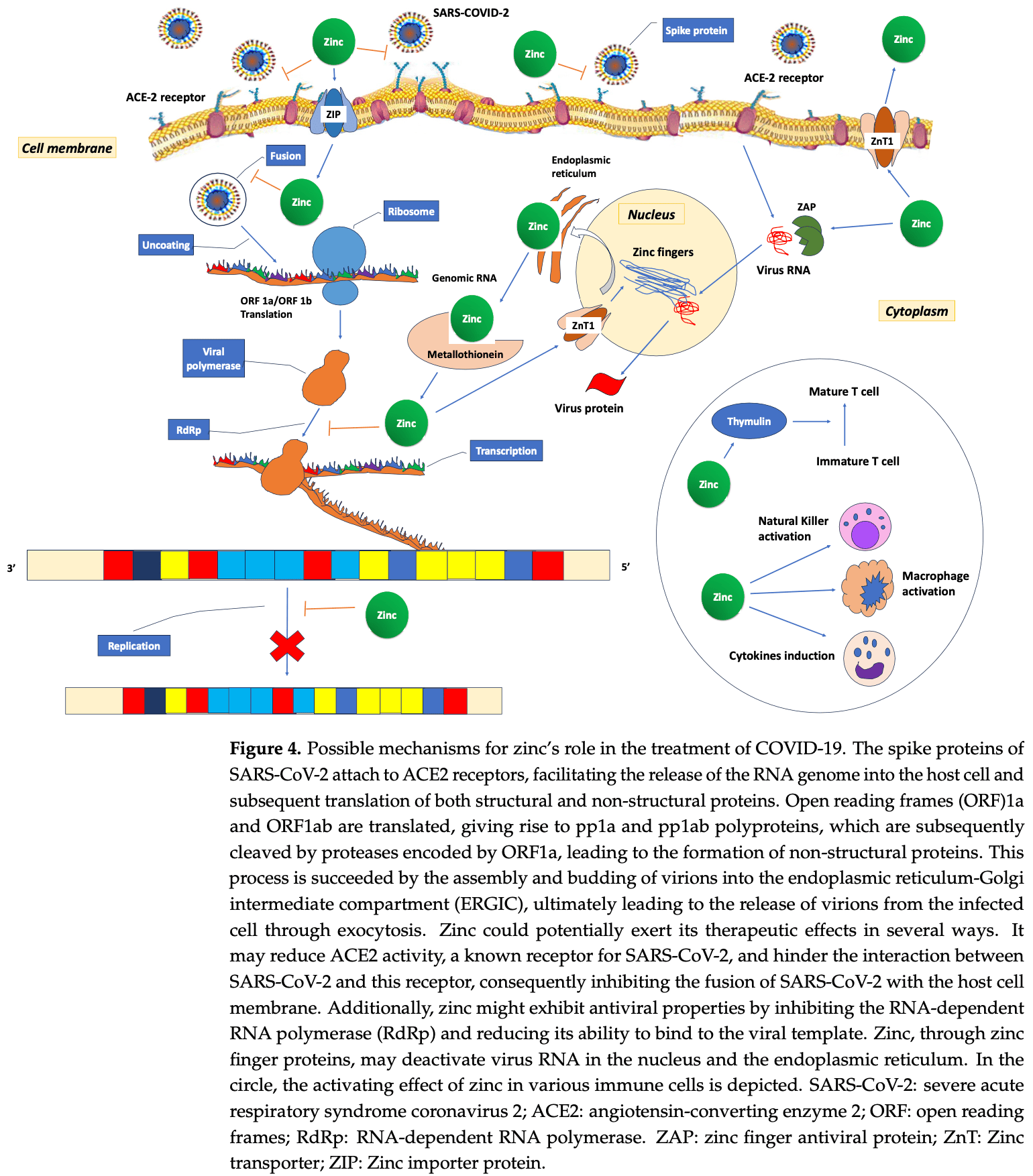
The Anti-Oxidative, Anti-Inflammatory, Anti-Apoptotic, and Anti-Necroptotic Role of Zinc in COVID-19 and Sepsis
et al., Antioxidants, doi:10.3390/antiox12111942, Oct 2023
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000028 from 47 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of the anti-oxidative, anti-inflammatory, anti-apoptotic, and anti-necroptotic role of zinc in COVID-19 and sepsis. Authors discuss how zinc functions as a structural component in proteins, a catalytic co-factor in enzymes, and plays a regulatory role in processes like DNA synthesis and protein-DNA interactions. Authors examine how zinc acts as an antioxidant, affects inflammation through pathways like NF-κB, regulates immune function, and modulates programmed cell death mechanisms like apoptosis and necroptosis. They summarize clinical data showing declines in zinc levels are associated with worse outcomes in sepsis and COVID-19. Authors review only a small subset of the clinical evidence, for more complete coverage see1.
2.
Smail et al., Antioxidant and oxidative enzymes, genetic variants, and cofactors as prognostic biomarkers of COVID-19 severity and mortality: a systematic review, Frontiers in Molecular Biosciences, doi:10.3389/fmolb.2025.1700263.
3.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
4.
Fazli et al., Possible Link between Gut Microbiota, Diet, and COVID-19 Infection, Journal of Medical Bacteriology, 12:4, jmb.tums.ac.ir/index.php/jmb/article/view/525.
5.
Mu et al., Anti-inflammatory and Nutritional Interventions Against SARS-CoV-2: A Comprehensive Review, Journal of Agriculture and Food Research, doi:10.1016/j.jafr.2024.101422.
6.
Jin et al., The nutritional roles of zinc for immune system and COVID-19 patients, Frontiers in Nutrition, doi:10.3389/fnut.2024.1385591.
7.
Briassoulis et al., The Anti-Oxidative, Anti-Inflammatory, Anti-Apoptotic, and Anti-Necroptotic Role of Zinc in COVID-19 and Sepsis, Antioxidants, doi:10.3390/antiox12111942.
8.
Winn et al., Effect of any form of steroids in comparison with that of other medications on the duration of olfactory dysfunction in patients with COVID-19: A systematic review of randomized trials and quasi-experimental studies, PLOS ONE, doi:10.1371/journal.pone.0288285.
9.
Schloss et al., Nutritional deficiencies that may predispose to long COVID, Inflammopharmacology, doi:10.1007/s10787-023-01183-3.
10.
Arora et al., Global Dietary and Herbal Supplement Use during COVID-19—A Scoping Review, Nutrients, doi:10.3390/nu15030771.
11.
Wang et al., Zinc and COVID-19: Immunity, Susceptibility, Severity and Intervention, Critical Reviews in Food Science and Nutrition, doi:10.1080/10408398.2022.2119932.
12.
Foshati et al., Antioxidants and clinical outcomes of patients with coronavirus disease 2019: A systematic review of observational and interventional studies, Food Science & Nutrition, doi:10.1002/fsn3.3034.
13.
DiGuilio et al., Micronutrient Improvement of Epithelial Barrier Function in Various Disease States: A Case for Adjuvant Therapy, International Journal of Molecular Sciences, doi:10.3390/ijms23062995.
14.
Wessels et al., Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of Corona Virus Disease 19, British Journal of Nutrition, doi:10.1017/S0007114521000738.
15.
Sethuram et al., Potential Role of Zinc in the COVID-19 Disease Process and its Probable Impact on Reproduction, Reproductive Sciences, doi:10.1007/s43032-020-00400-6.
16.
Joachimiak et al., Zinc against COVID-19? Symptom surveillance and deficiency risk groups, PLOS Neglected Tropical Diseases, doi:10.1371/journal.pntd.0008895.
Briassoulis et al., 31 Oct 2023, peer-reviewed, 5 authors.
Contact: briasoug@uoc.gr (corresponding author), stavroula.ilia@uoc.gr, briaspan@med.uoa.gr, med1p1130027@med.uoc.gr, ggbriass@med.uoc.gr.
The Anti-Oxidative, Anti-Inflammatory, Anti-Apoptotic, and Anti-Necroptotic Role of Zinc in COVID-19 and Sepsis
Antioxidants, doi:10.3390/antiox12111942
Zinc is a structural component of proteins, functions as a catalytic co-factor in DNA synthesis and transcription of hundreds of enzymes, and has a regulatory role in protein-DNA interactions of zinc-finger proteins. For many years, zinc has been acknowledged for its anti-oxidative and anti-inflammatory functions. Furthermore, zinc is a potent inhibitor of caspases-3, -7, and -8, modulating the caspase-controlled apoptosis and necroptosis. In recent years, the immunomodulatory role of zinc in sepsis and COVID-19 has been investigated. Both sepsis and COVID-19 are related to various regulated cell death (RCD) pathways, including apoptosis and necroptosis. Lack of zinc may have a negative effect on many immune functions, such as oxidative burst, cytokine production, chemotaxis, degranulation, phagocytosis, and RCD. While plasma zinc concentrations decline swiftly during both sepsis and COVID-19, this reduction is primarily attributed to a redistribution process associated with the inflammatory response. In this response, hepatic metallothionein production increases in reaction to cytokine release, which is linked to inflammation, and this protein effectively captures and stores zinc in the liver. Multiple regulatory mechanisms come into play, influencing the uptake of zinc, the binding of zinc to blood albumin and red blood cells, as well as the buffering and modulation of cytosolic zinc levels. Decreased zinc levels are associated with increasing severity of organ dysfunction, prolonged hospital stay and increased mortality in septic and COVID-19 patients. Results of recent studies focusing on these topics are summarized and discussed in this narrative review. Existing evidence currently does not support pharmacological zinc supplementation in patients with sepsis or COVID-19. Complementation and repletion should follow current guidelines for micronutrients in critically ill patients. Further research investigating the pharmacological mechanism of zinc in programmed cell death caused by invasive infections and its therapeutic potential in sepsis and COVID-19 could be worthwhile.
Conflicts of Interest: The authors declare no conflict of interest.
