, Sarker, Afrin, Richi, Zhao, Traditional herbal medicines, bioactive metabolites, and plant products against covid-19: update on clinical trials and mechanism of actions, Front Pharmacol, doi:10.3389/fphar.2021.671498
Badria, Abu-Karam, Mikhaeil, Anti-herpes activity of isolated compounds from frankincense, Biosci Biotechnol Res Asia
Baram, Karima, Shateri, Functional improvement and immune-inflammatory cytokines profile of ischaemic stroke patients after treatment with boswellic acids: a randomized, double-blind, placebo-controlled, pilot trial, Inflammopharmacology, doi:10.1007/s10787-019-00627-z
Beghelli, Isani, Roncada, Antioxidant and ex vivo immune system regulatory properties of Boswellia serrate extracts, Oxid Med Cell Longev, doi:10.1155/2017/7468064
Bignardi, Vengrus, Aquino, Cerci, Use of hydroxychloroquine and chloroquine in patients with COVID-19: a meta-analysis of randomized clinical trials, Pathog Glob Health, doi:10.1080/20477724.2021.1884807
Caliebe, Scior, Ammon, Binding of boswellic acids to functional proteins of the SARS-CoV-2 virus: Bioinformatic studies, Arch Pharm, doi:10.1002/ardp.202100160
Cheng, Hu, Zuo, Predictors of progression from moderate to severe coronavirus disease 2019: a retrospective cohort, Clin Microbiol Infect
Choi, Tian, Manohar, Harraz, Park, Human GAPDH is a target of aspirin's primary metabolite salicylic acid and its derivatives, PLoS ONE, doi:10.1371/journal.pone.0143447
Choi, Tian, Song, Venereau, Preti et al., Aspirin's active metabolite salicylic acid targets high mobility group box 1 to modulate inflammatory responses, Mol Med, doi:10.2119/molmed.2015.00148
Ding, Wei, Fu, Wang, Wu, Natural products that target the NLRP3 inflammasome to treat fibrosis, Front Pharmacol, doi:10.3389/fphar.2020.591393
Diomede, Beeg, Gamba, Fumagalli, Gobbi et al., Can antiviral activity of licorice help fight COVID-19 infection?, Biomolecules, doi:10.3390/biom11060855
Efferth, Oesch, Anti-inflammatory and anti-cancer activities of frankincense: targets, treatments and toxicities, Semin Cancer Biol, doi:10.1016/j.semcancer.2020.01.015
Eltahir, Fawzy, Mohamed, Antioxidant, antiinflammatory and anti-fibrotic effects of Boswellia serrate gum resin in CCl4-induced hepatotoxicity, Exp Ther Med, doi:10.3892/etm.2019.8353
Gomaa, Mohamed, Abd-Ellatief, Gomaa, Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly, Inflammopharmacology, doi:10.1007/s10787-021-00841-8
Gomaa, Ya, The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions, Phytomedicine plus, doi:10.1016/j.phyplu.2021.100043
Goswami, Mahapatra, Banerjee, Kar, Ojha et al., Boswellia serrata oleo-gum-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-кB and p38 MAP kinase signaling, Phytomedicine, doi:10.1016/j.phymed.2018.10.016
Guan, Liang, Zhao, Liang, Chen, China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis, Eur Respir J, doi:10.1183/13993003.00547-2020
Huan, Xu, Zhang, Guo, Pan et al., Research progress on the antiviral activity of glycyrrhizin and its derivatives in liquorice, Front Pharmacol, doi:10.3389/fphar.2021.680674
Jin, Cai, Cheng, A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version, Mil Med Res
Kadhim, Washeel Salman, Zarzoor, Kadhum, Inhibition of SARS-CoV-2 reproduction using Boswellia carterii: a theoretical study, J Mol Liq, doi:10.1016/j.molliq.2021.116440
Kimmatkar, Thawani, Hingorani, Khiyani, Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee-a randomized double blind placebo controlled trial, Phytomedicine, doi:10.1078/094471103321648593
Li, Xu, Wang, Zhang, Zhang et al., Glycyrrhizic acid inhibits SARS-CoV-2 infection by blocking spike proteinmediated cell attachment, Molecules, doi:10.3390/molecules26206090
Liu, Yu, Anti-NLRP3 inflammasome natural compounds: an update, Biomedicines, doi:10.3390/biomedicines9020136
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial, RMD Open, doi:10.1136/rmdopen-2020-001455
Majeed, Majeed, Narayanan, Nagabhushanam, A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee, Phytother Res, doi:10.1002/ptr.6338
Majeed, Nagabhushanam, Lawrence, Nallathambi, Thiyagarajan et al., Boswellia serrata extract containing 30% 3-acetyl-11-keto-boswellic acid attenuates inflammatory mediators and preserves extracellular matrix in collagen-induced arthritis, Front Physiol, doi:10.3389/fphys.2021.735247
Maroon, Bost, Maroon, Natural anti-inflammatory agents for pain relief, Surg Neurol Int
