Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial
et al., RMD Open, doi:10.1136/rmdopen-2020-001455, Aug 2020 (preprint)
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
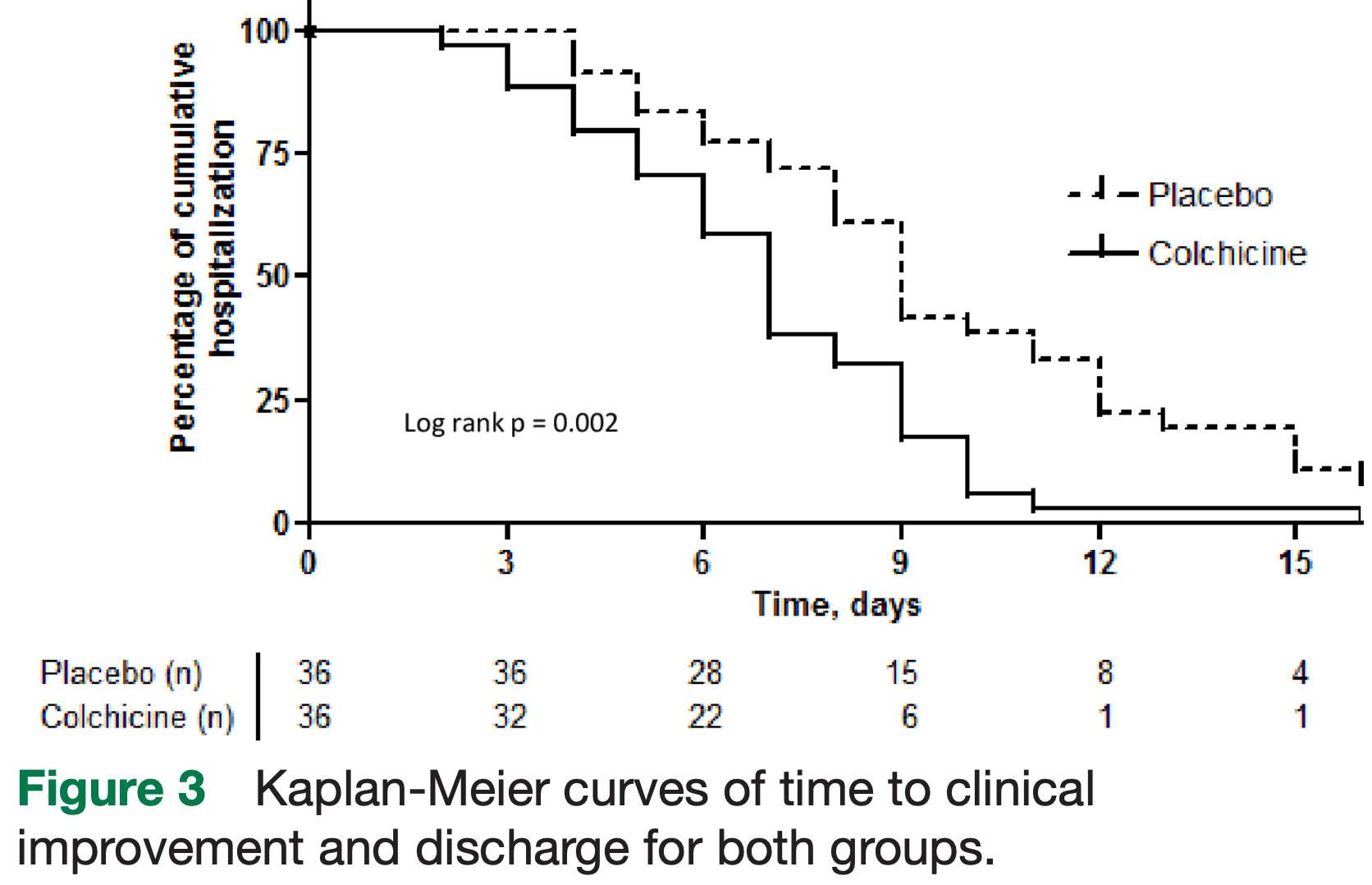
RCT with 36 colchicine and 36 control patients, showing reduced length of hospitalization and oxygen therapy with treatment.