References
Adrie, Bachelet, Vayssier-Taussat, Russo-Marie, Bouchaert et al., Mitochondrial Membrane Potential and Apoptosis Peripheral Blood Monocytes in Severe Human Sepsis, Am. J. Respir. Crit. Care Med, doi:10.1164/ajrccm.164.3.2009088
Alker, Haase, Zinc and Sepsis, Nutrients, doi:10.3390/nu10080976
Almasaud, Chalabi, Arfaj, Qarni, Alkroud et al., Association of Serum Zinc and Inflammatory Markers with the Severity of COVID-19 Infection in Adult Patients, Nutrients, doi:10.3390/nu15020340
Arslan, Scheidereit, The Prevalence of TNFα-Induced Necrosis over Apoptosis Is Determined by TAK1-RIP1 Interplay, PLoS ONE, doi:10.1371/journal.pone.0026069
Aydemir, Liuzzi, Mcclellan, Cousins, Zinc Transporter ZIP8 (SLC39A8) and Zinc Influence IFN-Gamma Expression in Activated Human T Cells, J. Leukoc. Biol, doi:10.1189/jlb.1208759
Bao, Liu, Lee, Besecker, Lai et al., Zinc Modulates the Innate Immune Response in Vivo to Polymicrobial Sepsis through Regulation of NF-kappaB, Am. J. Physiol. Lung Cell. Mol. Physiol, doi:10.1152/ajplung.00368.2009
Bao, Prasad, Beck, Fitzgerald, Snell et al., Zinc Decreases C-Reactive Protein, Lipid Peroxidation, and Inflammatory Cytokines in Elderly Subjects: A Potential Implication of Zinc as an Atheroprotective Agent, Am. J. Clin. Nutr, doi:10.3945/ajcn.2009.28836
Barnett, Blindauer, Kassaar, Khazaipoul, Martin et al., Allosteric Modulation of Zinc Speciation by Fatty Acids, Biochim. Biophys. Acta, doi:10.1016/j.bbagen.2013.05.028
Beck, Prasad, Kaplan, Fitzgerald, Brewer, Changes in Cytokine Production and T Cell Subpopulations in Experimentally Induced Zinc-Deficient Humans, Am. J. Physiol, doi:10.1152/ajpendo.1997.272.6.E1002
Bednorz, Oelgeschläger, Kinnemann, Hartmann, Neumann et al., The Broader Context of Antibiotic Resistance: Zinc Feed Supplementation of Piglets Increases the Proportion of Multi-Resistant Escherichia Coli in Vivo, Int. J. Med. Microbiol. IJMM, doi:10.1016/j.ijmm.2013.06.004
Ben Abdallah, Mhalla, Trabelsi, Sekma, Youssef et al., Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am, doi:10.1093/cid/ciac807
Berger, Shenkin, Schweinlin, Amrein, Augsburger et al., None, Clin. Nutr. Edinb. Scotl, doi:10.1016/j.clnu.2022.02.015
Berger, Talwar, Shenkin, Pitfalls in the Interpretation of Blood Tests Used to Assess and Monitor Micronutrient Nutrition Status, Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr, doi:10.1002/ncp.10924
Besecker, Bao, Bohacova, Papp, Sadee et al., The Human Zinc Transporter SLC39A8 (Zip8) Is Critical in Zinc-Mediated Cytoprotection in Lung Epithelia, Am. J. Physiol. Lung Cell. Mol. Physiol, doi:10.1152/ajplung.00057.2008
Besecker, Exline, Hollyfield, Phillips, Disilvestro et al., A Comparison of Zinc Metabolism, Inflammation, and Disease Severity in Critically Ill Infected and Noninfected Adults Early after Intensive Care Unit Admission, Am. J. Clin. Nutr, doi:10.3945/ajcn.110.008417
Beyersmann, Haase, Functions of Zinc in Signaling, Proliferation and Differentiation of Mammalian Cells, Biometals Int. J. Role Met. Ions Biol. Biochem. Med, doi:10.1023/a:1012905406548
Bhakat, Mantha, Mitra, Transcriptional Regulatory Functions of Mammalian AP-Endonuclease (APE1/Ref-1), an Essential Multifunctional Protein, Antioxid. Redox Signal, doi:10.1089/ars.2008.2198
Bhatnagar, Wadhwa, Aneja, Lodha, Kabra et al., Zinc as Adjunct Treatment in Infants Aged between 7 and 120 Days with Probable Serious Bacterial Infection: A Randomised, Double-Blind, Placebo-Controlled Trial, Lancet, doi:10.1016/S0140-6736(12)60477-2
Bin, Bhin, Seo, Kim, Lee et al., Requirement of Zinc Transporter SLC39A7/ZIP7 for Dermal Development to Fine-Tune Endoplasmic Reticulum Function by Regulating Protein Disulfide Isomerase, J. Investig. Dermatol, doi:10.1016/j.jid.2017.03.031
Bin, Seo, Kim, Function, Structure, and Transport Aspects of ZIP and ZnT Zinc Transporters in Immune Cells, J. Immunol. Res, doi:10.1155/2018/9365747
Black, Zinc Deficiency, Infectious Disease and Mortality in the Developing World, J. Nutr, doi:10.1093/jn/133.5.1485S
Blindauer, Harvey, Bunyan, Stewart, Sleep et al., Structure, Properties, and Engineering of the Major Zinc Binding Site on Human Albumin, J. Biol. Chem, doi:10.1074/jbc.M109.003459
Bolognese, Yang, Hansen, Denning, Nicastro et al., Inhibition of Necroptosis Attenuates Lung Injury and Improves Survival in Neonatal Sepsis, Surgery, doi:10.1016/j.surg.2018.02.017
Bonaventura, Benedetti, Albarède, Miossec, Zinc and Its Role in Immunity and Inflammation, Autoimmun. Rev, doi:10.1016/j.autrev.2014.11.008
Boone, Turer, Lee, Ahmad, Wheeler et al., The Ubiquitin-Modifying Enzyme A20 Is Required for Termination of Toll-like Receptor Responses, Nat. Immunol, doi:10.1038/ni1110
Boudreault, Pinilla-Vera, Englert, Kho, Isabelle et al., Zinc Deficiency Primes the Lung for Ventilator-Induced Injury, JCI Insight, doi:10.1172/jci.insight.86507
Bray, Bettger, The Physiological Role of Zinc as an Antioxidant, Free Radic. Biol. Med, doi:10.1016/0891-5849(90)90076-U
Briassoulis, Briassoulis, Ilia, If, Get, Good Nutrition, You Will Become Happy; If You Get a Bad One, You Will Become an ICU Philosopher, Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc, doi:10.1097/PCC.0000000000001774
Briassoulis, Briassoulis, Ilia, Nutrition Is More Than the Sum of Its Parts, Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc, doi:10.1097/PCC.0000000000001717
Briassoulis, Ilia, Vitamin D Deficiency in Sepsis: "Body Humors" Imbalance or Sepsis "Epiphenomenon"?, Crit. Care Med, doi:10.1097/CCM.0000000000002122
Bryan, Baregzay, Spicer, Singal, Khaper, Redox-Inflammatory Synergy in the Metabolic Syndrome, Can. J. Physiol. Pharmacol, doi:10.1139/cjpp-2012-0295
Cai, Li, Zhou, Wang, Li et al., Staphylococcus Aureus Facilitates Its Survival in Bovine Macrophages by Blocking Autophagic Flux, J. Cell. Mol. Med, doi:10.1111/jcmm.15027
Cander, Dundar, Gul, Girisgin, Prognostic Value of Serum Zinc Levels in Critically Ill Patients, J. Crit. Care, doi:10.1016/j.jcrc.2010.06.002
Carcillo, Dean, Holubkov, Berger, Meert et al., The Randomized Comparative Pediatric Critical Illness Stress-Induced Immune Suppression (CRISIS) Prevention Trial, Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc, doi:10.1097/PCC.0b013e31823896ae
Catrysse, Vereecke, Beyaert, Van Loo, A20 in Inflammation and Autoimmunity, Trends Immunol, doi:10.1016/j.it.2013.10.005
Chai, Zakrzewski, Günzel, Pieper, Wang et al., High-Dose Dietary Zinc Oxide Mitigates Infection with Transmissible Gastroenteritis Virus in Piglets, BMC Vet. Res, doi:10.1186/1746-6148-10-75
Chan, Luz, Moriwaki, Programmed Necrosis in the Cross Talk of Cell Death and Inflammation, Annu. Rev. Immunol, doi:10.1146/annurev-immunol-032414-112248
Cheng, Abrams, Toh, Wang, Wang et al., The Critical Roles and Mechanisms of Immune Cell Death in Sepsis, Front. Immunol, doi:10.3389/fimmu.2020.01918
Chinni, El-Khoury, Perera, Bellomo, Jones et al., Zinc Supplementation as an Adjunct Therapy for COVID-19: Challenges and Opportunities, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14826
Choi, Liu, Pan, Zinc Deficiency and Cellular Oxidative Stress: Prognostic Implications in Cardiovascular Diseases, Acta Pharmacol. Sin, doi:10.1038/aps.2018.25
Choi, Price, Ryter, Choi, Necroptosis: A Crucial Pathogenic Mediator of Human Disease, JCI Insight, doi:10.1172/jci.insight.128834
Choi, Yoo, Lee, Park, Jeon, Plasma Mitochondrial DNA and Necroptosis as Prognostic Indicators in Critically Ill Patients with Sepsis, Biomedicines, doi:10.3390/biomedicines10102386
Chousterman, Swirski, Weber, Cytokine Storm and Sepsis Disease Pathogenesis, Semin. Immunopathol, doi:10.1007/s00281-017-0639-8
Christian, Smith, Carmody, The Regulation of NF-κB Subunits by Phosphorylation, Cells, doi:10.3390/cells5010012
Clegg, Hanna, Niles, Momma, Keen, Zinc Deficiency-Induced Cell Death, IUBMB Life, doi:10.1080/15216540500264554
Colvin, Bush, Volitakis, Fontaine, Thomas et al., Insights into Zn2+ Homeostasis in Neurons from Experimental and Modeling Studies, Am. J. Physiol. Cell Physiol, doi:10.1152/ajpcell.00541.2007
Colvin, Holmes, Fontaine, Maret, Cytosolic Zinc Buffering and Muffling: Their Role in Intracellular Zinc Homeostasis, Met. Integr. Biometal Sci, doi:10.1039/b926662c
Cousins, Gastrointestinal Factors Influencing Zinc Absorption and Homeostasis, Int. J. Vitam. Nutr. Res. Int. Z. Vitam. Ernahrungsforschung J. Int. Vitaminol. Nutr, doi:10.1024/0300-9831/a000030
Cousins, Leinart, Tissue-Specific Regulation of Zinc Metabolism and Metallothionein Genes by Interleukin 1, FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol, doi:10.1096/fasebj.2.13.2458983
Cvijanovich, King, Flori, Gildengorin, Vinks et al., Safety and Dose Escalation Study of Intravenous Zinc Supplementation in Pediatric Critical Illness, JPEN J. Parenter. Enteral Nutr, doi:10.1177/0148607115572193
Demircan, Chillon, Bracken, Bulgarelli, Campi et al., Association of COVID-19 Mortality with Serum Selenium, Zinc and Copper: Six Observational Studies across Europe, Front. Immunol, doi:10.3389/fimmu.2022.1022673
Devaux, Lagier, Raoult, New Insights Into the Physiopathology of COVID-19: SARS-CoV-2-Associated Gastrointestinal Illness, Front. Med, doi:10.3389/fmed.2021.640073
Do Marreiro, Cruz, Morais, Beserra, Severo et al., Zinc and Oxidative Stress: Current Mechanisms, Antioxidants, doi:10.3390/antiox6020024
Dresen, Pimiento, Patel, Heyland, Rice et al., Overview of Oxidative Stress and the Role of Micronutrients in Critical Illness, JPEN J. Parenter. Enter. Nutr, doi:10.1002/jpen.2421
Duncan, Talwar, Mcmillan, Stefanowicz, O'reilly, Quantitative Data on the Magnitude of the Systemic Inflammatory Response and Its Effect on Micronutrient Status Based on Plasma Measurements, Am. J. Clin. Nutr, doi:10.3945/ajcn.111.023812
Dziedzic, Su, Jean Barrett, Najafov, Mookhtiar et al., ABIN-1 Regulates RIPK1 Activation by Linking Met1 Ubiquitylation with Lys63 Deubiquitylation in TNF-RSC, Nat. Cell Biol, doi:10.1038/s41556-017-0003-1
Dørup, Flyvbjerg, Everts, Clausen, Role of Insulin-like Growth Factor-1 and Growth Hormone in Growth Inhibition Induced by Magnesium and Zinc Deficiencies, Br. J. Nutr, doi:10.1079/BJN19910051
Eide, The Oxidative Stress of Zinc Deficiency, Met. Integr. Biometal Sci, doi:10.1039/c1mt00064k
El-Baset, Mazen, Abdul-Maksoud, Kattaia, The Therapeutic Prospect of Zinc Oxide Nanoparticles in Experimentally Induced Diabetic Nephropathy, Tissue Barriers, doi:10.1080/21688370.2022.2069966