Mehta, Mcauley, Brown, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Mollica, Marchis, Spitaleri, Dallacosta, Pennacchini, Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities, Chem Biol, doi:10.1016/j.chembiol.2007.03.007
Murck, Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19 infection?, Front Immunol, doi:10.3389/fimmu.2020.01239
Ng, Khaw, Ong, Goh, Kifli, Licorice: a potential herb in overcoming SARS-CoV-2 infections, J Evid Based Integr Med, doi:10.1177/2515690X21996662
Rabaan, Sh, Muhammad, Khan, Sule, Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm, Vaccines, doi:10.3390/vaccines9050436
Rayner, Dron, Park, Accelerating clinical evaluation of repurposed combination therapies for COVID-19, Am J Trop Med Hyg
Rejekia, Sarnadia, Wihastutia, Fazharyastia, Convalescent plasma therapy in patients with moderate-to-severe COVID-19: a study from Indonesia for clinical research in lowand middle-income countries, Eclinical Medicine
Renda, Gökkaya, Şöhretoğlu, Immunomodulatory properties of triterpenes, Phytochem Rev, doi:10.1007/s11101-021-09785-x
Reyes, Hu, Teperman, Anti-inflammatory therapy for COVID-19 infection: the case for colchicine, Ann Rheum Dis
Rodrigues, De Sa, Ishimoto, Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J Exp Med
Roy, Menon, Evaluation of bioactive compounds from Boswellia serrata against SARS-CoV-2, Vegetos, doi:10.1007/s42535-021-00318-7
Roy, Parama, Banik, Bordoloi, Devi, An update on pharmacological potential of boswellic acids against chronic diseases, Int J Mol Sci, doi:10.3390/ijms20174101
Ruan, Yang, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med
Si, Meng, Tian, Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes, Sci Adv, doi:10.1126/sciadv.aau8408
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant
Sun, He, Huang, Thilakavathy, Lim et al., Glycyrrhizic acid: a natural plant ingredient as a drug candidate to treat COVID-19, Front Pharmacol, doi:10.3389/fphar.2021.707205
Van De Sand, Bormann, Alt, Schipper, Heilingloh, Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease, Viruses, doi:10.3390/v13040609
Von Rhein, Weidner, Hens, Curcumin and Boswellia serrata gum resin extract inhibit chikungunya and vesicular stomatitis virus infections in vitro, Antiviral Res, doi:10.1016/j.antiviral.2015.11.007
Who, Clinical management of COVID-19
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention, JAMA
Xiao, Tian, Wang, Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives, Med Res Rev, doi:10.1002/med.21484
Xu, Li, Ju, Lai, Lu, A multicenter, randomized, double-blind, placebo-controlled study of compound glycyrrhizin capsules combined with a topical corticosteroid in adults with chronic eczema, Evid Based Complement Alternat Med, doi:10.1155/2020/6127327
Yi, Li, Lai, Zhang, Kuang, Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection, J Adv Res, doi:10.1016/j.jare.2021.11.012
Zhang, Huang, -Z, Qiu, Wu et al., Traditional uses, pharmacological effects, and molecular mechanisms of licorice in potential therapy of COVID-19, Front Pharmacol, doi:10.3389/fphar.2021.719758
Zhang, Penninger, Li, Zhong, Slutsky, Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target, Intensive Care Med, doi:10.1007/s00134-020-05985-9
Zhang, Xiang, Huo, Zhou, Jiang et al., Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy, Signal Transduct Target Ther, doi:10.1038/s41392-021-00653-w
Zhao, Zhao, NLRP3 inflammasome-a key player in antiviral responses, Front Immunol
Zheng, Huang, Lai, Liu, Jiang et al., Glycyrrhizic acid for covid-19: findings of targeting pivotal inflammatory pathways triggered by SARS-CoV-2, Front Pharmacol, doi:10.3389/fphar.2021.631206
DOI record:
{
"DOI": "10.1007/s10787-022-00939-7",
"ISSN": [
"0925-4692",
"1568-5608"
],
"URL": "http://dx.doi.org/10.1007/s10787-022-00939-7",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Recent evidence points to a potential therapeutic role for glycyrrhizin(GR) and boswellic acids (BA) in the treatment of COVID-19 but conclusive evidence is lacking. Our aim is to investigate the efficacy of GR + BA versus placebo for the treatment of hospitalized patients with moderate SARS-CoV-2 or COVID-19 variants infection. The current study is a randomized, double-blind, placebo-controlled, single-center trial. Patients with SARS-CoV-2 or COVID-19 variants diagnosed by PCR test who were admitted to Sohag University hospital were eligible if they were at least 18 years of age and had moderate symptoms. Patients