|
risk of death, 80.0% lower, RR 0.20, p = 0.49, treatment 0 of 36 (0.0%), control 2 of 36 (5.6%), NNT 18, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 50.0% lower, RR 0.50, p = 0.67, treatment 2 of 36 (5.6%), control 4 of 36 (11.1%), NNT 18.
|
|
hospitalization time, 22.2% lower, relative time 0.78, p < 0.01, treatment 36, control 36.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lopes et al., 12 Aug 2020, Double Blind Randomized Controlled Trial, Brazil, peer-reviewed, baseline oxygen required 93.0%, median age 54.5 (treatment) 55.0 (control), 34 authors, study period 11 April, 2020 - 30 August, 2020, average treatment delay 9.5 (treatment) 8.0 (control) days, dosage 1.5mg days 1-5, 1mg days 6-10.
Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial
RMD Open, doi:10.1136/rmdopen-2020-001455
Objective To evaluate whether the addition of colchicine to standard treatment for COVID-19 results in better outcomes. Design We present the results of a randomised, doubleblinded, placebo-controlled clinical trial of colchicine for the treatment of moderate to severe COVID-19, with 75 patients allocated 1:1 from 11 April to 30 August 2020. Colchicine regimen was 0.5 mg thrice daily for 5 days, then 0.5 mg twice daily for 5 days. The primary endpoints were the need for supplemental oxygen, time of hospitalisation, need for admission and length of stay in intensive care unit and death rate. Results Seventy-two patients (36 for placebo and 36 for colchicine) completed the study. Median (and IQR) time of need for supplemental oxygen was 4.0 (2.0-6.0) days for the colchicine group and 6.5 (4.0-9.0) days for the placebo group (p<0.001). Median (IQR) time of hospitalisation was 7.0 (5.0-9.0) days for the colchicine group and 9.0 (7.0-12.0) days for the placebo group (p=0.003). At day 2, 67% versus 86% of patients maintained the need for supplemental oxygen, while at day 7, the values were 9% versus 42%, in the colchicine and the placebo groups, respectively (log rank; p=0.001). Two patients died, both in placebo group. Diarrhoea was more frequent in the colchicine group (p=0.26). Conclusion Colchicine reduced the length of both, supplemental oxygen therapy and hospitalisation. The drug was safe and well tolerated. Once death was an uncommon event, it is not possible to ensure that colchicine reduced mortality of COVID-19. Trial registration number RBR-8jyhxh.
How might this impact on clinical practice?? ► This is the first RCT on colchicine for COVID-19. Colchicine may be considered as an adjunctive therapy for hospitalised patients with moderate to severe COVID-19.
Competing interests None declared. Patient consent for publication Not required. Ethics approval This study was approved by National Ethics Committee (CONEP; CAAE: 30248420.9.3001.5403). Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data are available on reasonable request. Data types: deidentified participant data. How to access data: renedroliveira@ gmail. com. When available: With publication. Additional information who can access the data: researchers whose proposed use of the data has been approved. Types of analyses: any purpose. Mechanisms of data availability: with investigator support, after approval of a proposal with a signed data access agreement.
Open access This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https:// creativecommons. org/ licenses/ by/ 4. 0/.