El-Gindy, Zahran, Ahmed, Ali, Mohamed et al., Counteract Severe Heat Stress by Including Different Forms of Zinc in the Rabbit Bucks, Diet. Sci. Rep, doi:10.1038/s41598-023-39928-3
El-Kossi, Ibrahim, Hassanin, Hamad, Rashed et al., Ameliorative Effects of Zinc Oxide, in Either Conventional or Nanoformulation, Against Bisphenol A Toxicity on Reproductive Performance, Oxidative Status, Gene Expression and Histopathology in Adult Male Rats, Biol. Trace Elem. Res, doi:10.1007/s12011-023-03830-w
Elmes, Jones, Paneth, Cell Zinc: A Comparison of Histochemical and Microanalytical Techniques, Histochem. J, doi:10.1007/BF01006887
Evans, Ovaa, Hamon, Kilshaw, Hamm et al., Zinc-Finger Protein A20, a Regulator of Inflammation and Cell Survival, Has de-Ubiquitinating Activity, Biochem. J, doi:10.1042/bj20031377
Fauster, Rebsamen, Willmann, César-Razquin, Girardi et al., Systematic Genetic Mapping of Necroptosis Identifies SLC39A7 as Modulator of Death Receptor Trafficking, Cell Death Differ, doi:10.1038/s41418-018-0192-6
Fenech, Bull, Van Klinken, Protective Effects of Micronutrient Supplements, Phytochemicals and Phytochemical-Rich Beverages and Foods Against DNA Damage in Humans: A Systematic Review of Randomized Controlled Trials and Prospective Studies, Adv. Nutr, doi:10.1016/j.advnut.2023.08.004
Feng, Wu, Duan, Wang, Zhong et al., Structural Characterization and Anti-Inflammatory Effects of Enteromorpha Prolifera Polysaccharide-Fe/Zn Complexes, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2023.127166
Fleischmann-Struzek, Mellhammar, Rose, Cassini, Rudd et al., Incidence and Mortality of Hospital-and ICU-Treated Sepsis: Results from an Updated and Expanded Systematic Review and Meta-Analysis, Intensive Care Med, doi:10.1007/s00134-020-06151-x
Fraker, King, Reprogramming of the Immune System during Zinc Deficiency, Annu. Rev. Nutr, doi:10.1146/annurev.nutr.24.012003.132454
Galluzzi, Vitale, Aaronson, Abrams, Adam et al., Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018, Cell Death Differ, doi:10.1038/s41418-017-0012-4
Gammoh, Rink, Zinc in Infection and Inflammation, Nutrients, doi:10.3390/nu9060624
Geng, Ito, Shi, Amin, Chu et al., Regulation of RIPK1 Activation by TAK1-Mediated Phosphorylation Dictates Apoptosis and Necroptosis, Nat. Commun, doi:10.1038/s41467-017-00406-w
Grzywacz, Gdula-Argasi Ńska, Muszy Ńska, Tyszka-Czochara, Librowski et al., Metal Responsive Transcription Factor 1 (MTF-1) Regulates Zinc Dependent Cellular Processes at the Molecular Level, Acta Biochim. Pol, doi:10.18388/abp.2015_1038
Gudivada, Kumar, Sriram, Baby, Shariff et al., Antioxidant Micronutrient Supplements for Adult Critically Ill Patients: A Bayesian Multiple Treatment Comparisons Meta-Analysis, Clin. Nutr. ESPEN, doi:10.1016/j.clnesp.2021.12.015
Gumus, Gulbahce-Mutlu, Unal, Baltaci, Unlukal et al., Marginal Maternal Zinc Deficiency Produces Liver Damage and Altered Zinc Transporter Expression in Offspring Male Rats, Biol. Trace Elem. Res, doi:10.1007/s12011-023-03824-8
Gurung, Man, Kanneganti, A20 Is a Regulator of Necroptosis, Nat. Immunol, doi:10.1038/ni.3174
Guttek, Reinhold, Grüngreiff, Schraven, Reinhold, Zinc Aspartate Induces Proliferation of Resting and Antigen-Stimulated Human PBMC under High-Density Cell Culture Condition, J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS, doi:10.1016/j.jtemb.2023.127152
Haase, Rink, The Immune System and the Impact of Zinc during Aging, Immun. Ageing A, doi:10.1186/1742-4933-6-9
Hambidge, Micronutrient Bioavailability: Dietary Reference Intakes and a Future Perspective, Am. J. Clin. Nutr, doi:10.3945/ajcn.2010.28674B
Hambidge, Miller, Westcott, Sheng, Krebs, Zinc Bioavailability and Homeostasis, Am. J. Clin. Nutr, doi:10.3945/ajcn.2010.28674I
Han, Boyle, Manning, Firestein, AP-1 and NF-kappaB Regulation in Rheumatoid Arthritis and Murine Collagen-Induced Arthritis, Autoimmunity, doi:10.3109/08916939808995367
Handing, Shabalin, Kassaar, Khazaipoul, Blindauer et al., Circulatory Zinc Transport Is Controlled by Distinct Interdomain Sites on Mammalian Albumins, Chem. Sci, doi:10.1039/C6SC02267G
Harrington, Choi, Nakahira, Mitochondrial DNA in Sepsis, Curr. Opin. Crit. Care, doi:10.1097/MCC.0000000000000427
Hasan, Rink, Haase, Chelation of Free Zn 2+ Impairs Chemotaxis, Phagocytosis, Oxidative Burst, Degranulation, and Cytokine Production by Neutrophil Granulocytes, Biol. Trace Elem. Res, doi:10.1007/s12011-015-0515-0
He, Yuan, Zuo, Li, Sun et al., Berberine Induces ZIP14 Expression and Modulates Zinc Redistribution to Protect Intestinal Mucosal Barrier during Polymicrobial Sepsis, Life Sci, doi:10.1016/j.lfs.2019.116697
Heidarvand, Hosseini, Kazemi, Andalib, Sami et al., Differentially Expressed Inflammatory Cell Death-Related Genes and the Serum Levels of IL-6 Are Determinants for Severity of Coronaviruses Diseases-2019 (COVID-19), Adv. Biomed. Res, doi:10.4103/abr.abr_232_22
Heller, Sun, Hackler, Seelig, Seibert et al., Prediction of Survival Odds in COVID-19 by Zinc, Age and Selenoprotein P as Composite Biomarker, Redox Biol, doi:10.1016/j.redox.2020.101764
Heyland, Jones, Cvijanovich, Wong, Zinc Supplementation in Critically Ill Patients: A Key Pharmaconutrient?, JPEN J. Parenter. Enteral Nutr, doi:10.1177/0148607108322402
Heyninck, De Valck, Vanden Berghe, Van Criekinge, Contreras et al., The Zinc Finger Protein A20 Inhibits TNF-Induced NF-kappaB-Dependent Gene Expression by Interfering with an RIP-or TRAF2-Mediated Transactivation Signal and Directly Binds to a Novel NF-kappaB-Inhibiting Protein ABIN, J. Cell Biol, doi:10.1083/jcb.145.7.1471
Hoeger, Simon, Beeker, Marx, Haase et al., Persistent Low Serum Zinc Is Associated with Recurrent Sepsis in Critically Ill Patients-A Pilot Study, PLoS ONE, doi:10.1371/journal.pone.0176069
Holly, Smith, Paneth, Cells during Viral Infection and Pathogenesis, Viruses, doi:10.3390/v10050225
Imran, Fatima, Alzahrani, Suhail, Alshammari et al., Development of Therapeutic and Prophylactic Zinc Compositions for Use against COVID-19: A Glimpse of the Trends, Inventions, and Patents, Nutrients, doi:10.3390/nu14061227
Iommarini, Porcelli, Gasparre, Kurelac, Non-Canonical Mechanisms Regulating Hypoxia-Inducible Factor 1 Alpha in Cancer, Front. Oncol, doi:10.3389/fonc.2017.00286
Ischia, Bolton, Patel, Why Is It Worth Testing the Ability of Zinc to Protect against Ischaemia Reperfusion Injury for Human Application, Met. Integr. Biometal Sci, doi:10.1039/c9mt00079h
Iwata, Incefy, Tanaka, Fernandes, Menendez-Botet et al., Circulating Thymic Hormone Levels in Zinc Deficiency, Cell. Immunol, doi:10.1016/0008-8749(79)90318-6
Jarosz, Olbert, Wyszogrodzka, Młyniec, Librowski, Antioxidant and Anti-Inflammatory Effects of Zinc. Zinc-Dependent NF-κB Signaling, Inflammopharmacology, doi:10.1007/s10787-017-0309-4
Jimenez-Rondan, Ruggiero, Mckinley, Koh, Roberts et al., Enterocyte-Specific Deletion of Metal Transporter Zip14 (Slc39a14) Alters Intestinal Homeostasis through Epigenetic Mechanisms, Am. J. Physiol. Gastrointest. Liver Physiol, doi:10.1152/ajpgi.00244.2022
Jorgensen, Rayamajhi, Miao, Programmed Cell Death as a Defence against Infection, Nat. Rev. Immunol, doi:10.1038/nri.2016.147
Jothimani, Kailasam, Danielraj, Nallathambi, Ramachandran et al., COVID-19: Poor Outcomes in Patients with Zinc Deficiency, Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis, doi:10.1016/j.ijid.2020.09.014
Kadac-Czapska, Knez, Grembecka, Food and Human Safety: The Impact of Microplastics, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2022.2132212
Kang, Wang, Potential Therapeutic Value of Necroptosis Inhibitor for the Treatment of COVID-19, Eur. J. Med. Res, doi:10.1186/s40001-022-00913-7
Karakike, Giamarellos-Bourboulis, Kyprianou, Fleischmann-Struzek, Pletz et al., Coronavirus Disease 2019 as Cause of Viral Sepsis: A Systematic Review and Meta-Analysis, Crit. Care Med, doi:10.1097/CCM.0000000000005195
Keen, Gershwin, Zinc Deficiency and Immune Function, Annu. Rev. Nutr, doi:10.1146/annurev.nu.10.070190.002215
Kim, Kim, Lee, Ahn, Zinc-Induced NF-kappaB Inhibition Can Be Modulated by Changes in the Intracellular Metallothionein Level, Toxicol. Appl. Pharmacol, doi:10.1016/S0041-008X(03)00167-4
King, Cidlowski, Cell Cycle Regulation and Apoptosis, Annu. Rev. Physiol, doi:10.1146/annurev.physiol.60.1.601
King, Osati-Ashtiani, Fraker, Apoptosis Plays a Distinct Role in the Loss of Precursor Lymphocytes during Zinc Deficiency in Mice, J. Nutr, doi:10.1093/jn/132.5.974
Kitala, Tanski, Godlewski, Krajewska-Włodarczyk, Gromadzi Ński et al., Copper and Zinc Particles as Regulators of Cardiovascular System Function-A Review, Nutrients, doi:10.3390/nu15133040
Knez, Boy, Existing Knowledge on Zn Status Biomarkers (1963-2021) with a Particular Focus on FADS1 and FADS2 Diagnostic Performance and Recommendations for Further Research, Front. Nutr, doi:10.3389/fnut.2022.1057156
Koekkoek, Berger, An Update on Essential Micronutrients in Critical Illness, Curr. Opin. Crit. Care, doi:10.1097/MCC.0000000000001062
Koekkoek, Van Zanten, Antioxidant Vitamins and Trace Elements in Critical Illness, Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr, doi:10.1177/0884533616653832
Kohlgrüber, Upadhye, Dyballa-Rukes, Mcnamara, Altschmied, Regulation of Transcription Factors by Reactive Oxygen Species and Nitric Oxide in Vascular Physiology and Pathology, Antioxid. Redox Signal, doi:10.1089/ars.2016.6946
Koner, Banerjee, Hasan, Saha, Antioxidant Activity of Endogenously Produced Nitric Oxide against the Zinc Oxide Nanoparticle-Induced Oxidative Stress in Primary Hepatocytes of Air-Breathing Catfish, Clarias Magur, Nitric Oxide Biol. Chem, doi:10.1016/j.niox.2018.12.010
Kubota, Kuroda, Sone, Neuropsychiatric Aspects of Long COVID: A Comprehensive Review, Psychiatry Clin. Neurosci, doi:10.1111/pcn.13508
Kumar, Kumar, Singh, Gite, Patole et al., Exploring Mitigating Role of Zinc Nanoparticles on Arsenic, Ammonia and Temperature Stress Using Molecular Signature in Fish, J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS, doi:10.1016/j.jtemb.2022.127076
Kumar, Rajagopalan, Sarkar, Dorward, Peterson et al., Zinc-Induced Polymerization of Killer-Cell Ig-like Receptor into Filaments Promotes Its Inhibitory Function at Cytotoxic Immunological Synapses, Mol. Cell, doi:10.1016/j.molcel.2016.03.009
Kwak, Choi, Kim, Lee, Park et al., SARS-CoV-2 Infection Induces HMGB1 Secretion Through Post-Translational Modification and PANoptosis, Immune Netw, doi:10.4110/in.2023.23.e26