were randomly assigned to receive oral GR capsule (60 mg) and BA (200 mg) twice daily for 14 days or a matching placebo. All patients also received treatment with the institutional protocol for COVID-19. The primary outcome was mortality and time to recovery. Secondary outcome was clinical status score, 14 days after receiving study drugs. Adverse events from use of study drugs have been evaluated for up to 14 days. The trial is registered at ClinicalTrials.gov (Identifier NCT04487964). During the 6-month enrollment period (June-November, 2021) only 50 patients (54% women; median age 60 years, IQR 54–65) met eligibility and were randomly assigned. Evaluation of the primary outcome at 14 days showed that there were five deaths in the placebo group and no deaths in the GR + BA group. With regard to recovery time, it was significantly shorter (<jats:italic>p</jats:italic> = 0.0001) in the group receiving GR + BA capsule compared to the placebo group (median 7.0; IQR 6.0–8.0 days vs. median 12.5; IQR 12–20 days). Clinical status on the ordinal score scale as a secondary outcome showed a significant difference between the GR + BA group (median (IQR) score, 2 [2–3]) and placebo groups (mean (IQR) score, 3 [3–5.5]). There was a significant decrease in CRB (<jats:italic>p</jats:italic> = 0.000041) in GR + BA compared with the placebo group. In conclusion, this safe, inexpensive, antiviral, immunomodulating and anti-inflammatory combination may be considered for use in mild to moderate infections of SARS-CoV-2 or COVID-19 variants. The study is limited by the small sample size; therefore, larger randomized trials are required.</jats:p>",
"alternative-id": [
"939"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "4 February 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "8 February 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "1 March 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "All authors declare no competing interests."
},
{
"group": {
"label": "Ethical approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The study was approved by the local ethical committee of the Faculty of Medicine, Assiut University, Egypt, complying with international standards of clinical trials (IRB No: 17101070; 6/5/2020) and was registered on ClinicalTrials.gov (Identifier NCT04487964)."
},
{
"group": {
"label": "Consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Informed consent to participate in the study has been obtained from participants and a form of consent is included as supplement."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6462-0917",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gomaa",
"given": "Adel A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mohamed",
"given": "Hamdy S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abd-ellatief",
"given": "Rasha B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomaa",
"given": "Mohamed A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hammam",
"given": "Doaa S.",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04487964",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": [
"Inflammopharmacology"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T17:03:13Z",
"timestamp": 1646154193000
},
"deposited": {
"date-parts": [
[
2022,
3,
25
]
],
"date-time": "2022-03-25T12:52:04Z",
"timestamp": 1648212724000
},
"funder": [
{
"DOI": "10.13039/501100009169",
"doi-asserted-by": "crossref",
"name": "Assiut University"
}
],
"indexed": {
"date-parts": [
[
2022,
4,
6
]
],
"date-time": "2022-04-06T02:11:00Z",
"timestamp": 1649211060561
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0925-4692"
},
{
"type": "electronic",
"value": "1568-5608"
}
],
"issue": "2",
"issued": {
"date-parts": [
[
2022,
3,
1
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:00:00Z",
"timestamp": 1646092800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:00:00Z",
"timestamp": 1646092800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s10787-022-00939-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s10787-022-00939-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s10787-022-00939-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "477-486",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
3,
1
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
4
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1007/s00894-021-04816-y",
"author": "S Ahmad",
"doi-asserted-by": "publisher",