References
Broz, Dixit, Inflammasomes: mechanism of assembly, regulation and signalling, Nat Rev Immunol, doi:10.1038/nri.2016.58
Chawla, Nguyen, Goh, Macrophage-Mediated inflammation in metabolic disease, Nat Rev Immunol, doi:10.1038/nri3071
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.13136
Esser, Legrand-Poels, Piette, Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes, Diabetes Res Clin Pract, doi:10.1016/j.diabres.2014.04.006
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19 -Preliminary Report, N Engl J Med, doi:10.1056/NEJMoa2021436
Iwasaki, Medzhitov, Control of adaptive immunity by the innate immune system, Nat Immunol, doi:10.1038/ni.3123
Jin, Cai, Cheng, A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version), Mil Med Res, doi:10.1186/s40779-020-0233-6
Kajikawa, Higashi, Tomiyama, Effect of short-term colchicine treatment on endothelial function in patients with coronary artery disease, Int J Cardiol, doi:10.1016/j.ijcard.2019.01.054
Liantinioti, Argyris, Protogerou, The role of colchicine in the treatment of autoinflammatory diseases, Curr Pharm Des, doi:10.2174/1381612824666180116095658
Mandel, Harari, Gurevich, Cytokine prediction of mortality in COVID19 patients, Cytokine, doi:10.1016/j.cyto.2020.155190
Mi, Zhong, Huang, Gender, age and comorbidities as the main prognostic factors in patients with COVID-19 pneumonia, Am J Transl Res
Niel, Scherrmann, Colchicine today, Joint Bone Spine, doi:10.1016/j.jbspin.2006.03.006
Nuki, Colchicine: its mechanism of action and efficacy in crystalinduced inflammation, Curr Rheumatol Rep, doi:10.1007/s11926-008-0036-3
Piantoni, Colombo, Airò, The rationale for the use of colchicine in COVID-19: comments on the letter by Cumhur Cure M et al, Clin Rheumatol, doi:10.1007/s10067-020-05232-y
Rocha, Libby, Obesity, inflammation, and atherosclerosis, Nat Rev Cardiol, doi:10.1038/nrcardio.2009.55
Rodrigues, De Sá, Ishimoto, Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J Exp Med, doi:10.1084/jem.20201707
Rodriguez-Morales, Cardona, Ocampo, Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis, Travel Med Infect Dis
Ruan, Yang, Wang, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med, doi:10.1007/s00134-020-05991-x
Safi, Kallas, Bardawil, Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behçet's disease, J Dermatol Sci, doi:10.1016/j.jdermsci.2018.08.010
Scarsi, Piantoni, Colombo, Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217712
Tardif, Kouz, Waters, Efficacy and safety of low-dose colchicine after myocardial infarction, N Engl J Med, doi:10.1056/NEJMoa1912388
Veras, Pontelli, Silva, SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology, J Exp Med, doi:10.1084/jem.20201129
Wu, Huang, Sun, Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19, mSphere, doi:10.1128/mSphere.00362-20
Wu, Mcgoogan, Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648
Yang, Hu, Zhu, Obesity aggravates COVID-19: a systematic review and meta-analysis, J Med Virol
Zhao, Zhao, Nlrp3 Inflammasome-A key player in antiviral responses, Front Immunol, doi:10.3389/fimmu.2020.00211
DOI record:
{
"DOI": "10.1136/rmdopen-2020-001455",
"ISSN": [
"2056-5933"
],
"URL": "http://dx.doi.org/10.1136/rmdopen-2020-001455",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate whether the addition of colchicine to standard treatment for COVID-19 results in better outcomes.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>We present the results of a randomised, double-blinded, placebo-controlled clinical trial of colchicine for the treatment of moderate to severe COVID-19, with 75 patients allocated 1:1 from 11 April to 30 August 2020. Colchicine regimen was 0.5 mg thrice daily for 5 days, then 0.5 mg twice daily for 5 days. The primary endpoints were the need for supplemental oxygen, time of hospitalisation, need for admission and length of stay in intensive care unit and death rate.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Seventy-two patients (36 for placebo and 36 for colchicine) completed the study. Median (and IQR) time of need for supplemental oxygen was 4.0 (2.0–6.0) days for the colchicine group and 6.5 (4.0–9.0) days for the placebo group (p<0.001). Median (IQR) time of hospitalisation was 7.0 (5.0–9.0) days for the colchicine group and 9.0 (7.0–12.0) days for the placebo group (p=0.003). At day 2, 67% versus 86% of patients maintained the need for supplemental oxygen, while at day 7, the values were 9% versus 42%, in the colchicine and the placebo groups, respectively (log rank; p=0.001). Two patients died, both in placebo group. Diarrhoea was more frequent in the colchicine group (p=0.26).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Colchicine reduced the length of both, supplemental oxygen therapy and hospitalisation. The drug was safe and well tolerated. Once death was an uncommon event, it is not possible to ensure that colchicine reduced mortality of COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Trial registration number</jats:title><jats:p>RBR-8jyhxh.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/rmdopen-2020-001455"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5060-4289",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lopes",
"given": "Maria Isabel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bonjorno",