Lahiri, Abraham, Activation of Pattern Recognition Receptors Up-Regulates Metallothioneins, Thereby Increasing Intracellular Accumulation of Zinc, Autophagy, and Bacterial Clearance by Macrophages, Gastroenterology, doi:10.1053/j.gastro.2014.06.024
Laity, Lee, Wright, Zinc Finger Proteins: New Insights into Structural and Functional Diversity, Curr. Opin. Struct. Biol, doi:10.1016/S0959-440X(00)00167-6
Lee, Bang, Lee, Lee, Kang et al., Serum Concentrations of Trace Elements Zinc, Copper, Selenium, and Manganese in Critically Ill Patients, Biol. Trace Elem. Res, doi:10.1007/s12011-018-1429-4
Legarda-Addison, Hase, O'donnell, Ting, NEMO/IKKgamma Regulates an Early NF-kappaB-Independent Cell-Death Checkpoint during TNF Signaling, Cell Death Differ, doi:10.1038/cdd.2009.41
Li, Ma, Zhu, Zhang, Zhao et al., Zinc Improves Neurological Recovery by Promoting Angiogenesis via the Astrocyte-Mediated HIF-1α/VEGF Signaling Pathway in Experimental Stroke, CNS Neurosci. Ther, doi:10.1111/cns.13918
Li, Mcquade, Siemer, Napetschnig, Moriwaki et al., The RIP1/RIP3 Necrosome Forms a Functional Amyloid Signaling Complex Required for Programmed Necrosis, Cell, doi:10.1016/j.cell.2012.06.019
Liang, Xiang, Yang, Zhang, Guo et al., ZnT7 Can Protect MC3T3-E1 Cells from Oxidative Stress-Induced Apoptosis via PI3K/Akt and MAPK/ERK Signaling Pathways, Cell. Signal, doi:10.1016/j.cellsig.2013.02.003
Linko, Karlsson, Pettilä, Varpula, Okkonen et al., Serum Zinc in Critically Ill Adult Patients with Acute Respiratory Failure, Acta Anaesthesiol. Scand, doi:10.1111/j.1399-6576.2011.02425.x
Liu, Bao, Gálvez-Peralta, Pyle, Rudawsky et al., ZIP8 Regulates Host Defense through Zinc-Mediated Inhibition of NF-κB, Cell Rep, doi:10.1016/j.celrep.2013.01.009
Liu, Liu, Wu, Xi, Wei et al., Zinc Supplementation Protects against Diabetic Endothelial Dysfunction via GTP Cyclohydrolase 1 Restoration, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2019.11.046
Liu, Wen, Shao, Cui, Tang et al., ATF4 Knockdown in Macrophage Impairs Glycolysis and Mediates Immune Tolerance by Targeting HK2 and HIF-1α Ubiquitination in Sepsis, Clin. Immunol, doi:10.1016/j.clim.2023.109698
Liu, Xu, Wang, Liang, Li et al., Necroptosis Is Active and Contributes to Intestinal Injury in a Piglet Model with Lipopolysaccharide Challenge, Cell Death Dis, doi:10.1038/s41419-020-03365-1
Liuzzi, Lichten, Rivera, Blanchard, Aydemir et al., Interleukin-6 Regulates the Zinc Transporter Zip14 in Liver and Contributes to the Hypozincemia of the Acute-Phase Response, Proc. Natl. Acad. Sci, doi:10.1073/pnas.0502257102
Livingstone, Zinc, Physiology, Deficiency, and Parenteral Nutrition, Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr, doi:10.1177/0884533615570376
Lu, Haragopal, Slepchenko, Stork, Li, Intracellular Zinc Distribution in Mitochondria, ER and the Golgi Apparatus, Int. J. Physiol. Pathophysiol. Pharmacol
Maares, Haase, Zinc and Immunity: An Essential Interrelation, Arch. Biochem. Biophys, doi:10.1016/j.abb.2016.03.022
Malyar, Li, Liu, Abdulrahim, Farid et al., Selenium/Zinc-Enriched Probiotics Improve Serum Enzyme Activity, Antioxidant Ability, Inflammatory Factors and Related Gene Expression of Wistar Rats Inflated under Heat Stress, Life Sci, doi:10.1016/j.lfs.2020.117464
Mantzarlis, Tsolaki, Zakynthinos, Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies, Oxid. Med. Cell. Longev, doi:10.1155/2017/5985209
Mao, Li, Zhang, Wang, Liu, Plasma Mitochondrial DNA Levels Are Associated with Acute Lung Injury and Mortality in Septic Patients, BMC Pulm. Med, doi:10.1186/s12890-021-01437-2
Maret, Krezel, Cellular Zinc and Redox Buffering Capacity of Metallothionein/Thionein in Health and Disease, Mol. Med. Camb. Mass, doi:10.2119/2007-00036.Maret
Maret, Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life, Adv. Nutr, doi:10.3945/an.112.003038
Marino, Valla, Tume, Jotterand-Chaparro, Moullet et al., Considerations for Nutrition Support in Critically Ill Children with COVID-19 and Paediatric Inflammatory Multisystem Syndrome Temporally Associated with COVID-19, Clin. Nutr, doi:10.1016/j.clnu.2020.10.007
Martens, Hofmans, Declercq, Augustyns, Vandenabeele, Inhibitors Targeting RIPK1/RIPK3: Old and New Drugs, Trends Pharmacol. Sci, doi:10.1016/j.tips.2020.01.002
Martinez, Campa, Li, Fleetwood, Stewart et al., Low Plasma Zinc Is Associated with Higher Mitochondrial Oxidative Stress and Faster Liver Fibrosis Development in the Miami Adult Studies in HIV Cohort, J. Nutr, doi:10.3945/jn.116.243832
Mcneal, Legolvan, Chung, Ayala, The Dual Functions of Receptor Interacting Protein 1 in Fas-Induced Hepatocyte Death during Sepsis, Shock, doi:10.1097/SHK.0b013e31820b2db1
Miliaraki, Briassoulis, Ilia, Michalakakou, Karakonstantakis et al., Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations, Antioxidants, doi:10.3390/antiox11020231
Miliaraki, Briassoulis, Ilia, Polonifi, Mantzourani et al., Survivin and Caspases Serum Protein Levels and Survivin Variants mRNA Expression in Sepsis, Sci. Rep, doi:10.1038/s41598-020-78208-2
Mittal, Siddiqui, Tran, Reddy, Malik, Reactive Oxygen Species in Inflammation and Tissue Injury, Antioxid. Redox Signal, doi:10.1089/ars.2012.5149
Miyai, Hojyo, Ikawa, Kawamura, Irié et al., Zinc Transporter SLC39A10/ZIP10 Facilitates Antiapoptotic Signaling during Early B-Cell Development, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1323549111
Mocchegiani, Malavolta, Dyshomeostasis, Ageing and Neurodegeneration: Implications of A2M and Inflammatory Gene Polymorphisms, J. Alzheimers Dis. JAD, doi:10.3233/JAD-2007-12110
Nagar, Piao, Kim, Role of Mitochondrial Oxidative Stress in Sepsis, Acute Crit. Care, doi:10.4266/acc.2018.00157
Najafov, Luu, Mookhtiar, Mifflin, Xia et al., RIPK1 Promotes Energy Sensing by the mTORC1 Pathway, Mol. Cell, doi:10.1016/j.molcel.2020.11.008
Najjar, Saleh, Zelic, Nogusa, Shah et al., RIPK1 and RIPK3 Kinases Promote Cell-Death-Independent Inflammation by Toll-like Receptor 4, Immunity, doi:10.1016/j.immuni.2016.06.007
Neumann, Bruns, Rohde, Prajsnar, Foster et al., Intracellular Staphylococcus Aureus Eludes Selective Autophagy by Activating a Host Cell Kinase, Autophagy, doi:10.1080/15548627.2016.1226732
Newton, Wickliffe, Dugger, Maltzman, Roose-Girma et al., Cleavage of RIPK1 by Caspase-8 Is Crucial for Limiting Apoptosis and Necroptosis, Nature, doi:10.1038/s41586-019-1548-x
Nouira, Evaluation of the Efficacy and Safety of Zinc in Viral Infections; clinicaltrials
Ohashi, Kimura, Iwanaga, Furusawa, Irié et al., Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress, PLoS Genet, doi:10.1371/journal.pgen.1006349
Oshima, Turer, Callahan, Chai, Advincula et al., ABIN-1 Is a Ubiquitin Sensor That Restricts Cell Death and Sustains Embryonic Development, Nature, doi:10.1038/nature07575
Osman, Zinc Supplementation in Pediatric Sepsis; a Randomized Controlled Trial; clinicaltrials
Osuchowski, Winkler, Skirecki, Cajander, Shankar-Hari et al., The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00218-6
Palacios, Ramón-Luing, Ruiz, García-Martínez, Sánchez-Monciváis et al., COVID-19 Patients with High TNF/IFN-γ Levels Show Hallmarks of PANoptosis, an Inflammatory Cell Death, Microbes Infect, doi:10.1016/j.micinf.2023.105179
Pan, Mu, Yang, Sun, Wang et al., Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study, Am. J. Gastroenterol, doi:10.14309/ajg.0000000000000620
Pan, Sun, Chen, Tian, Chen et al., Immune Effects of PI3K/Akt/HIF-1α-Regulated Glycolysis in Polymorphonuclear Neutrophils during Sepsis, Crit. Care, doi:10.1186/s13054-022-03893-6
Partap, Sharma, Marathe, Wang, Shaikh et al., Vitamin D and Zinc Supplementation to Improve Treatment Outcomes among COVID-19 Patients in India: Results from a Double-Blind Randomized Placebo-Controlled Trial, Curr. Dev. Nutr, doi:10.1016/j.cdnut.2023.101971
Patel, Chinni, El-Khoury, Perera, Neto et al., A Pilot Double-Blind Safety and Feasibility Randomized Controlled Trial of High-Dose Intravenous Zinc in Hospitalized COVID-19 Patients, J. Med. Virol, doi:10.1002/jmv.26895
Patel, Webster, Varfolomeev, Kwon, Cheng et al., RIP1 Inhibition Blocks Inflammatory Diseases but Not Tumor Growth or Metastases, Cell Death Differ, doi:10.1038/s41418-019-0347-0
Peng, Liao, Qin, Zhu, Peng et al., Regulated Cell Death (RCD) in Cancer: Key Pathways and Targeted Therapies, Signal Transduct. Target. Ther, doi:10.1038/s41392-022-01110-y
Perkins, Integrating Cell-Signalling Pathways with NF-kappaB and IKK Function, Nat. Rev. Mol. Cell Biol, doi:10.1038/nrm2083
Pinti, Cevenini, Nasi, De Biasi, Salvioli et al., Circulating Mitochondrial DNA Increases with Age and Is a Familiar Trait: Implications for "Inflamm-Aging, Eur. J. Immunol, doi:10.1002/eji.201343921
Player, Torrence, The 2-5A System: Modulation of Viral and Cellular Processes through Acceleration of RNA Degradation, Pharmacol. Ther, doi:10.1016/S0163-7258(97)00167-8
Podany, Wright, Lamendella, Soybel, Kelleher, ZnT2-Mediated Zinc Import Into Paneth Cell Granules Is Necessary for Coordinated Secretion and Paneth Cell Function in Mice, Cell. Mol. Gastroenterol. Hepatol, doi:10.1016/j.jcmgh.2015.12.006
Polykratis, Hermance, Zelic, Roderick, Kim et al., Cutting Edge: RIPK1 Kinase Inactive Mice Are Viable and Protected from TNF-Induced Necroptosis in Vivo, J. Immunol, doi:10.4049/jimmunol.1400590
Polykratis, Martens, Eren, Shirasaki, Yamagishi et al., A20 Prevents Inflammasome-Dependent Arthritis by Inhibiting Macrophage Necroptosis through Its ZnF7 Ubiquitin-Binding Domain, Nat. Cell Biol, doi:10.1038/s41556-019-0324-3
Prasad, Bao, Beck, Sarkar, Zinc-Suppressed Inflammatory Cytokines by Induction of A20-Mediated Inhibition of Nuclear Factor-κB, Nutrition, doi:10.1016/j.nut.2010.08.010
Prasad, Beck, Bao, Fitzgerald, Snell et al., Zinc Supplementation Decreases Incidence of Infections in the Elderly: Effect of Zinc on Generation of Cytokines and Oxidative Stress, Am. J. Clin. Nutr, doi:10.1093/ajcn/85.3.837
Prasad, Beck, Bao, Snell, Fitzgerald, Duration and Severity of Symptoms and Levels of Plasma Interleukin-1 Receptor Antagonist, Soluble Tumor Necrosis Factor Receptor, and Adhesion Molecules in Patients with Common Cold Treated with Zinc Acetate, J. Infect. Dis, doi:10.1086/528803
Prasad, Effects of Zinc Deficiency on Th1 and Th2 Cytokine Shifts, J. Infect. Dis, doi:10.1086/315916
Prasad, Zinc Is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health, Front. Nutr, doi:10.3389/fnut.2014.00014
Qu, Wang, Qiu, Zhu, Guo et al., Pyroptosis, Ferroptosis in Sepsis and Treatment, Shock, doi:10.1097/SHK.0000000000001936
