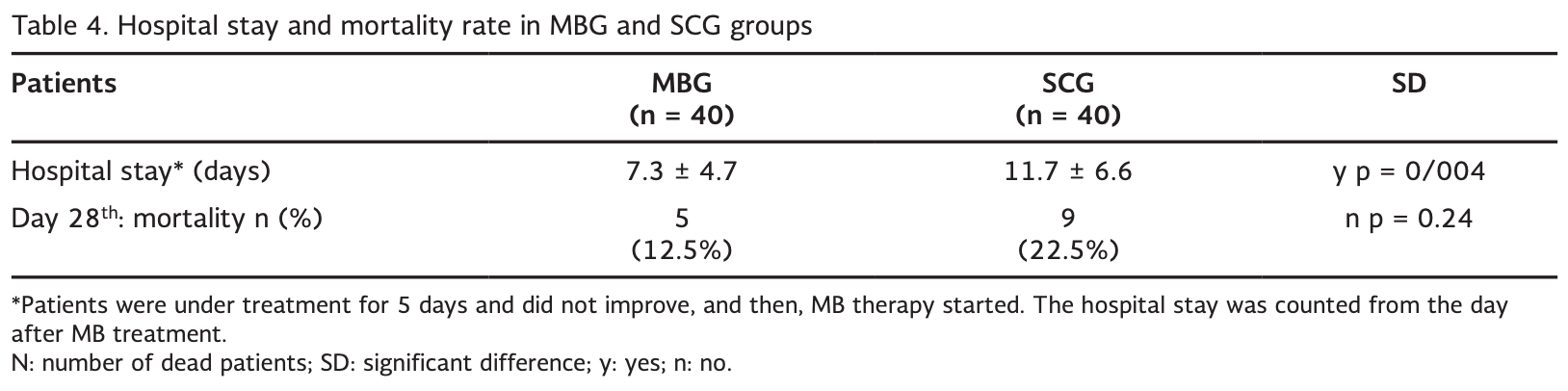
Methylene blue for treatment of hospitalized COVID-19 patients: a randomized, controlled, open-label clinical trial, phase 2
et al., Clinical and Translational Investigation, doi:10.24875/RIC.21000028, NCT04370288, Mar 2021
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 80 hospitalized patients with severe COVID-19, 40 treated with methylene blue + vitamin C + N-acetylcysteine, showing lower mortality, shorter hospitalization, and significantly improved SpO2 and respiratory distress with treatment.
This is the 5th of 20 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0016.
This is the 15th of 73 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000076.
|
risk of death, 44.4% lower, RR 0.56, p = 0.38, treatment 5 of 40 (12.5%), control 9 of 40 (22.5%), NNT 10.0.
|
|
hospitalization time, 37.6% lower, relative time 0.62, p = 0.004, treatment 40, control 40.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hamidi-Alamdari et al., 8 Mar 2021, Randomized Controlled Trial, Iran, peer-reviewed, 23 authors, study period 19 April, 2020 - 21 September, 2020, this trial uses multiple treatments in the treatment arm (combined with methylene blue and N-acetyl cysteine) - results of individual treatments may vary, trial NCT04370288 (history).
Methylene Blue for Treatment of Hospitalized COVID-19 Patients: A Randomized, Controlled, Open-Label Clinical Trial, Phase 2
Revista de investigaci�n Cl�nica, doi:10.24875/ric.21000028
Background: There is no pharmacological intervention on the treatment of hypoxemia and respiratory distress in COVID-19 patients. Objective: The objective of the study was to study the effect of the reduced form of methylene blue (MB) on the improvement of oxygen saturation (SpO 2 ) and respiratory rate (RR). Methods: In an academic medical center, 80 hospitalized patients with severe COVID-19 were randomly assigned to receive either oral MB along with standard of care (SOC) (MB group, n = 40) or SOC only (SOC group, n=40). The primary outcomes were SpO 2 and RR on the 3 rd and 5 th days. The secondary outcomes were hospital stay and mortality within 28 days. Results: In the MB group, a significant improvement in SpO 2 and RR was observed on the 3 rd day (for both, p < 0.0001) and also the 5 th day (for both, p < 0.0001). In the SOC group, there was no significant improvement in SpO 2 (p = 0.24) and RR (p = 0.20) on the 3 rd day, although there was a significant improvement of SpO 2 (p = 0.002) and RR (p = 0.01) on the 5 th day. In the MB group in comparison to the SOC group, the rate ratio of increased SpO 2 was 13.5 and 2.1 times on the 3 rd and 5 th days, respectively. In the MB group compared with the SOC group, the rate ratio of RR improvement was 10.1 and 3.7 times on the 3 rd and 5 th days, respectively. The hospital stay was significantly shortened in the MB group (p = 0.004), and the mortality was 12.5% and 22.5% in the MB and SOC groups, respectively. Conclusions: The addition of MB to the treatment protocols significantly improved SpO 2 and respiratory distress in COVID-19 patients, which resulted in decreased hospital stay and mortality. ClinicalTrials.gov: NCT04370288 (REV INVEST CLIN. 2021;73(3):XX-XX)
References
Alamdari, Moghaddam, Amini, Keramati, Zarmehri et al., Application of methylene blue -vitamin C -N-acetyl for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial, Eur J Pharmacol
Bojadzic, Alcazar, Buchwald, Methylene blue inhibits the SARS-CoV-2 spike-ACE2 protein-protein interaction-a mechanism that can contribute to its antiviral activity against COV-ID-19, Front Pharmacol
Chan, Yang, Kwan, Cheng, Lee et al., Structure-based optimization of FDA-approved drug methylene blue as a c-myc G-quadruplex DNA stabilizer, Biochimie
Haynes, Chan, Wong, Li, Wu et al., Facile oxidation of leucomethylene blue and dihydroflavins by artemisinins: relationship with flavoenzyme function and antimalarial mechanism of action, ChemMed Chem
Janssen, Ekström, Currow, Johnson, Maddocks et al., COVID-19: guidance on palliative care from a European Respiratory Society international task force, Eur Respir J
Kovacs, Prevention of cytopathic effect and propagation of poliovirus by methylene blue, Z Naturforsch B
Lin, Wang, Chen, Zhuang, Ke et al., Methylene blue mitigates acute neuroinflammation after spinal cord injury through inhibiting NLRP3 inflammasome activation in microglia, Front Cell Neurosci
May, Qu, Cobb, Reduction and uptake of methylene blue by human erythrocytes, Am J Physiol Cell Physiol
May, Qu, Whitesell, Generation of oxidant stress in cultured endothelial cells by methylene blue: protective effects of glucose and ascorbic acid, Biochem Pharmacol
Mayer, Brunner, Schmidt, Inhibition of nitric oxide synthesis by methylene blue, Biochem Pharmacol
Mcpherson, Henry's Clinical Diagnosis and Management by Laboratory Methods: First South Asia Edition
Miclescu, Wiklund, Methylene blue, an old drug with new indications, J Rom Anest Terap Int
Oz, Lorke, Hasan, Petroianu, Cellular and molecular actions of methylene blue in the nervous system, Med Res Rev