"first-page": "206",
"issue": "7",
"journal-title": "J Mol Model",
"key": "939_CR1",
"unstructured": "Ahmad S, Waheed Y, Abro A, Abbasi SW, Ismail S (2021) Molecular screening of glycyrrhizin-based inhibitors against ACE2 host receptor of SARS-CoV-2. J Mol Model 27(7):206. https://doi.org/10.1007/s00894-021-04816-y",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.671498",
"author": "S Alam",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "939_CR2",
"unstructured": "Alam S, Sarker MMR, Afrin S, Richi FT, Zhao C et al (2021) Traditional herbal medicines, bioactive metabolites, and plant products against covid-19: update on clinical trials and mechanism of actions. Front Pharmacol 12:671498. https://doi.org/10.3389/fphar.2021.671498",
"volume": "12",
"year": "2021"
},
{
"author": "FA Badria",
"first-page": "1",
"issue": "1",
"journal-title": "Biosci Biotechnol Res Asia",
"key": "939_CR3",
"unstructured": "Badria FA, Abu-Karam M, Mikhaeil BR et al (2003) Anti-herpes activity of isolated compounds from frankincense. Biosci Biotechnol Res Asia 1(1):1–10",
"volume": "1",
"year": "2003"
},
{
"DOI": "10.1007/s10787-019-00627-z",
"author": "SM Baram",
"doi-asserted-by": "publisher",
"first-page": "1101",
"journal-title": "Inflammopharmacology",
"key": "939_CR4",
"unstructured": "Baram SM, Karima S, Shateri S et al (2019) Functional improvement and immune-inflammatory cytokines profile of ischaemic stroke patients after treatment with boswellic acids: a randomized, double- blind, placebo-controlled, pilot trial. Inflammopharmacology 27:1101–1112. https://doi.org/10.1007/s10787-019-00627-z",
"volume": "27",
"year": "2019"
},
{
"DOI": "10.1155/2017/7468064",
"author": "D Beghelli",
"doi-asserted-by": "publisher",
"journal-title": "Oxid Med Cell Longev",
"key": "939_CR5",
"unstructured": "Beghelli D, Isani G, Roncada P et al (2017) Antioxidant and ex vivo immune system regulatory properties of Boswellia serrate extracts. Oxid Med Cell Longev. https://doi.org/10.1155/2017/7468064",
"year": "2017"
},
{
"DOI": "10.1080/20477724.2021.1884807",
"author": "PR Bignardi",
"doi-asserted-by": "publisher",
"first-page": "139",
"issue": "3",
"journal-title": "Pathog Glob Health",
"key": "939_CR6",
"unstructured": "Bignardi PR, Vengrus CS, Aquino BM, Cerci NA (2021) Use of hydroxychloroquine and chloroquine in patients with COVID-19: a meta-analysis of randomized clinical trials. Pathog Glob Health 115(3):139–150. https://doi.org/10.1080/20477724.2021.1884807",
"volume": "115",
"year": "2021"
},
{
"DOI": "10.1002/ardp.202100160",
"author": "RH Caliebe",
"doi-asserted-by": "publisher",
"first-page": "2100160",
"journal-title": "Arch Pharm",
"key": "939_CR7",
"unstructured": "Caliebe RH, Scior T, Ammon HP (2021) T (2021) Binding of boswellic acids to functional proteins of the SARS-CoV-2 virus: Bioinformatic studies. Arch Pharm 354:2100160. https://doi.org/10.1002/ardp.202100160",
"volume": "354",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2020.06.033",
"author": "B Cheng",
"doi-asserted-by": "crossref",
"first-page": "1400",
"issue": "10",
"journal-title": "Clin Microbiol Infect",
"key": "939_CR8",
"unstructured": "Cheng B, Hu J, Zuo X et al (2020) Predictors of progression from moderate to severe coronavirus disease 2019: a retrospective cohort. Clin Microbiol Infect 26(10):1400–1405",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0143447",
"author": "HW Choi",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "PLoS ONE",
"key": "939_CR9",
"unstructured": "Choi HW, Tian M, Manohar M, Harraz MM, Park SW et al (2015a) Human GAPDH is a target of aspirin’s primary metabolite salicylic acid and its derivatives. PLoS ONE 10(11):e0143447. https://doi.org/10.1371/journal.pone.0143447",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.2119/molmed.2015.00148",
"author": "HW Choi",
"doi-asserted-by": "publisher",
"first-page": "526",
"journal-title": "Mol Med",
"key": "939_CR10",
"unstructured": "Choi HW, Tian M, Song F, Venereau E, Preti A, Park SW et al (2015b) Aspirin’s active metabolite salicylic acid targets high mobility group box 1 to modulate inflammatory responses. Mol Med 21:526–535. https://doi.org/10.2119/molmed.2015.00148",
"volume": "21",
"year": "2015"
},
{
"DOI": "10.3389/fphar.2020.591393",
"author": "N Ding",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "939_CR11",