Rahimi, Mohammad, Zarei, Shokoohi, Oskoueian et al., Zinc Oxide Nanoparticles Synthesized Using Hyssopus officinalis L. Extract Induced Oxidative Stress and Changes the Expression of Key Genes Involved in Inflammatory and Antioxidant Systems, Biol. Res, doi:10.1186/s40659-022-00392-4
Rahman, Idid, Can Zn Be a Critical Element in COVID-19 Treatment?, Biol. Trace Elem. Res, doi:10.1007/s12011-020-02194-9
Ranaldi, Ferruzza, Canali, Leoni, Zalewski et al., Intracellular Zinc Is Required for Intestinal Cell Survival Signals Triggered by the Inflammatory Cytokine TNFα, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2012.06.020
Rao, Sethi, Ischia, Gibson, Galea et al., Protective Effect of Zinc Preconditioning against Renal Ischemia Reperfusion Injury Is Dose Dependent, PLoS ONE, doi:10.1371/journal.pone.0180028
Read, Obeid, Ahlenstiel, Ahlenstiel, The Role of Zinc in Antiviral Immunity, Adv. Nutr, doi:10.1093/advances/nmz013
Reiterer, Toborek, Hennig, Peroxisome Proliferator Activated Receptors Alpha and Gamma Require Zinc for Their Anti-Inflammatory Properties in Porcine Vascular Endothelial Cells, J. Nutr, doi:10.1093/jn/134.7.1711
Ri, Trace Metals in Endocrinology, Med. Clin. N. Am, doi:10.1016/s0025-7125(16)31861-2
Riegler, Benson, Long, Leal, Differential Activation of Programmed Cell Death in Patients with Severe SARS-CoV-2 Infection, Res. Sq, doi:10.21203/rs.3.rs-3059466/v1
Roscioli, Hamon, Lester, Murgia, Grant et al., Zinc-Rich Inhibitor of Apoptosis Proteins (IAPs) as Regulatory Factors in the Epithelium of Normal and Inflamed Airways, Biometals Int. J. Role Met. Ions Biol. Biochem. Med, doi:10.1007/s10534-013-9618-2
Rowe, Bobilya, Albumin Facilitates Zinc Acquisition by Endothelial Cells, Proc. Soc. Exp. Biol. Med, doi:10.1046/j.1525-1373.2000.22418.x
Rusch, Zhou, Silverman, Caspase-Dependent Apoptosis by 2 ,5 -Oligoadenylate Activation of RNase L Is Enhanced by IFN-Beta, J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res, doi:10.1089/107999000750053762
Sakaguchi, Iizuka, Furusawa, Ishikawa, Satoh et al., Role of Zn(2+) in Oxidative Stress Caused by Endotoxin Challenge, Eur. J. Pharmacol, doi:10.1016/S0014-2999(02)02223-9
Salzano, Saracino, Cardillo, Possible Adrenal Involvement in Long COVID Syndrome, Med. Kaunas Lith, doi:10.3390/medicina57101087
Scarpellini, Balsiger, Maurizi, Rinninella, Gasbarrini et al., Zinc and Gut Microbiota in Health and Gastrointestinal Disease under the COVID-19 Suggestion, BioFactors, doi:10.1002/biof.1829
Schifanella, Anderson, Wieking, Southern, Antinori et al., The Defenders of the Alveolus Succumb in COVID-19 Pneumonia to SARS-CoV-2 and Necroptosis, Pyroptosis, and PANoptosis, J. Infect. Dis, doi:10.1093/infdis/jiad056
Schloss, Nutritional Deficiencies That May Predispose to Long COVID, Inflammopharmacology, doi:10.1007/s10787-023-01183-3
Segars, Katler, Mcqueen, Kotlyar, Glenn et al., Prior and Novel Coronaviruses, Coronavirus Disease 2019 (COVID-19), and Human Reproduction: What Is Known?, Fertil. Steril, doi:10.1016/j.fertnstert.2020.04.025
Sekler, Sensi, Hershfinkel, Silverman, Mechanism and Regulation of Cellular Zinc Transport, Mol. Med. Camb. Mass, doi:10.2119/2007-00037.Sekler
Shan, Pan, Najafov, Yuan, Necroptosis in Development and Diseases, Genes Dev, doi:10.1101/gad.312561.118
Shankar, Prasad, Zinc and Immune Function: The Biological Basis of Altered Resistance to Infection, Am. J. Clin. Nutr, doi:10.1093/ajcn/68.2.447S
Shea-Budgell, Dojka, Nimmo, Lee, Xu, Marginal Zinc Deficiency Increased the Susceptibility to Acute Lipopolysaccharide-Induced Liver Injury in Rats, Exp. Biol. Med, doi:10.1177/153537020623100509
Shen, Shao, Li, Different Types of Cell Death and Their Shift in Shaping Disease, Cell Death Discov, doi:10.1038/s41420-023-01581-0
Shenkin, Berger, Micronutrients: A Low Blood Concentration Is Not Equivalent to Deficiency, Clin. Nutr, doi:10.1016/j.clnu.2022.09.015
Singer, Deutschman, Seymour, Shankar-Hari, Annane et al., The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), JAMA, doi:10.1001/jama.2016.0287
Skalny, Aschner, Lei, Gritsenko, Santamaria et al., Gut Microbiota as a Mediator of Essential and Toxic Effects of Zinc in the Intestines and Other Tissues, Int. J. Mol. Sci, doi:10.3390/ijms222313074
Slifierz, Friendship, Weese, Methicillin-Resistant Staphylococcus Aureus in Commercial Swine Herds Is Associated with Disinfectant and Zinc Usage, Appl. Environ. Microbiol, doi:10.1128/AEM.00036-15
Sobczyk, Gaunt, The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study, Nutrients, doi:10.3390/nu14020233
Souffriau, Timmermans, Vanderhaeghen, Wallaeys, Van Looveren et al., Zinc Inhibits Lethal Inflammatory Shock by Preventing Microbe-Induced Interferon Signature in Intestinal Epithelium, EMBO Mol. Med, doi:10.15252/emmm.201911917
Speir, Djajawi, Conos, Tye, Lawlor, Targeting RIP Kinases in Chronic Inflammatory Disease, Biomolecules, doi:10.3390/biom11050646
Srinivasan, Ndeezi, Mboijana, Kiguli, Bimenya et al., Zinc Adjunct Therapy Reduces Case Fatality in Severe Childhood Pneumonia: A Randomized Double Blind Placebo-Controlled Trial, BMC Med, doi:10.1186/1741-7015-10-14
St, Hospital, New York A Randomized, Placebo-Controlled Study Evaluating the Efficacy of Zinc for the Treatment of COVID-19 in the Outpatient Setting; clinicaltrials
Stambouli, Driss, Gargouri, Bahrini, Arfaoui et al., COVID-19 Prophylaxis with Doxycycline and Zinc in Health Care Workers: A Prospective, Randomized, Double-Blind Clinical Trial, Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis, doi:10.1016/j.ijid.2022.06.016
Stennicke, Salvesen, Biochemical Characteristics of Caspases-3, -6, -7, and -8, J. Biol. Chem, doi:10.1074/jbc.272.41.25719
Stewart, Frederickson, Howell, Gage, Cholinergic Denervation-Induced Increase of Chelatable Zinc in Mossy-Fiber Region of the Hippocampal Formation, Brain Res, doi:10.1016/0006-8993(84)90734-0
Su, Dziedzic, Hu, Barrett, Broekema et al., ABIN-1 Heterozygosity Sensitizes to Innate Immune Response in Both RIPK1-Dependent and RIPK1-Independent Manner, Cell Death Differ, doi:10.1038/s41418-018-0215-3
Suarez, )) and Standard of Care (SOC) vs. SOC in the Treatment of Non-Hospitalized Patients with COVID-19
Sun, Wang, Wang, He, Chen et al., Mixed Lineage Kinase Domain-like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase, Cell, doi:10.1016/j.cell.2011.11.031
Sun, Zhong, Zhang, Li, Sun et al., Zinc Deficiency Mediates Alcohol-Induced Apoptotic Cell Death in the Liver of Rats through Activating ER and Mitochondrial Cell Death Pathways, Am. J. Physiol. Gastrointest. Liver Physiol, doi:10.1152/ajpgi.00442.2014
Suresh, Balijepalli, Solanki, Aktay, Choudhary et al., Hypoxia-Inducible Factor 1α and Its Role in Lung Injury: Adaptive or Maladaptive, Inflammation, doi:10.1007/s10753-022-01769-z
Tak, Firestein, NF-kappaB: A Key Role in Inflammatory Diseases, J. Clin. Investig, doi:10.1172/JCI11830
Tang, Kang, Berghe, Vandenabeele, Kroemer, The Molecular Machinery of Regulated Cell Death, Cell Res, doi:10.1038/s41422-019-0164-5
Thambiayya, Kaynar, St Croix, Pitt, Functional Role of Intracellular Labile Zinc in Pulmonary Endothelium, Pulm. Circ, doi:10.4103/2045-8932.105032
Thimmulappa, Lee, Rangasamy, Reddy, Yamamoto et al., Nrf2 Is a Critical Regulator of the Innate Immune Response and Survival during Experimental Sepsis, J. Clin. Investig, doi:10.1172/JCI25790
Thokala, Bodiga, Kudle, Bodiga, Comparative Response of Cardiomyocyte ZIPs and ZnTs to Extracellular Zinc and TPEN, Biol. Trace Elem. Res, doi:10.1007/s12011-019-01671-0
Thomas, Coles, Deitmer, Homeostatic Muffling, Nature, doi:10.1038/350564b0
Tian, Liu, Li, Zhao, Shereen et al., HIF-1α Promotes SARS-CoV-2 Infection and Aggravates Inflammatory Responses to COVID-19, Signal Transduct. Target. Ther, doi:10.1038/s41392-021-00726-w
Tojo, Yamamoto, Tamada, Mihara, Abe et al., Early Alveolar Epithelial Cell Necrosis Is a Potential Driver of COVID-19-Induced Acute Respiratory Distress Syndrome, iScience, doi:10.1016/j.isci.2022.105748
Tomasa-Irriguible, Bielsa-Berrocal, Bordejé-Laguna, Tural-Llàcher, Barallat et al., Low Levels of Few Micronutrients May Impact COVID-19 Disease Progression: An Observational Study on the First Wave, Metabolites, doi:10.3390/metabo11090565
Truong-Tran, Carter, Ruffin, Zalewski, The Role of Zinc in Caspase Activation and Apoptotic Cell Death, Biometals Int. J. Role Met. Ions Biol. Biochem. Med, doi:10.1023/a:1012993017026
Van Es, Sato, Van De Wetering, Lyubimova, Yee Nee et al., Dll1+ Secretory Progenitor Cells Revert to Stem Cells upon Crypt Damage, Nat. Cell Biol, doi:10.1038/ncb2581
Van Huffel, Delaei, Heyninck, De Valck, Beyaert, Identification of a Novel A20-Binding Inhibitor of Nuclear Factor-Kappa B Activation Termed ABIN-2, J. Biol. Chem, doi:10.1074/jbc.M100048200
Vankrunkelsven, Gunst, Amrein, Bear, Berger et al., Monitoring and Parenteral Administration of Micronutrients, Phosphate and Magnesium in Critically Ill Patients: The VITA-TRACE Survey, Clin. Nutr, doi:10.1016/j.clnu.2020.06.005
Vasquez, Zuniga, Rodriguez, Oxidative Stress and Pathogenesis in Malaria, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2021.768182
Velthuis, Van Den Worm, Sims, Baric, Snijder et al., Zn(2+) Inhibits Coronavirus and Arterivirus RNA Polymerase Activity in Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture, PLoS Pathog, doi:10.1371/journal.ppat.1001176
Vinceti, Filippini, Fairweather-Tait, A Potential Role for Zinc to Enhance Treatment for Coronavirus Disease 2019 (COVID-19)?, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am, doi:10.1093/cid/ciac849
Vruwink, Fletcher, Keen, Golub, Hendrickx et al., Moderate Zinc Deficiency in Rhesus Monkeys. An Intrinsic Defect of Neutrophil Chemotaxis Corrected by Zinc Repletion, J. Immunol, doi:10.4049/jimmunol.146.1.244
Wang, Chai, Guo, Wang, Liao et al., A New Perspective on the Potential Application of RIPK1 in the Treatment of Sepsis, Immunotherapy, doi:10.2217/imt-2022-0219
Wang, Liu, Ma, Zhang, The Pathogenesis of COVID-19-Related Taste Disorder and Treatments, J. Dent. Res, doi:10.1177/00220345231182926
Wang, Zhang, Li, Wu, He et al., Toll-like Receptor 4-Mediated Endoplasmic Reticulum Stress Induces Intestinal Paneth Cell Damage in Mice Following CLP-Induced Sepsis, Sci. Rep, doi:10.1038/s41598-022-19614-6
Weisel, Berger, Thorn, Taylor, Peterfy et al., A Randomized, Placebo-Controlled Experimental Medicine Study of RIPK1 Inhibitor GSK2982772 in Patients with Moderate to Severe Rheumatoid Arthritis, Arthritis Res. Ther, doi:10.1186/s13075-021-02468-0
