Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome
et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-217712, Sep 2020
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
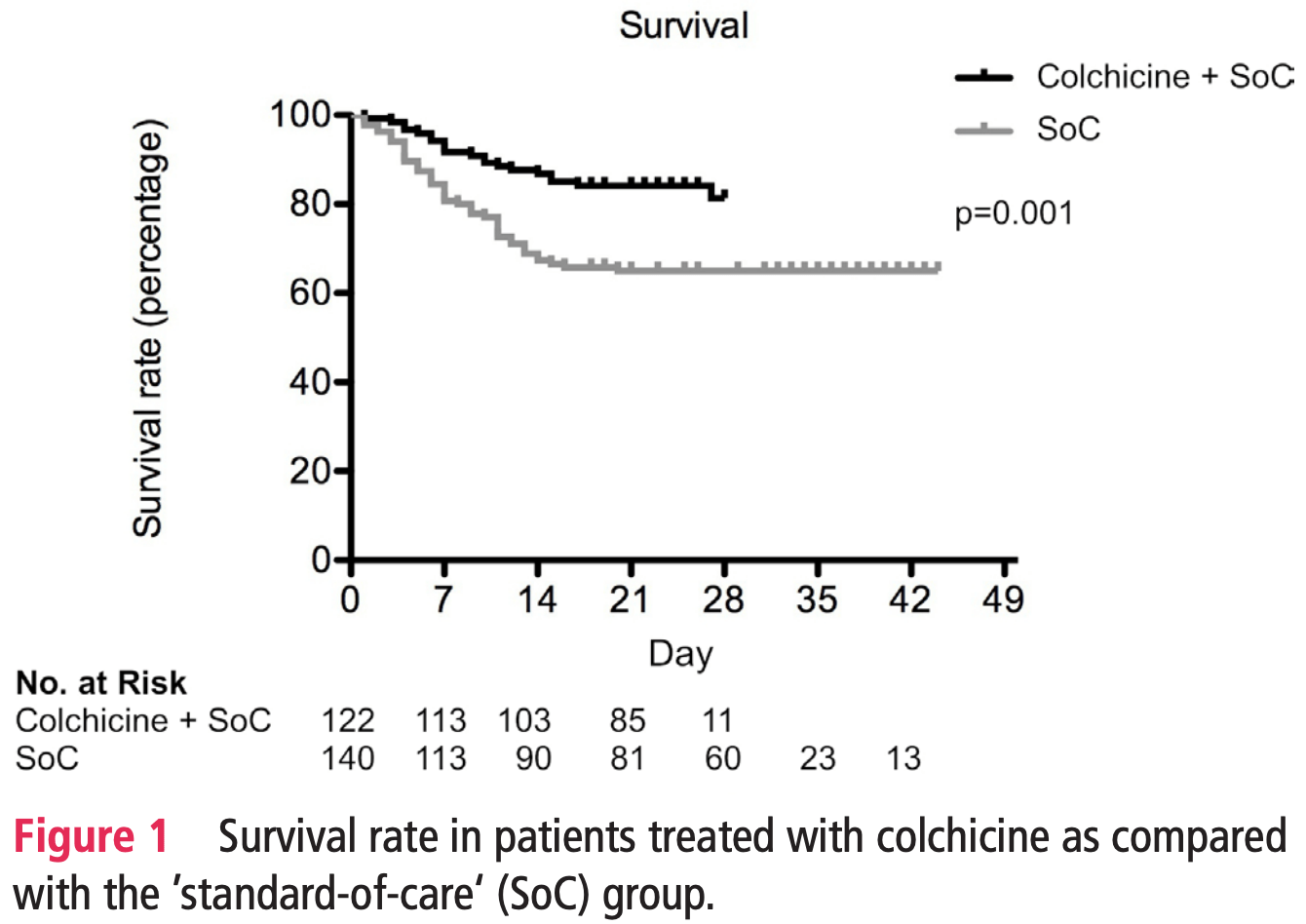
Retrospective 122 colchicine patients and 140 control patients in Italy, showing lower mortality with treatment.
Confounding may be significant: the groups were recruited during different time periods of a rapidly evolving epidemic - the control group during the chaotic peak of Lombardy's COVID crisis (March 5 - 19, 2020) when hospitals were overwhelmed and clinical protocols were still being developed, versus the treatment group later (March 19 - April 5) when care had improved.
This study is excluded in the after exclusion results of meta-analysis:
significant confounding by time possible due to separation of groups in different time periods.
|
risk of death, 84.9% lower, HR 0.15, p < 0.001, treatment 122, control 140.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Scarsi et al., 14 Sep 2020, retrospective, Italy, peer-reviewed, 28 authors, dosage 1mg daily.
Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome
Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-217712
Objectives The outbreak of COViD-19 posed the issue of urgently identifying treatment strategies. Colchicine was considered for this purpose based on well-recognised anti-inflammatory effects and potential antiviral properties. in the present study, colchicine was proposed to patients with COViD-19, and its effects compared with 'standard-of-care' (soC). Methods in the public hospital of esine, northern italy, 140 consecutive inpatients, with virologically and radiographically confirmed COViD-19 admitted in the period 5-19 March 2020, were treated with 'soC' (hydroxychloroquine and/or intravenous dexamethasone; and/or lopinavir/ritonavir). They were compared with 122 consecutive inpatients, admitted between 19 March and 5 april 2020, treated with colchicine (1 mg/day) and soC (antiviral drugs were stopped before colchicine, due to potential interaction). results Patients treated with colchicine had a better survival rate as compared with soC at 21 days of followup (84.2% (se=3.3%) vs 63.6% (se=4.1%), p=0.001). Cox proportional hazards regression survival analysis showed that a lower risk of death was independently associated with colchicine treatment (HR=0.151 (95% Ci 0.062 to 0.368), p<0.0001), whereas older age, worse PaO2/FiO2, and higher serum levels of ferritin at entry were associated with a higher risk. Conclusion This proof-of-concept study may support the rationale of use of colchicine for the treatment of COViD-19. efficacy and safety must be determined in controlled clinical trials.
Key messages What is already known about this subject? ► Considering the hypothesis that COVID-19 in its worst manifestations resembles a secondary, viral-driven haemophagocitic lymphohistiocytosis syndrome, a rationale for the use of antirheumatic drugs that are used for autoinflammatory disease have been placed in the algorithm of treatment.
What does this study add? ► Based on anti-inflammatory and potential antiviral properties, colchicine was administered to hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome (ARDS). ► The survival rate of patients treated with colchicine was significantly higher as compared with that of patients treated with standard of care only (84.2% vs 63.6%).
How might this impact on clinical practice or future developments? ► This proof-of-concept study supports the rationale of testing colchicine in clinical trials for the treatment of COVID-19 pneumonia with ARDS.
Epidemiology FiO 2 , lower CRP, ferritin and neutrophil count). Indeed, Cox proportional hazards regression survival analysis showed the independent association of colchicine treatment with survival and that of older age, higher serum levels of ferritin and more severe hypoxaemia at admission with death. In conclusion, our report can be considered as a proof-ofconcept study supporting the possible use of colchicine in the treatment of the early phase of COVID-19 with the purpose of preventing the host's autoinflammatory response. Properly designed trials will determine the efficacy and safety of colchicine and the best protocol in terms of dosage and timing of administration in patients with COVID-19. Such trials have been approved in Greece, 18 Italy 19 and Canada. 20 Author affiliations Contributors all authors were involved in drafting the article or revising it critically for intellectual content. Ms, sP, eC, Pa, RF and la had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. study conception and design: Ms, sP, eC, Pa, DR, MMi, aT, FF, RF and la. acquisition of data: Ms, eC, DR, MMi, VB, MB, DB, PC, M-sC, eD, GG, DG, M-lG, RM, PM, MMe, sM, aM, ls, MT, aV and RF. analysis and interpretation of data: Ms, sP, eC, Pa, DR, MMi, aT, aB, FF, RF and la.
Ethics approval The off-label use of colchicine was supported by the hospital administration as the emergency situation of COViD-19,..