Perkins, Couper, Connolly, Baillie, Bradley et al., RECOVERY-respiratory support: respiratory strategies for patients with suspected or proven COVID-19 respiratory failure; continuous positive airway pressure, high-flow nasal oxygen, and standard care: a structured summary of a study protocol for a randomised controlled trial, Trials
Prakash, Saini, Mullick, Pawar, Green urine: a cause for concern?, J Anaesthesiol Clin Pharmacol
Riedel, Lang, Oetjen, Schlapp, Shibata, Inhibition of oxygen radical formation by methylene blue, aspirin, or alphalipoic acid, prevents bacterial-lipopolysaccharide-induced fever, Mol Cel Biochem
Salaris, Babbs, Voorhees, Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. A potential new drug for the attenuation of ischemia/reperfusion injury, Biochem Pharmacol
Shenoy, Luchtel, Gulani, Considerations for target oxygen saturation in COVID-19 patients: are we under-shooting?, BMC Med
Smith, Bushek, Leclaire, Prosser, COVID-19 Drug Therapy, Clinical Drug Information
Woo, Heil, A prospective evaluation of methylene blue and gentian violet dressing for management of chronic wounds with local infection, Int Wound J
DOI record:
{
"DOI": "10.24875/ric.21000028",
"ISSN": [
"0034-8376"
],
"URL": "http://dx.doi.org/10.24875/RIC.21000028",
"assertion": [
{
"label": "Content Type",
"name": "content_type",
"value": "Original Articles"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"value": "2021-01-17"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"value": "2021-03-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"value": "2021-05-13"
}
],
"author": [
{
"affiliation": [],
"family": "Hamidi-Alamdari",
"given": "Daryoush",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hafizi-Lotfabadi",
"given": "Saied",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bagheri-Moghaddam",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Safari",
"given": "Hossin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mozdourian",
"given": "Mahnaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Javidarabshahi",
"given": "Zahra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peivandi-Yazdi",
"given": "Arash",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali-Zeraati",
"given": "Abass",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sedaghat",
"given": "Alireza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poursadegh",
"given": "Farid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barazandeh-Ahmadabadi",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agheli-Rad",
"given": "Marzieh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tavousi",
"given": "Seyed M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vojouhi",
"given": "Shohreh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amini",
"given": "Shahram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amini",
"given": "Mahnaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Majid-Hosseini",
"given": "Seyed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tavanaee-Sani",
"given": "Ashraf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghiabi",
"given": "Amin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nabavi-Mahalli",
"given": "Shima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morovatdar",
"given": "Negar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajabi",
"given": "Omid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koliakos",
"given": "George",
"sequence": "additional"
}
],
"container-title": "Revista de investigaci�n Cl�nica",
"container-title-short": "RIC",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.clinicalandtranslationalinvestigation.com"
]
},
"created": {
"date-parts": [
[
2021,
5,
12
]
],
"date-time": "2021-05-12T16:32:37Z",
"timestamp": 1620837157000
},
"deposited": {
"date-parts": [
[
2022,
3,
4
]
],
"date-time": "2022-03-04T08:45:50Z",
"timestamp": 1646383550000
},
"indexed": {
"date-parts": [
[
2023,
12,
23
]
],
"date-time": "2023-12-23T05:30:09Z",
"timestamp": 1703309409521
},
"is-referenced-by-count": 7,
"issue": "3",
"issued": {
"date-parts": [
[
2021,
5,
13
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2021,
5,
13
]
]
},
"published-print": {
"date-parts": [
[
2021,
5,
13
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.clinicalandtranslationalinvestigation.com/frame_esp.php?id=375",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10499",
"original-title": [],
"prefix": "10.24875",
"published": {
"date-parts": [
[
2021,
5,
13
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
13
]
]
},
"published-print": {
"date-parts": [
[
2021,
5,
13
]
]
},
"publisher": "Publicidad Permanyer, SLU",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.clinicalandtranslationalinvestigation.com/frame_esp.php?id=375"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Methylene Blue for Treatment of Hospitalized COVID-19 Patients: A Randomized, Controlled, Open-Label Clinical Trial, Phase 2",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.24875/ric.policy",
"volume": "73"
}