Wessels, Cousins, Zinc Dyshomeostasis during Polymicrobial Sepsis in Mice Involves Zinc Transporter Zip14 and Can Be Overcome by Zinc Supplementation, Am. J. Physiol. Gastrointest. Liver Physiol, doi:10.1152/ajpgi.00179.2015
Wessels, Maywald, Rink, Zinc as a Gatekeeper of Immune Function, Nutrients, doi:10.3390/nu9121286
West, Shadel, Mitochondrial DNA in Innate Immune Responses and Inflammatory Pathology, Nat. Rev. Immunol, doi:10.1038/nri.2017.21
Wiscovitch-Russo, Ibáñez-Prada, Serrano-Mayorga, Sievers, Engelbride et al., Necroptosis Drives Major Adverse Cardiovascular Events During Severe COVID-19, Res. Sq, doi:10.21203/rs.3.rs-2468706/v1
Wu, Zhu, Zeng, Gu, Miao et al., Extracellular Mitochondrial DNA Promote NLRP3 Inflammasome Activation and Induce Acute Lung Injury through TLR9 and NF-κB, J. Thorac. Dis, doi:10.21037/jtd.2019.10.26
Xia, Sun, Chen, Pineda, Jiang et al., Direct Activation of Protein Kinases by Unanchored Polyubiquitin Chains, Nature, doi:10.1038/nature08247
Yang, Li, Yu, Zhang, Xu et al., Regulation of RIP3 by the Transcription Factor Sp1 and the Epigenetic Regulator UHRF1 Modulates Cancer Cell Necroptosis, Cell Death Dis, doi:10.1038/cddis.2017.483
Yasui, Yasui, Suzuki, Saitou, Yamamoto et al., Analysis of the Predictive Factors for a Critical Illness of COVID-19 during Treatment-Relationship between Serum Zinc Level and Critical Illness of COVID-19, Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis, doi:10.1016/j.ijid.2020.09.008
Yoo, Im, Ko, Lee, Park et al., Association of Plasma Level of High-Mobility Group Box-1 with Necroptosis and Sepsis Outcomes, Sci. Rep, doi:10.1038/s41598-021-88970-6
Zago, Oteiza, The Antioxidant Properties of Zinc: Interactions with Iron and Antioxidants, Free Radic. Biol. Med, doi:10.1016/S0891-5849(01)00583-4
Zelic, Roderick, O'donnell, Lehman, Lim et al., RIP Kinase 1-Dependent Endothelial Necroptosis Underlies Systemic Inflammatory Response Syndrome, J. Clin. Investig, doi:10.1172/JCI96147
Zhang, Liu, Dai, Wang, Wu et al., Crosstalk between Regulated Necrosis and Micronutrition, Bridged by Reactive Oxygen Species, Front. Nutr, doi:10.3389/fnut.2022.1003340
Zhang, Raoof, Chen, Sumi, Sursal et al., Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury, Nature, doi:10.1038/nature08780
Zhang, Zhang, Li, Xing, Zhou et al., A Zinc-Modified Anemarrhena Asphodeloides Polysaccharide Complex Enhances Immune Activity via the NF-κB and MAPK Signaling Pathways, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2023.126017
Zhao, Gong, Wang, Fu, Zhang et al., A Zinc-and Calcium-Rich Lysosomal Nanoreactor Rescues Monocyte/Macrophage Dysfunction under Sepsis, Adv. Sci, doi:10.1002/advs.202205097
Zhong, Wake, Liu, Gao, Teshigawara et al., Effects of Histidine-Rich Glycoprotein on Erythrocyte Aggregation and Hemolysis: Implications for a Role under Septic Conditions, J. Pharmacol. Sci, doi:10.1016/j.jphs.2017.11.003
Zhong, Zhang, Wang, Xing, Ri et al., Zinc Finger Protein 91 Mediates Necroptosis by Initiating RIPK1-RIPK3-MLKL Signal Transduction in Response to TNF Receptor 1 Ligation, Toxicol. Lett, doi:10.1016/j.toxlet.2021.12.015
DOI record:
{
"DOI": "10.3390/antiox12111942",
"ISSN": [
"2076-3921"
],
"URL": "http://dx.doi.org/10.3390/antiox12111942",
"abstract": "<jats:p>Zinc is a structural component of proteins, functions as a catalytic co-factor in DNA synthesis and transcription of hundreds of enzymes, and has a regulatory role in protein–DNA interactions of zinc-finger proteins. For many years, zinc has been acknowledged for its anti-oxidative and anti-inflammatory functions. Furthermore, zinc is a potent inhibitor of caspases-3, -7, and -8, modulating the caspase-controlled apoptosis and necroptosis. In recent years, the immunomodulatory role of zinc in sepsis and COVID-19 has been investigated. Both sepsis and COVID-19 are related to various regulated cell death (RCD) pathways, including apoptosis and necroptosis. Lack of zinc may have a negative effect on many immune functions, such as oxidative burst, cytokine production, chemotaxis, degranulation, phagocytosis, and RCD. While plasma zinc concentrations decline swiftly during both sepsis and COVID-19, this reduction is primarily attributed to a redistribution process associated with the inflammatory response. In this response, hepatic metallothionein production increases in reaction to cytokine release, which is linked to inflammation, and this protein effectively captures and stores zinc in the liver. Multiple regulatory mechanisms come into play, influencing the uptake of zinc, the binding of zinc to blood albumin and red blood cells, as well as the buffering and modulation of cytosolic zinc levels. Decreased zinc levels are associated with increasing severity of organ dysfunction, prolonged hospital stay and increased mortality in septic and COVID-19 patients. Results of recent studies focusing on these topics are summarized and discussed in this narrative review. Existing evidence currently does not support pharmacological zinc supplementation in patients with sepsis or COVID-19. Complementation and repletion should follow current guidelines for micronutrients in critically ill patients. Further research investigating the pharmacological mechanism of zinc in programmed cell death caused by invasive infections and its therapeutic potential in sepsis and COVID-19 could be worthwhile.</jats:p>",
"alternative-id": [
"antiox12111942"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0928-6183",
"affiliation": [
{
"name": "Postgraduate Program “Emergency and Intensive Care in Children, Adolescents, and Young Adults”, School of Medicine, University of Crete, 71003 Heraklion, Greece"
}
],
"authenticated-orcid": false,
"family": "Briassoulis",
"given": "George",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-9789-9002",
"affiliation": [
{
"name": "Second Department of Anesthesiology, Attikon University Hospital, School of Medicine, National and Kapodistrian University of Athens, 12462 Athens, Greece"
}
],
"authenticated-orcid": false,
"family": "Briassoulis",
"given": "Panagiotis",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0936-368X",
"affiliation": [
{
"name": "Postgraduate Program “Emergency and Intensive Care in Children, Adolescents, and Young Adults”, School of Medicine, University of Crete, 71003 Heraklion, Greece"
},
{
"name": "Paediatric Intensive Care Unit, University Hospital, School of Medicine, University of Crete, 71110 Heraklion, Greece"
}
],
"authenticated-orcid": false,
"family": "Ilia",
"given": "Stavroula",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7622-202X",
"affiliation": [
{
"name": "Paediatric Intensive Care Unit, University Hospital, School of Medicine, University of Crete, 71110 Heraklion, Greece"
}
],
"authenticated-orcid": false,
"family": "Miliaraki",
"given": "Marianna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department “MAKKA”, First Department of Paediatrics, “Aghia Sophia” Children’s Hospital, School of Medicine, National and Kapodistrian University of Athens, 11527 Athens, Greece"
}
],
"family": "Briassouli",
"given": "Efrossini",
"sequence": "additional"
}
],
"container-title": "Antioxidants",
"container-title-short": "Antioxidants",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
31
]
],
"date-time": "2023-10-31T16:44:03Z",
"timestamp": 1698770643000
},
"deposited": {
"date-parts": [
[
2023,
10,
31
]
],
"date-time": "2023-10-31T18:13:14Z",
"timestamp": 1698775994000
},
"indexed": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T07:24:22Z",
"timestamp": 1698823462343
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
10,
31
]
]
},
"journal-issue": {
"issue": "11",
"published-online": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
31
]
],
"date-time": "2023-10-31T00:00:00Z",
"timestamp": 1698710400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-3921/12/11/1942/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1942",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
10,
31
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
31
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1007/s00134-020-06151-x",
"article-title": "Incidence and Mortality of Hospital- and ICU-Treated Sepsis: Results from an Updated and Expanded Systematic Review and Meta-Analysis",
"author": "Mellhammar",
"doi-asserted-by": "crossref",
"first-page": "1552",
"journal-title": "Intensive Care Med.",
"key": "ref_1",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1001/jama.2016.0287",
"article-title": "The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)",
"author": "Singer",
"doi-asserted-by": "crossref",
"first-page": "801",
"journal-title": "JAMA",
"key": "ref_2",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.1097/CCM.0000000000005195",
"article-title": "Coronavirus Disease 2019 as Cause of Viral Sepsis: A Systematic Review and Meta-Analysis",
"author": "Karakike",
"doi-asserted-by": "crossref",
"first-page": "2042",
"journal-title": "Crit. Care Med.",
"key": "ref_3",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00218-6",
"article-title": "The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity",
"author": "Osuchowski",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "Lancet Respir. Med.",
"key": "ref_4",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41418-017-0012-4",
"article-title": "Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018",
"author": "Galluzzi",
"doi-asserted-by": "crossref",
"first-page": "486",
"journal-title": "Cell Death Differ.",
"key": "ref_5",
"volume": "25",
"year": "2018"
},
{
"DOI": "10.1038/nri.2016.147",
"article-title": "Programmed Cell Death as a Defence against Infection",
"author": "Jorgensen",
"doi-asserted-by": "crossref",
"first-page": "151",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_6",
"volume": "17",
"year": "2017"
},
{
"DOI": "10.1097/SHK.0000000000001936",
"article-title": "Necroptosis, Pyroptosis, Ferroptosis in Sepsis and Treatment",
"author": "Qu",
"doi-asserted-by": "crossref",
"first-page": "161",
"journal-title": "Shock",
"key": "ref_7",
"volume": "57",
"year": "2022"
},
{
"DOI": "10.3390/antiox11020231",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Miliaraki, M., Briassoulis, P., Ilia, S., Michalakakou, K., Karakonstantakis, T., Polonifi, A., Bastaki, K., Briassouli, E., Vardas, K., and Pistiki, A. (2022). Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations. Antioxidants, 11."