"given": "Leticia P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giannini",
"given": "Marcela C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amaral",
"given": "Natalia B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Menezes",
"given": "Pamella Indira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dib",
"given": "Saulo Musse",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gigante",
"given": "Samara Libich",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benatti",
"given": "Maira N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rezek",
"given": "Uebe C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Emrich-Filho",
"given": "Laerte L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sousa",
"given": "Betania A A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almeida",
"given": "Sergio C L",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8430-8357",
"affiliation": [],
"authenticated-orcid": false,
"family": "Luppino Assad",
"given": "Rodrigo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Veras",
"given": "Flavio P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schneider",
"given": "Ayda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodrigues",
"given": "Tamara S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leiria",
"given": "Luiz O S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cunha",
"given": "Larissa D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alves-Filho",
"given": "Jose C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cunha",
"given": "Thiago M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arruda",
"given": "Eurico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miranda",
"given": "Carlos H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pazin-Filho",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auxiliadora-Martins",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borges",
"given": "Marcos C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fonseca",
"given": "Benedito A L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bollela",
"given": "Valdes R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Del-Ben",
"given": "Cristina M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cunha",
"given": "Fernando Q",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zamboni",
"given": "Dario S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santana",
"given": "Rodrigo C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vilar",
"given": "Fernando C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Louzada-Junior",
"given": "Paulo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0229-6864",
"affiliation": [],
"authenticated-orcid": false,
"family": "Oliveira",
"given": "Rene D R",
"sequence": "additional"
}
],
"container-title": "RMD Open",
"container-title-short": "RMD Open",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
6
]
],
"date-time": "2021-02-06T18:54:11Z",
"timestamp": 1612637651000
},
"deposited": {
"date-parts": [
[
2021,
4,
20
]
],
"date-time": "2021-04-20T19:07:42Z",
"timestamp": 1618945662000
},
"funder": [
{
"DOI": "10.13039/501100003593",
"award": [
"425075/2016-8"
],
"doi-asserted-by": "publisher",
"name": "Conselho Nacional de Desenvolvimento Científico e Tecnológico"
},
{
"DOI": "10.13039/501100001807",
"award": [
"2013/08216-2",
"2020/04964-8",
"2020/05288-6",
"2020/05601-6"
],
"doi-asserted-by": "publisher",
"name": "Fundação de Amparo à Pesquisa do Estado de São Paulo"
},
{
"DOI": "10.13039/501100002322",
"doi-asserted-by": "publisher",
"name": "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T18:26:54Z",
"timestamp": 1712687214314
},
"is-referenced-by-count": 162,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
2
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2021,
2,
4
]
]
},
"published-print": {
"date-parts": [
[
2021,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 2,
"start": {
"date-parts": [
[
2021,
2,
3
]
],
"date-time": "2021-02-03T00:00:00Z",
"timestamp": 1612310400000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/rmdopen-2020-001455",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e001455",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2021,
2
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
4
]
]
},
"published-print": {
"date-parts": [
[
2021,
2
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1007/s00134-020-05991-x",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.1"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.2"
},
{
"DOI": "10.1016/j.cyto.2020.155190",
"article-title": "Cytokine prediction of mortality in COVID19 patients",
"author": "Mandel",
"doi-asserted-by": "crossref",
"journal-title": "Cytokine",
"key": "2021042008451130000_7.1.e001455.3",
"volume": "134",
"year": "2020"
},
{
"DOI": "10.1128/mSphere.00362-20",
"article-title": "Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "e00362",
"journal-title": "mSphere",
"key": "2021042008451130000_7.1.e001455.4",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101623",
"doi-asserted-by": "crossref",
"key": "2021042008451130000_7.1.e001455.5",
"unstructured": "Rodriguez-Morales J , Cardona-Ospina A , Gutiérrez-Ocampo E . Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020."