"unstructured": "Ding N, Wei B, Fu X, Wang C, Wu Y (2020) Natural products that target the NLRP3 inflammasome to treat fibrosis. Front Pharmacol 11:591393. https://doi.org/10.3389/fphar.2020.591393",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3390/biom11060855",
"author": "L Diomede",
"doi-asserted-by": "publisher",
"first-page": "855",
"issue": "6",
"journal-title": "Biomolecules",
"key": "939_CR12",
"unstructured": "Diomede L, Beeg M, Gamba A, Fumagalli O, Gobbi M, Salmona M (2021) Can antiviral activity of licorice help fight COVID-19 infection? Biomolecules 11(6):855. https://doi.org/10.3390/biom11060855",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.semcancer.2020.01.015",
"author": "T Efferth",
"doi-asserted-by": "publisher",
"journal-title": "Semin Cancer Biol",
"key": "939_CR13",
"unstructured": "Efferth T, Oesch F (2020) Anti-inflammatory and anti-cancer activities of frankincense: targets, treatments and toxicities. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.01.015",
"year": "2020"
},
{
"DOI": "10.3892/etm.2019.8353",
"author": "HM Eltahir",
"doi-asserted-by": "publisher",
"first-page": "1313",
"journal-title": "Exp Ther Med",
"key": "939_CR14",
"unstructured": "Eltahir HM, Fawzy MA, Mohamed EM et al (2020) Antioxidant, anti-inflammatory and anti-fibrotic effects of Boswellia serrate gum resin in CCl4-induced hepatotoxicity. Exp Ther Med 19:1313–1321. https://doi.org/10.3892/etm.2019.8353",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.phyplu.2021.100043",
"author": "AA Gomaa",
"doi-asserted-by": "publisher",
"journal-title": "Phytomedicine plus",
"key": "939_CR15",
"unstructured": "Gomaa AA, Abdel-Wadood YA (2021) The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomedicine plus 1:100043. https://doi.org/10.1016/j.phyplu.2021.100043",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.1007/s10787-021-00841-8",
"author": "AA Gomaa",
"doi-asserted-by": "publisher",
"first-page": "1033",
"issue": "4",
"journal-title": "Inflammopharmacology",
"key": "939_CR16",
"unstructured": "Gomaa AA, Mohamed HS, Abd-Ellatief RB, Gomaa MA (2021) Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly. Inflammopharmacology 29(4):1033–1048. https://doi.org/10.1007/s10787-021-00841-8",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1016/j.phymed.2018.10.016",
"author": "D Goswami",
"doi-asserted-by": "publisher",
"first-page": "94",
"journal-title": "Phytomedicine",
"key": "939_CR17",
"unstructured": "Goswami D, Mahapatra AD, Banerjee S, Kar A, Ojha D, Mukherjee PK, Chattopadhyay D (2018) Boswellia serrata oleo-gum-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-кB and p38 MAP kinase signaling. Phytomedicine 51:94–103. https://doi.org/10.1016/j.phymed.2018.10.016",
"volume": "51",
"year": "2018"
},
{
"DOI": "10.1183/13993003.00547-2020",
"author": "WJ Guan",
"doi-asserted-by": "publisher",
"journal-title": "Eur Respir J 55:2000547.",
"key": "939_CR500",
"unstructured": "Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS et al (2020) China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55:2000547. https://doi.org/10.1183/13993003.00547-2020",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.680674",
"author": "C Huan",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "939_CR18",
"unstructured": "Huan C, Xu Y, Zhang W, Guo T, Pan H, Gao S (2021) Research progress on the antiviral activity of glycyrrhizin and its derivatives in liquorice. Front Pharmacol 12:680674. https://doi.org/10.3389/fphar.2021.680674",
"volume": "12",
"year": "2021"
},
{
"author": "Y-H Jin",
"first-page": "4",
"journal-title": "Mil Med Res",
"key": "939_CR19",
"unstructured": "Jin Y-H, Cai L, Cheng Z-S et al (2020) 2020) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version. Mil Med Res 7:4",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.molliq.2021.116440",
"author": "MM Kadhim",
"doi-asserted-by": "publisher",
"journal-title": "J Mol Liq",
"key": "939_CR20",
"unstructured": "Kadhim MM, Washeel Salman A, Mrebee Zarzoor A, Kadhum WR (2021) Inhibition of SARS-CoV-2 reproduction using Boswellia carterii: a theoretical study. J Mol Liq 337:116440. https://doi.org/10.1016/j.molliq.2021.116440",
"volume": "337",
"year": "2021"
},
{
"DOI": "10.1078/094471103321648593",
"author": "N Kimmatkar",
"doi-asserted-by": "publisher",
"first-page": "3",
"issue": "1",
"journal-title": "Phytomedicine",
"key": "939_CR21",
"unstructured": "Kimmatkar N, Thawani V, Hingorani L, Khiyani R (2003) Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee—a randomized double blind placebo controlled trial. Phytomedicine 10(1):3–7. https://doi.org/10.1078/094471103321648593",