},
{
"DOI": "10.1002/jpen.2421",
"article-title": "Overview of Oxidative Stress and the Role of Micronutrients in Critical Illness",
"author": "Dresen",
"doi-asserted-by": "crossref",
"first-page": "S38",
"journal-title": "JPEN J. Parenter. Enter. Nutr.",
"key": "ref_9",
"volume": "47",
"year": "2023"
},
{
"DOI": "10.1016/j.clnu.2022.02.015",
"article-title": "ESPEN Micronutrient Guideline",
"author": "Berger",
"doi-asserted-by": "crossref",
"first-page": "1357",
"journal-title": "Clin. Nutr. Edinb. Scotl.",
"key": "ref_10",
"volume": "41",
"year": "2022"
},
{
"DOI": "10.1093/advances/nmz013",
"article-title": "The Role of Zinc in Antiviral Immunity",
"author": "Read",
"doi-asserted-by": "crossref",
"first-page": "696",
"journal-title": "Adv. Nutr.",
"key": "ref_11",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.abb.2016.03.022",
"article-title": "Zinc and Immunity: An Essential Interrelation",
"author": "Maares",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "Arch. Biochem. Biophys.",
"key": "ref_12",
"volume": "611",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0180028",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Rao, K., Sethi, K., Ischia, J., Gibson, L., Galea, L., Xiao, L., Yim, M., Chang, M., Papa, N., and Bolton, D. (2017). Protective Effect of Zinc Preconditioning against Renal Ischemia Reperfusion Injury Is Dose Dependent. PLoS ONE, 12."
},
{
"DOI": "10.1164/ajrccm.164.3.2009088",
"article-title": "Mitochondrial Membrane Potential and Apoptosis Peripheral Blood Monocytes in Severe Human Sepsis",
"author": "Adrie",
"doi-asserted-by": "crossref",
"first-page": "389",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "ref_14",
"volume": "164",
"year": "2001"
},
{
"DOI": "10.4266/acc.2018.00157",
"article-title": "Role of Mitochondrial Oxidative Stress in Sepsis",
"author": "Nagar",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "Acute Crit. Care",
"key": "ref_15",
"volume": "33",
"year": "2018"
},
{
"DOI": "10.1093/ajcn/85.3.837",
"article-title": "Zinc Supplementation Decreases Incidence of Infections in the Elderly: Effect of Zinc on Generation of Cytokines and Oxidative Stress",
"author": "Prasad",
"doi-asserted-by": "crossref",
"first-page": "837",
"journal-title": "Am. J. Clin. Nutr.",
"key": "ref_16",
"volume": "85",
"year": "2007"
},
{
"DOI": "10.1155/2017/5985209",
"article-title": "Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies",
"author": "Mantzarlis",
"doi-asserted-by": "crossref",
"first-page": "5985209",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "ref_17",
"volume": "2017",
"year": "2017"
},
{
"DOI": "10.1039/c1mt00064k",
"article-title": "The Oxidative Stress of Zinc Deficiency",
"author": "Eide",
"doi-asserted-by": "crossref",
"first-page": "1124",
"journal-title": "Met. Integr. Biometal Sci.",
"key": "ref_18",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.3390/nu15133040",
"doi-asserted-by": "crossref",
"key": "ref_19",
"unstructured": "Kitala, K., Tanski, D., Godlewski, J., Krajewska-Włodarczyk, M., Gromadziński, L., and Majewski, M. (2023). Copper and Zinc Particles as Regulators of Cardiovascular System Function-A Review. Nutrients, 15."
},
{
"DOI": "10.1007/s10787-017-0309-4",
"article-title": "Antioxidant and Anti-Inflammatory Effects of Zinc. Zinc-Dependent NF-κB Signaling",
"author": "Jarosz",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "Inflammopharmacology",
"key": "ref_20",
"volume": "25",
"year": "2017"
},
{
"DOI": "10.1172/JCI25790",
"article-title": "Nrf2 Is a Critical Regulator of the Innate Immune Response and Survival during Experimental Sepsis",
"author": "Thimmulappa",
"doi-asserted-by": "crossref",
"first-page": "984",
"journal-title": "J. Clin. Investig.",
"key": "ref_21",
"volume": "116",
"year": "2006"
},
{
"DOI": "10.1038/s41598-023-39928-3",
"article-title": "Counteract Severe Heat Stress by Including Different Forms of Zinc in the Rabbit Bucks’ Diet",
"author": "Zahran",
"doi-asserted-by": "crossref",
"first-page": "12987",
"journal-title": "Sci. Rep.",
"key": "ref_22",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/S0014-2999(02)02223-9",
"article-title": "Role of Zn(2+) in Oxidative Stress Caused by Endotoxin Challenge",
"author": "Sakaguchi",
"doi-asserted-by": "crossref",
"first-page": "309",
"journal-title": "Eur. J. Pharmacol.",
"key": "ref_23",
"volume": "451",
"year": "2002"
},
{
"DOI": "10.1038/aps.2018.25",
"article-title": "Zinc Deficiency and Cellular Oxidative Stress: Prognostic Implications in Cardiovascular Diseases",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "1120",
"journal-title": "Acta Pharmacol. Sin.",
"key": "ref_24",
"volume": "39",
"year": "2018"
},
{
"DOI": "10.1016/S0891-5849(01)00583-4",
"article-title": "The Antioxidant Properties of Zinc: Interactions with Iron and Antioxidants",
"author": "Zago",
"doi-asserted-by": "crossref",
"first-page": "266",
"journal-title": "Free Radic. Biol. Med.",
"key": "ref_25",
"volume": "31",
"year": "2001"
},
{
"DOI": "10.1016/0891-5849(90)90076-U",
"article-title": "The Physiological Role of Zinc as an Antioxidant",
"author": "Bray",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Free Radic. Biol. Med.",
"key": "ref_26",
"volume": "8",
"year": "1990"
},
{
"DOI": "10.3390/antiox6020024",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "do Marreiro, D.N., Cruz, K.J.C., Morais, J.B.S., Beserra, J.B., Severo, J.S., and de Oliveira, A.R.S. (2017). Zinc and Oxidative Stress: Current Mechanisms. Antioxidants, 6."
},
{
"DOI": "10.3389/fnut.2014.00014",
"article-title": "Zinc Is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health",
"author": "Prasad",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Front. Nutr.",
"key": "ref_28",
"volume": "1",
"year": "2014"
},
{
"DOI": "10.1016/j.jtemb.2022.127076",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Kumar, N., Kumar, S., Singh, A.K., Gite, A., Patole, P.B., and Thorat, S.T. (2022). Exploring Mitigating Role of Zinc Nanoparticles on Arsenic, Ammonia and Temperature Stress Using Molecular Signature in Fish. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS, 74."
},
{
"DOI": "10.1186/s40659-022-00392-4",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Rahimi, G., Mohammad, K.S., Zarei, M., Shokoohi, M., Oskoueian, E., Poorbagher, M.R.M., and Karimi, E. (2022). Zinc Oxide Nanoparticles Synthesized Using Hyssopus officinalis L. Extract Induced Oxidative Stress and Changes the Expression of Key Genes Involved in Inflammatory and Antioxidant Systems. Biol. Res., 55."
},
{
"DOI": "10.1080/21688370.2022.2069966",
"article-title": "The Therapeutic Prospect of Zinc Oxide Nanoparticles in Experimentally Induced Diabetic Nephropathy",
"author": "Mazen",
"doi-asserted-by": "crossref",
"first-page": "2069966",
"journal-title": "Tissue Barriers",
"key": "ref_31",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.lfs.2020.117464",
"article-title": "Selenium/Zinc-Enriched Probiotics Improve Serum Enzyme Activity, Antioxidant Ability, Inflammatory Factors and Related Gene Expression of Wistar Rats Inflated under Heat Stress",
"author": "Malyar",
"doi-asserted-by": "crossref",
"first-page": "117464",
"journal-title": "Life Sci.",
"key": "ref_32",
"volume": "248",
"year": "2020"
},
{
"DOI": "10.1016/j.niox.2018.12.010",
"article-title": "Antioxidant Activity of Endogenously Produced Nitric Oxide against the Zinc Oxide Nanoparticle-Induced Oxidative Stress in Primary Hepatocytes of Air-Breathing Catfish, Clarias Magur",
"author": "Koner",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Nitric Oxide Biol. Chem.",
"key": "ref_33",
"volume": "84",
"year": "2019"
},
{
"DOI": "10.1139/cjpp-2012-0295",
"article-title": "Redox-Inflammatory Synergy in the Metabolic Syndrome",
"author": "Bryan",
"doi-asserted-by": "crossref",
"first-page": "22",
"journal-title": "Can. J. Physiol. Pharmacol.",
"key": "ref_34",
"volume": "91",
"year": "2013"
},
{
"DOI": "10.1089/ars.2008.2198",
"article-title": "Transcriptional Regulatory Functions of Mammalian AP-Endonuclease (APE1/Ref-1), an Essential Multifunctional Protein",
"author": "Bhakat",
"doi-asserted-by": "crossref",
"first-page": "621",
"journal-title": "Antioxid. Redox Signal.",
"key": "ref_35",
"volume": "11",
"year": "2009"
},
{
"DOI": "10.1089/ars.2012.5149",
"article-title": "Reactive Oxygen Species in Inflammation and Tissue Injury",
"author": "Mittal",
"doi-asserted-by": "crossref",
"first-page": "1126",
"journal-title": "Antioxid. Redox Signal.",
"key": "ref_36",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1089/ars.2016.6946",
"article-title": "Regulation of Transcription Factors by Reactive Oxygen Species and Nitric Oxide in Vascular Physiology and Pathology",
"author": "Upadhye",
"doi-asserted-by": "crossref",
"first-page": "679",
"journal-title": "Antioxid. Redox Signal.",
"key": "ref_37",
"volume": "26",
"year": "2017"
},
{
"DOI": "10.3390/cells5010012",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Christian, F., Smith, E.L., and Carmody, R.J. (2016). The Regulation of NF-κB Subunits by Phosphorylation. Cells, 5."
},
{
"DOI": "10.1172/JCI11830",
"article-title": "NF-kappaB: A Key Role in Inflammatory Diseases",
"author": "Tak",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "J. Clin. Investig.",
"key": "ref_39",
"volume": "107",
"year": "2001"
},
{
"DOI": "10.1038/cdd.2009.41",
"article-title": "NEMO/IKKgamma Regulates an Early NF-kappaB-Independent Cell-Death Checkpoint during TNF Signaling",
"author": "Hase",
"doi-asserted-by": "crossref",
"first-page": "1279",
"journal-title": "Cell Death Differ.",
"key": "ref_40",
"volume": "16",
"year": "2009"
},
{
"DOI": "10.1371/journal.pone.0026069",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Arslan, S.Ç., and Scheidereit, C. (2011). The Prevalence of TNFα-Induced Necrosis over Apoptosis Is Determined by TAK1-RIP1 Interplay. PLoS ONE, 6."