},
{
"DOI": "10.1084/jem.20201129",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.6"
},
{
"DOI": "10.3389/fimmu.2020.00211",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.7"
},
{
"DOI": "10.1038/nri.2016.58",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.8"
},
{
"DOI": "10.1038/ni.3123",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.9"
},
{
"DOI": "10.2174/1381612824666180116095658",
"article-title": "The role of colchicine in the treatment of autoinflammatory diseases",
"author": "Liantinioti",
"doi-asserted-by": "crossref",
"first-page": "690",
"journal-title": "Curr Pharm Des",
"key": "2021042008451130000_7.1.e001455.10",
"volume": "24",
"year": "2018"
},
{
"DOI": "10.1007/s11926-008-0036-3",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.11"
},
{
"DOI": "10.1016/j.jdermsci.2018.08.010",
"article-title": "Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behçet's disease",
"author": "Safi",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "J Dermatol Sci",
"key": "2021042008451130000_7.1.e001455.12",
"volume": "92",
"year": "2018"
},
{
"DOI": "10.1007/s10067-020-05232-y",
"article-title": "The rationale for the use of colchicine in COVID-19: comments on the letter by Cumhur Cure M et al",
"author": "Piantoni",
"doi-asserted-by": "crossref",
"first-page": "2489",
"journal-title": "Clin Rheumatol",
"key": "2021042008451130000_7.1.e001455.13",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.14"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"article-title": "Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial",
"author": "Deftereos",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "2021042008451130000_7.1.e001455.15",
"volume": "3",
"year": "2020"
},
{
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "2021042008451130000_7.1.e001455.16",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.17"
},
{
"DOI": "10.1186/s40779-020-0233-6",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.18"
},
{
"article-title": "Gender, age and comorbidities as the main prognostic factors in patients with COVID-19 pneumonia",
"author": "Mi",
"first-page": "6537",
"journal-title": "Am J Transl Res",
"key": "2021042008451130000_7.1.e001455.19",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.jbspin.2006.03.006",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.20"
},
{
"DOI": "10.1016/j.diabres.2014.04.006",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.21"
},
{
"DOI": "10.1038/nrcardio.2009.55",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.22"
},
{
"DOI": "10.1038/nri3071",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.23"
},
{
"DOI": "10.1002/jmv.26237",
"doi-asserted-by": "crossref",
"key": "2021042008451130000_7.1.e001455.24",
"unstructured": "Yang J , Hu J , Zhu C . Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol 2020;30."
},
{
"DOI": "10.1056/NEJMoa1912388",
"doi-asserted-by": "publisher",
"key": "2021042008451130000_7.1.e001455.25"
},
{
"DOI": "10.1016/j.ijcard.2019.01.054",
"article-title": "Effect of short-term colchicine treatment on endothelial function in patients with coronary artery disease",
"author": "Kajikawa",
"doi-asserted-by": "crossref",
"first-page": "35",
"journal-title": "Int J Cardiol",
"key": "2021042008451130000_7.1.e001455.26",
"volume": "281",
"year": "2019"
},
{
"DOI": "10.1084/jem.20201707",
"article-title": "Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients",
"author": "Rodrigues",
"doi-asserted-by": "crossref",
"journal-title": "J Exp Med",
"key": "2021042008451130000_7.1.e001455.27",
"volume": "218",
"year": "2021"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://rmdopen.bmj.com/lookup/doi/10.1136/rmdopen-2020-001455"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy",
"Rheumatology"
],
"subtitle": [],
"title": "Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "7"
}