References
Aifa, sperimentazioni cliniche -COViD-19
Angelidis, Kotsialou, Kossyvakis, Colchicine pharmacokinetics and mechanism of action, Curr Pharm Des, doi:10.2174/1381612824666180123110042
Brucato A, The role of colchicine in pericardial syndromes, Curr Pharm Des, doi:10.2174/1381612824666180116101823
Colcorona, Trial requirements, doi:10.1136/annrheumdis
Deftereos, Giannopoulos, The Greek study in the effects of colchicine in COViD-19 complications prevention (GReCCO-19 study): rationale and study design, Hellenic J Cardiol, doi:10.1016/j.hjc.2020.03.002
Ferro, Baldini, COViD-19: the new challenge for rheumatologists, Clin Exp Rheumatol
Gandolfini I, Delsante, Fiaccadori E, COViD-19 in kidney transplant recipients, Am J Transplant, doi:10.1111/ajt.15891
Grasselli, Pesenti A, Cecconi, Critical care utilization for the COViD-19 outbreak in lombardy, italy: early experience and forecast during an emergency response, JAMA, doi:10.1001/jama.2020.4031
Gultekin N, Kucukates E, Microtubule inhibition therapy by colchicine in severe myocarditis especially caused by epstein-barr and cytomegalovirus co-infection during a two-year period: a novel therapeutic approach, J Pak Med Assoc
Ishizuka, Hisada, Monden, a case of non-specific interstitial pneumonia effectively treated with a combination of prednisolone and colchicine, in which granulation tissue was extensive, Respirology, doi:10.1111/j.1440-1843.2005.00733.x
Karamanou, Tsoucalas, Pantos, isolating colchicine in 19th century: an old drug revisited, Curr Pharm Des, doi:10.2174/1381612824666180115105850
Maestroni S, Valenti A, is colchicine really harmful in viral myocarditis?, Int J Cardiol, doi:10.1016/j.ijcard.2016.10.031
Mehta, Mcauley, Brown, COViD-19: consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Milewska A, Nowak, Owczarek, entry of human coronavirus nl63 into the cell, J Virol, doi:10.1128/JVI.01933-17
Nieto ; Torres, Jl, Verdiá-Báguena, Jimenez-Guardeño, severe acute respiratory syndrome coronavirus e protein transports calcium ions and activates the nlRP3 inflammasome, Virology, doi:10.1016/j.virol.2015.08.010
Pereira Eg, Guimarães, Bottino, sarcoidosis and chronic hepatitis C: treatment with prednisone and colchicine, An Bras Dermatol, doi:10.1590/abd1806-4841.20164029
Stewart S, Yang, Atkins, adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials, Arthritis Res Ther, doi:10.1186/s13075-020-2120-7
Valeriani E, influenza B virus infection complicated by lifethreatening pericarditis: a unique case-report and literature review, BMC Infect Dis, doi:10.1186/s12879-018-3606-7
Zhang, Wu, Li, Cytokine release syndrome in severe COViD-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105954
DOI record:
{
"DOI": "10.1136/annrheumdis-2020-217712",
"ISSN": [
"0003-4967",
"1468-2060"
],
"URL": "http://dx.doi.org/10.1136/annrheumdis-2020-217712",
"abstract": "<jats:sec><jats:title>Objectives</jats:title><jats:p>The outbreak of COVID-19 posed the issue of urgently identifying treatment strategies. Colchicine was considered for this purpose based on well-recognised anti-inflammatory effects and potential antiviral properties. In the present study, colchicine was proposed to patients with COVID-19, and its effects compared with ‘standard-of-care’ (SoC).</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>In the public hospital of Esine, northern Italy, 140 consecutive inpatients, with virologically and radiographically confirmed COVID-19 admitted in the period 5–19 March 2020, were treated with ‘SoC’ (hydroxychloroquine and/or intravenous dexamethasone; and/or lopinavir/ritonavir). They were compared with 122 consecutive inpatients, admitted between 19 March and 5 April 2020, treated with colchicine (1 mg/day) and SoC (antiviral drugs were stopped before colchicine, due to potential interaction).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Patients treated with colchicine had a better survival rate as compared with SoC at 21 days of follow-up (84.2% (SE=3.3%)<jats:italic>vs</jats:italic>63.6% (SE=4.1%), p=0.001). Cox proportional hazards regression survival analysis showed that a lower risk of death was independently associated with colchicine treatment (HR=0.151 (95% CI 0.062 to 0.368), p<0.0001), whereas older age, worse PaO2/FiO2, and higher serum levels of ferritin at entry were associated with a higher risk.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>This proof-of-concept study may support the rationale of use of colchicine for the treatment of COVID-19. Efficacy and safety must be determined in controlled clinical trials.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/annrheumdis-2020-217712"
],
"author": [
{
"affiliation": [],
"family": "Scarsi",
"given": "Mirko",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-0913-0197",
"affiliation": [],
"authenticated-orcid": false,
"family": "Piantoni",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colombo",
"given": "Enrico",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5241-1918",
"affiliation": [],
"authenticated-orcid": false,
"family": "Airó",
"given": "Paolo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richini",
"given": "Donata",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miclini",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bertasi",
"given": "Valeria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bianchi",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bottone",
"given": "Damiano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Civelli",
"given": "Patrizia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cotelli",
"given": "Maria-Sofia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Damiolini",
"given": "Ezio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galbassini",
"given": "Gloria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gatta",