"volume": "10",
"year": "2003"
},
{
"DOI": "10.3390/molecules26206090",
"author": "J Li",
"doi-asserted-by": "publisher",
"first-page": "6090",
"issue": "20",
"journal-title": "Molecules",
"key": "939_CR22",
"unstructured": "Li J, Xu D, Wang L, Zhang M, Zhang G, Li E, He S (2021) Glycyrrhizic acid inhibits SARS-CoV-2 infection by blocking spike protein-mediated cell attachment. Molecules 26(20):6090. https://doi.org/10.3390/molecules26206090",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.3390/biomedicines9020136",
"author": "B Liu",
"doi-asserted-by": "publisher",
"first-page": "136",
"journal-title": "Biomedicines",
"key": "939_CR23",
"unstructured": "Liu B, Yu J (2021) Anti-NLRP3 inflammasome natural compounds: an update. Biomedicines 9:136. https://doi.org/10.3390/biomedicines9020136",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"author": "MI Lopes",
"doi-asserted-by": "publisher",
"journal-title": "RMD Open",
"key": "939_CR24",
"unstructured": "Lopes MI, Bonjorno LP, Giannini MC et al (2021) Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open 7:e001455. https://doi.org/10.1136/rmdopen-2020-001455",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1002/ptr.6338",
"author": "M Majeed",
"doi-asserted-by": "publisher",
"first-page": "1457",
"issue": "5",
"journal-title": "Phytother Res",
"key": "939_CR25",
"unstructured": "Majeed M, Majeed S, Narayanan NK, Nagabhushanam K (2019) A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother Res 33(5):1457–1468. https://doi.org/10.1002/ptr.6338 (Epub 2019 Mar 6)",
"volume": "33",
"year": "2019"
},
{
"DOI": "10.3389/fphys.2021.735247",
"author": "M Majeed",
"doi-asserted-by": "publisher",
"journal-title": "Front Physiol",
"key": "939_CR26",
"unstructured": "Majeed M, Nagabhushanam K, Lawrence L, Nallathambi R, Thiyagarajan V, Mundkur L (2021) Boswellia serrata extract containing 30% 3-acetyl-11-keto-boswellic acid attenuates inflammatory mediators and preserves extracellular matrix in collagen-induced arthritis. Front Physiol 12:735247. https://doi.org/10.3389/fphys.2021.735247",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.4103/2152-7806.73804",
"author": "JC Maroon",
"doi-asserted-by": "crossref",
"first-page": "80",
"journal-title": "Surg Neurol Int",
"key": "939_CR27",
"unstructured": "Maroon JC, Bost JW, Maroon A (2010) Natural anti-inflammatory agents for pain relief. Surg Neurol Int 1:80",
"volume": "1",
"year": "2010"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "crossref",
"first-page": "1033",
"journal-title": "Lancet",
"key": "939_CR28",
"unstructured": "Mehta P, McAuley DF, Brown M et al (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395:1033–1034",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.chembiol.2007.03.007",
"author": "L Mollica",
"doi-asserted-by": "publisher",
"first-page": "431",
"journal-title": "Chem Biol",
"key": "939_CR29",
"unstructured": "Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D et al (2007) Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 14:431–441. https://doi.org/10.1016/j.chembiol.2007.03.007",
"volume": "14",
"year": "2007"
},
{
"DOI": "10.3389/fimmu.2020.01239",
"author": "H Murck",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "Front Immunol",
"key": "939_CR30",
"unstructured": "Murck H (2020) Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19 infection? Front Immunol 11:1239. https://doi.org/10.3389/fimmu.2020.01239",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1177/2515690X21996662",
"author": "SL Ng",
"doi-asserted-by": "publisher",
"journal-title": "J Evid Based Integr Med",
"key": "939_CR31",
"unstructured": "Ng SL, Khaw KY, Ong YS, Goh HP, Kifli N (2021) Licorice: a potential herb in overcoming SARS-CoV-2 infections. J Evid Based Integr Med. https://doi.org/10.1177/2515690X21996662",
"year": "2021"
},
{
"DOI": "10.3390/vaccines9050436",
"author": "AA Rabaan",
"doi-asserted-by": "publisher",
"first-page": "436",
"journal-title": "Vaccines",
"key": "939_CR32",
"unstructured": "Rabaan AA, Al-Ahmed SH, Muhammad J, Khan A, Sule AA et al (2021) Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines 9:436. https://doi.org/10.3390/vaccines9050436",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.4269/ajtmh.20-0995",
"author": "CR Rayner",
"doi-asserted-by": "crossref",
"first-page": "1364",
"journal-title": "Am J Trop Med Hyg",
"key": "939_CR33",
"unstructured": "Rayner CR, Dron L, Park JJH et al (2020) Accelerating clinical evaluation of repurposed combination therapies for COVID-19. Am J Trop Med Hyg 103:1364–1366",
"volume": "103",
"year": "2020"
},
{
"author": "MS Rejekia",
"first-page": "10093",
"journal-title": "Eclinical Medicine",
"key": "939_CR34",
"unstructured": "Rejekia MS, Sarnadia N, Wihastutia R, Fazharyastia V et al (2021) Convalescent plasma therapy in patients with moderate-to-severe COVID-19: a study from Indonesia for clinical research in low- and middle-income countries. Eclinical Medicine 36:10093",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1007/s11101-021-09785-x",
"author": "G Renda",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Phytochem Rev",
"key": "939_CR35",
"unstructured": "Renda G, Gökkaya İ, Şöhretoğlu D (2021) Immunomodulatory properties of triterpenes. Phytochem Rev 18:1–27. https://doi.org/10.1007/s11101-021-09785-x",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1136/annrheumdis-2020-219174",
"author": "AZ Reyes",
"doi-asserted-by": "crossref",
"first-page": "550",
"journal-title": "Ann Rheum Dis",
"key": "939_CR36",
"unstructured": "Reyes AZ, Hu KA, Teperman J et al (2021) Anti-inflammatory therapy for COVID-19 infection: the case for colchicine. Ann Rheum Dis 80:550–557",
"volume": "80",
"year": "2021"
},
{
"DOI": "10.1084/jem.20201707",
"author": "TS Rodrigues",
"doi-asserted-by": "crossref",
"first-page": "e20201707",
"journal-title": "J Exp Med",
"key": "939_CR37",
"unstructured": "Rodrigues TS, de Sa KSG, Ishimoto AY et al (2021) Inflammasomes are activated in response to SARS-CoV- 2 infection and are associated with COVID-19 severity in patients. J Exp Med 218:e20201707",
"volume": "218",
"year": "2021"
},
{
"DOI": "10.1007/s42535-021-00318-7",
"author": "A Roy",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Vegetos",
"key": "939_CR38",
"unstructured": "Roy A, Menon T (2021) Evaluation of bioactive compounds from Boswellia serrata against SARS-CoV-2. Vegetos 17:1–11. https://doi.org/10.1007/s42535-021-00318-7",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.3390/ijms20174101",
"author": "NK Roy",
"doi-asserted-by": "publisher",
"first-page": "4101",
"issue": "17",
"journal-title": "Int J Mol Sci",
"key": "939_CR39",
"unstructured": "Roy NK, Parama D, Banik K, Bordoloi D, Devi AK et al (2019) An update on pharmacological potential of boswellic acids against chronic diseases. Int J Mol Sci 20(17):4101. https://doi.org/10.3390/ijms20174101",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"author": "Q Ruan",
"doi-asserted-by": "crossref",
"first-page": "846",
"journal-title": "Intensive Care Med",
"key": "939_CR40",
"unstructured": "Ruan Q, Yang K, Wang W et al (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46:846–848",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1126/sciadv.aau8408",
"author": "L Si",
"doi-asserted-by": "publisher",
"journal-title": "Sci Adv",
"key": "939_CR41",
"unstructured": "Si L, Meng K, Tian Z et al (2018) Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes. Sci Adv. https://doi.org/10.1126/sciadv.aau8408",
"year": "2018"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"author": "HK Siddiqi",
"doi-asserted-by": "crossref",
"first-page": "405",
"issue": "5",
"journal-title": "J Heart Lung Transplant",
"key": "939_CR42",
"unstructured": "Siddiqi HK, Mehra MR (2020) COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 39(5):405–407",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.707205",
"author": "Z Sun",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "939_CR43",
"unstructured": "Sun Z, He G, Huang N, Thilakavathy K, Lim JCW, Kumar SS, Xiong C (2021) Glycyrrhizic acid: a natural plant ingredient as a drug candidate to treat COVID-19. Front Pharmacol 12:707205. https://doi.org/10.3389/fphar.2021.707205",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3390/v13040609",
"author": "L van de Sand",
"doi-asserted-by": "publisher",
"first-page": "609",
"issue": "4",
"journal-title": "Viruses",
"key": "939_CR44",
"unstructured": "van de Sand L, Bormann M, Alt M, Schipper L, Heilingloh CS et al (2021) Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses 13(4):609. https://doi.org/10.3390/v13040609",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2015.11.007",
"author": "C Von Rhein",
"doi-asserted-by": "publisher",
"first-page": "51",