"given": "Diego",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghirardelli",
"given": "Maria-Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Magri",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malamani",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendeni",
"given": "Monia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molinari",
"given": "Stefano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morotti",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salada",
"given": "Luisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turla",
"given": "Marinella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vender",
"given": "Angiola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tincani",
"given": "Angela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7566-5600",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brucato",
"given": "Antonio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3678-6124",
"affiliation": [],
"authenticated-orcid": false,
"family": "Franceschini",
"given": "Franco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Furloni",
"given": "Roberto",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9107-3218",
"affiliation": [],
"authenticated-orcid": false,
"family": "Andreoli",
"given": "Laura",
"sequence": "additional"
}
],
"container-title": "Annals of the Rheumatic Diseases",
"container-title-short": "Ann Rheum Dis",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
30
]
],
"date-time": "2020-07-30T16:08:41Z",
"timestamp": 1596125321000
},
"deposited": {
"date-parts": [
[
2022,
11,
5
]
],
"date-time": "2022-11-05T05:35:48Z",
"timestamp": 1667626548000
},
"indexed": {
"date-parts": [
[
2024,
3,
14
]
],
"date-time": "2024-03-14T23:58:12Z",
"timestamp": 1710460692403
},
"is-referenced-by-count": 116,
"issue": "10",
"issued": {
"date-parts": [
[
2020,
7,
30
]
]
},
"journal-issue": {
"issue": "10",
"published-online": {
"date-parts": [
[
2020,
9,
14
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
30
]
],
"date-time": "2020-07-30T00:00:00Z",
"timestamp": 1596067200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/annrheumdis-2020-217712",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "1286-1289",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2020,
7,
30
]
]
},
"published-online": {
"date-parts": [
[
2020,
7,
30
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1001/jama.2020.4031",
"article-title": "Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "2021051223100662000_79.10.1286.1",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.2"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105954",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.3"
},
{
"DOI": "10.55563/clinexprheumatol/r3k9l6",
"article-title": "COVID-19: the new challenge for rheumatologists",
"author": "Ferro",
"doi-asserted-by": "crossref",
"first-page": "175",
"journal-title": "Clin Exp Rheumatol",
"key": "2021051223100662000_79.10.1286.4",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.2174/1381612824666180123110042",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.5"
},
{
"DOI": "10.2174/1381612824666180116101823",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.6"
},
{
"DOI": "10.2174/1381612824666180115105850",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.7"
},
{
"DOI": "10.1128/JVI.01933-17",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.8"
},
{
"article-title": "Microtubule inhibition therapy by colchicine in severe myocarditis especially caused by epstein-barr and cytomegalovirus co-infection during a two-year period: a novel therapeutic approach",
"author": "Gultekin",
"first-page": "1420",
"journal-title": "J Pak Med Assoc",
"key": "2021051223100662000_79.10.1286.9",
"volume": "64",
"year": "2014"
},
{
"DOI": "10.1186/s12879-018-3606-7",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.10"
},
{
"DOI": "10.1111/j.1440-1843.2005.00733.x",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.11"
},
{
"DOI": "10.1590/abd1806-4841.20164029",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.12"
},
{
"DOI": "10.1186/s13075-020-2120-7",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.13"
},
{
"article-title": "Vademecum for the treatment of people with COVID-19. edition 2.0, 13 March 2020",
"first-page": "143",
"journal-title": "Infez Med",
"key": "2021051223100662000_79.10.1286.14",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1111/ajt.15891",
"article-title": "COVID-19 in kidney transplant recipients",
"author": "Gandolfini",
"doi-asserted-by": "crossref",
"journal-title": "Am J Transplant",
"key": "2021051223100662000_79.10.1286.15",
"year": "2020"
},
{
"DOI": "10.1016/j.virol.2015.08.010",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.16"
},
{
"DOI": "10.1016/j.ijcard.2016.10.031",
"doi-asserted-by": "publisher",
"key": "2021051223100662000_79.10.1286.17"
},
{
"DOI": "10.1016/j.hjc.2020.03.002",
"article-title": "The Greek study in the effects of colchicine in COVID-19 complications prevention (GRECCO-19 study): rationale and study design",
"author": "Deftereos",
"doi-asserted-by": "crossref",
"journal-title": "Hellenic J Cardiol",
"key": "2021051223100662000_79.10.1286.18",
"year": "2020"
},
{
"key": "2021051223100662000_79.10.1286.19",
"unstructured": "AIFA . Sperimentazioni cliniche - COVID-19. Available: https://www.aifa.gov.it/sperimentazioni-cliniche-covid-19"
},
{
"key": "2021051223100662000_79.10.1286.20",
"unstructured": "ColCorona . Trial requirements. Available: https://www.colcorona.org"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://ard.bmj.com/lookup/doi/10.1136/annrheumdis-2020-217712"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Biochemistry, Genetics and Molecular Biology",
"Immunology",
"Immunology and Allergy",
"Rheumatology"
],
"subtitle": [],
"title": "Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "79"
}