"journal-title": "Antiviral Res",
"key": "939_CR45",
"unstructured": "Von Rhein C, Weidner T, Hens L et al (2016) Curcumin and Boswellia serrata gum resin extract inhibit chikungunya and vesicular stomatitis virus infections in vitro. Antiviral Res 125:51–57. https://doi.org/10.1016/j.antiviral.2015.11.007",
"volume": "125",
"year": "2016"
},
{
"key": "939_CR46",
"unstructured": "WHO (2020) Clinical management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed 18 Feb 2020"
},
{
"key": "939_CR47",
"unstructured": "WHO R&D (2020) Blueprintnovel Coronavirus COVID-19 Therapeutic Trial Synopsis. 2020 https://www.who.int/blueprint/priority-diseases/key-action/COVID-1918022020. Accessed 18 Feb 2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"author": "Z Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA",
"key": "939_CR48",
"unstructured": "Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA 323:1239–1242",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/med.21484",
"author": "S Xiao",
"doi-asserted-by": "publisher",
"first-page": "951",
"issue": "3",
"journal-title": "Med Res Rev",
"key": "939_CR49",
"unstructured": "Xiao S, Tian Z, Wang Y et al (2018) Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med Res Rev 38(3):951–976. https://doi.org/10.1002/med.21484",
"volume": "38",
"year": "2018"
},
{
"DOI": "10.1155/2020/6127327",
"author": "W Xu",
"doi-asserted-by": "publisher",
"first-page": "6127327",
"journal-title": "Evid Based Complement Alternat Med",
"key": "939_CR50",
"unstructured": "Xu W, Li Y, Ju M, Lai W, Lu X et al (2020) A multicenter, randomized, double-blind, placebo-controlled study of compound glycyrrhizin capsules combined with a topical corticosteroid in adults with chronic eczema. Evid Based Complement Alternat Med 2020:6127327. https://doi.org/10.1155/2020/6127327",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1016/j.jare.2021.11.012",
"author": "Y Yi",
"doi-asserted-by": "publisher",
"first-page": "201",
"journal-title": "J Adv Res",
"key": "939_CR51",
"unstructured": "Yi Y, Li J, Lai X, Zhang M, Kuang Y et al (2021) Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection. J Adv Res 36:201–210. https://doi.org/10.1016/j.jare.2021.11.012",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1007/s00134-020-05985-9",
"author": "H Zhang",
"doi-asserted-by": "publisher",
"first-page": "586",
"issue": "4",
"journal-title": "Intensive Care Med",
"key": "939_CR52",
"unstructured": "Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46(4):586–590. https://doi.org/10.1007/s00134-020-05985-9",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00653-w",
"author": "Q Zhang",
"doi-asserted-by": "publisher",
"first-page": "233",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "939_CR53",
"unstructured": "Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S, Wang Q, Yu F (2021a) Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther 6(1):233. https://doi.org/10.1038/s41392-021-00653-w",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.719758",
"author": "Q-h Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "939_CR54",
"unstructured": "Zhang Q-h, Huang H-z, Qiu M, Wu Z-f, Xin Z-c et al (2021b) Traditional uses, pharmacological effects, and molecular mechanisms of licorice in potential therapy of COVID-19. Front Pharmacol 12:719758. https://doi.org/10.3389/fphar.2021.719758",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.00211",
"author": "C Zhao",
"doi-asserted-by": "crossref",
"first-page": "211",
"journal-title": "Front Immunol",
"key": "939_CR55",
"unstructured": "Zhao C, Zhao W (2020) NLRP3 inflammasome—a key player in antiviral responses. Front Immunol 11:211",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.631206",
"author": "W Zheng",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "939_CR56",
"unstructured": "Zheng W, Huang X, Lai Y, Liu X, Jiang Y, Zhan S (2021) Glycyrrhizic acid for covid-19: findings of targeting pivotal inflammatory pathways triggered by SARS-CoV-2. Front Pharmacol 12:631206. https://doi.org/10.3389/fphar.2021.631206",
"volume": "12",
"year": "2021"
}
],
"reference-count": 57,
"references-count": 57,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s10787-022-00939-7"
}
},
"score": 1,
"short-container-title": [
"Inflammopharmacol"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology",
"Immunology"
],
"subtitle": [],
"title": [
"Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "30"
}