},
{
"DOI": "10.1038/nature08247",
"article-title": "Direct Activation of Protein Kinases by Unanchored Polyubiquitin Chains",
"author": "Xia",
"doi-asserted-by": "crossref",
"first-page": "114",
"journal-title": "Nature",
"key": "ref_42",
"volume": "461",
"year": "2009"
},
{
"DOI": "10.3109/08916939808995367",
"article-title": "AP-1 and NF-kappaB Regulation in Rheumatoid Arthritis and Murine Collagen-Induced Arthritis",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "197",
"journal-title": "Autoimmunity",
"key": "ref_43",
"volume": "28",
"year": "1998"
},
{
"DOI": "10.1016/j.autrev.2014.11.008",
"article-title": "Zinc and Its Role in Immunity and Inflammation",
"author": "Bonaventura",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Autoimmun. Rev.",
"key": "ref_44",
"volume": "14",
"year": "2015"
},
{
"DOI": "10.1016/j.lfs.2019.116697",
"article-title": "Berberine Induces ZIP14 Expression and Modulates Zinc Redistribution to Protect Intestinal Mucosal Barrier during Polymicrobial Sepsis",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "116697",
"journal-title": "Life Sci.",
"key": "ref_45",
"volume": "233",
"year": "2019"
},
{
"DOI": "10.1016/j.ijbiomac.2023.126017",
"doi-asserted-by": "crossref",
"key": "ref_46",
"unstructured": "Zhang, S., Zhang, Q., Li, C., Xing, N., Zhou, P., and Jiao, Y. (2023). A Zinc-Modified Anemarrhena Asphodeloides Polysaccharide Complex Enhances Immune Activity via the NF-κB and MAPK Signaling Pathways. Int. J. Biol. Macromol., 249."
},
{
"DOI": "10.1016/S0041-008X(03)00167-4",
"article-title": "Zinc-Induced NF-kappaB Inhibition Can Be Modulated by Changes in the Intracellular Metallothionein Level",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "Toxicol. Appl. Pharmacol.",
"key": "ref_47",
"volume": "190",
"year": "2003"
},
{
"DOI": "10.1038/nrm2083",
"article-title": "Integrating Cell-Signalling Pathways with NF-kappaB and IKK Function",
"author": "Perkins",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_48",
"volume": "8",
"year": "2007"
},
{
"DOI": "10.1016/j.celrep.2013.01.009",
"article-title": "ZIP8 Regulates Host Defense through Zinc-Mediated Inhibition of NF-κB",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "386",
"journal-title": "Cell Rep.",
"key": "ref_49",
"volume": "3",
"year": "2013"
},
{
"DOI": "10.1016/j.it.2013.10.005",
"article-title": "A20 in Inflammation and Autoimmunity",
"author": "Catrysse",
"doi-asserted-by": "crossref",
"first-page": "22",
"journal-title": "Trends Immunol.",
"key": "ref_50",
"volume": "35",
"year": "2014"
},
{
"DOI": "10.3945/ajcn.2009.28836",
"article-title": "Zinc Decreases C-Reactive Protein, Lipid Peroxidation, and Inflammatory Cytokines in Elderly Subjects: A Potential Implication of Zinc as an Atheroprotective Agent",
"author": "Bao",
"doi-asserted-by": "crossref",
"first-page": "1634",
"journal-title": "Am. J. Clin. Nutr.",
"key": "ref_51",
"volume": "91",
"year": "2010"
},
{
"DOI": "10.1152/ajplung.00368.2009",
"article-title": "Zinc Modulates the Innate Immune Response in Vivo to Polymicrobial Sepsis through Regulation of NF-kappaB",
"author": "Bao",
"doi-asserted-by": "crossref",
"first-page": "L744",
"journal-title": "Am. J. Physiol. Lung Cell. Mol. Physiol.",
"key": "ref_52",
"volume": "298",
"year": "2010"
},
{
"DOI": "10.1152/ajpgi.00244.2022",
"article-title": "Enterocyte-Specific Deletion of Metal Transporter Zip14 (Slc39a14) Alters Intestinal Homeostasis through Epigenetic Mechanisms",
"author": "Ruggiero",
"doi-asserted-by": "crossref",
"first-page": "G159",
"journal-title": "Am. J. Physiol. Gastrointest. Liver Physiol.",
"key": "ref_53",
"volume": "324",
"year": "2023"
},
{
"DOI": "10.1007/s12011-023-03830-w",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "El-Kossi, D.M.M.H., Ibrahim, S.S., Hassanin, K.M.A., Hamad, N., Rashed, N.A., and Abdel-Wahab, A. (2023). Ameliorative Effects of Zinc Oxide, in Either Conventional or Nanoformulation, Against Bisphenol A Toxicity on Reproductive Performance, Oxidative Status, Gene Expression and Histopathology in Adult Male Rats. Biol. Trace Elem. Res."
},
{
"DOI": "10.1038/cddis.2017.483",
"article-title": "Regulation of RIP3 by the Transcription Factor Sp1 and the Epigenetic Regulator UHRF1 Modulates Cancer Cell Necroptosis",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "e3084",
"journal-title": "Cell Death Dis.",
"key": "ref_55",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1016/S0959-440X(00)00167-6",
"article-title": "Zinc Finger Proteins: New Insights into Structural and Functional Diversity",
"author": "Laity",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Curr. Opin. Struct. Biol.",
"key": "ref_56",
"volume": "11",
"year": "2001"
},
{
"DOI": "10.1093/jn/134.7.1711",
"article-title": "Peroxisome Proliferator Activated Receptors Alpha and Gamma Require Zinc for Their Anti-Inflammatory Properties in Porcine Vascular Endothelial Cells",
"author": "Reiterer",
"doi-asserted-by": "crossref",
"first-page": "1711",
"journal-title": "J. Nutr.",
"key": "ref_57",
"volume": "134",
"year": "2004"
},
{
"DOI": "10.1016/j.nut.2010.08.010",
"article-title": "Zinc-Suppressed Inflammatory Cytokines by Induction of A20-Mediated Inhibition of Nuclear Factor-κB",
"author": "Prasad",
"doi-asserted-by": "crossref",
"first-page": "816",
"journal-title": "Nutrition",
"key": "ref_58",
"volume": "27",
"year": "2011"
},
{
"DOI": "10.1083/jcb.145.7.1471",
"article-title": "The Zinc Finger Protein A20 Inhibits TNF-Induced NF-kappaB-Dependent Gene Expression by Interfering with an RIP- or TRAF2-Mediated Transactivation Signal and Directly Binds to a Novel NF-kappaB-Inhibiting Protein ABIN",
"author": "Heyninck",
"doi-asserted-by": "crossref",
"first-page": "1471",
"journal-title": "J. Cell Biol.",
"key": "ref_59",
"volume": "145",
"year": "1999"
},
{
"DOI": "10.1074/jbc.M100048200",
"article-title": "Identification of a Novel A20-Binding Inhibitor of Nuclear Factor-Kappa B Activation Termed ABIN-2",
"author": "Delaei",
"doi-asserted-by": "crossref",
"first-page": "30216",
"journal-title": "J. Biol. Chem.",
"key": "ref_60",
"volume": "276",
"year": "2001"
},
{
"DOI": "10.1097/MCC.0000000000000427",
"article-title": "Mitochondrial DNA in Sepsis",
"author": "Harrington",
"doi-asserted-by": "crossref",
"first-page": "284",
"journal-title": "Curr. Opin. Crit. Care",
"key": "ref_61",
"volume": "23",
"year": "2017"
},
{
"DOI": "10.3389/fimmu.2020.01918",
"article-title": "The Critical Roles and Mechanisms of Immune Cell Death in Sepsis",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "1918",
"journal-title": "Front. Immunol.",
"key": "ref_62",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3390/biomedicines10102386",
"doi-asserted-by": "crossref",
"key": "ref_63",
"unstructured": "Choi, H., Yoo, H., Lee, J.Y., Park, J., and Jeon, K. (2022). Plasma Mitochondrial DNA and Necroptosis as Prognostic Indicators in Critically Ill Patients with Sepsis. Biomedicines, 10."
},
{
"DOI": "10.1038/nri.2017.21",
"article-title": "Mitochondrial DNA in Innate Immune Responses and Inflammatory Pathology",
"author": "West",
"doi-asserted-by": "crossref",
"first-page": "363",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_64",
"volume": "17",
"year": "2017"
},
{
"DOI": "10.1016/j.advnut.2023.08.004",
"doi-asserted-by": "crossref",
"key": "ref_65",
"unstructured": "Fenech, M.F., Bull, C.F., and Van Klinken, B.J.-W. (2023). Protective Effects of Micronutrient Supplements, Phytochemicals and Phytochemical-Rich Beverages and Foods Against DNA Damage in Humans: A Systematic Review of Randomized Controlled Trials and Prospective Studies. Adv. Nutr."
},
{
"DOI": "10.3945/jn.116.243832",
"article-title": "Low Plasma Zinc Is Associated with Higher Mitochondrial Oxidative Stress and Faster Liver Fibrosis Development in the Miami Adult Studies in HIV Cohort",
"author": "Martinez",
"doi-asserted-by": "crossref",
"first-page": "556",
"journal-title": "J. Nutr.",
"key": "ref_66",
"volume": "147",
"year": "2017"
},
{
"DOI": "10.21037/jtd.2019.10.26",
"article-title": "Extracellular Mitochondrial DNA Promote NLRP3 Inflammasome Activation and Induce Acute Lung Injury through TLR9 and NF-κB",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "4816",
"journal-title": "J. Thorac. Dis.",
"key": "ref_67",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1002/eji.201343921",
"article-title": "Circulating Mitochondrial DNA Increases with Age and Is a Familiar Trait: Implications for “Inflamm-Aging”",
"author": "Pinti",
"doi-asserted-by": "crossref",
"first-page": "1552",
"journal-title": "Eur. J. Immunol.",
"key": "ref_68",
"volume": "44",
"year": "2014"
},
{
"DOI": "10.1038/nature08780",
"article-title": "Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "104",
"journal-title": "Nature",
"key": "ref_69",
"volume": "464",
"year": "2010"
},
{
"DOI": "10.1186/s12890-021-01437-2",
"doi-asserted-by": "crossref",
"key": "ref_70",
"unstructured": "Mao, J.-Y., Li, D.-K., Zhang, H.-M., Wang, X.-T., and Liu, D.-W. (2021). Plasma Mitochondrial DNA Levels Are Associated with Acute Lung Injury and Mortality in Septic Patients. BMC Pulm. Med., 21."
},
{
"DOI": "10.1016/j.ijbiomac.2023.127166",
"doi-asserted-by": "crossref",
"key": "ref_71",
"unstructured": "Feng, Y., Wu, Y., Duan, R., Wang, P., Zhong, X., and Wu, X. (2023). Structural Characterization and Anti-Inflammatory Effects of Enteromorpha Prolifera Polysaccharide-Fe/Zn Complexes. Int. J. Biol. Macromol., 253."
},
{
"article-title": "Functions of Zinc in Signaling, Proliferation and Differentiation of Mammalian Cells",
"author": "Beyersmann",
"first-page": "331",
"journal-title": "Biometals Int. J. Role Met. Ions Biol. Biochem. Med.",
"key": "ref_72",
"volume": "14",
"year": "2001"
},
{
"DOI": "10.1007/s12011-015-0515-0",
"article-title": "Chelation of Free Zn2+ Impairs Chemotaxis, Phagocytosis, Oxidative Burst, Degranulation, and Cytokine Production by Neutrophil Granulocytes",
"author": "Hasan",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Biol. Trace Elem. Res.",
"key": "ref_73",
"volume": "171",
"year": "2016"
},
{
"DOI": "10.1016/j.molcel.2016.03.009",
"article-title": "Zinc-Induced Polymerization of Killer-Cell Ig-like Receptor into Filaments Promotes Its Inhibitory Function at Cytotoxic Immunological Synapses",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Mol. Cell",
"key": "ref_74",
"volume": "62",
"year": "2016"
},
{
"DOI": "10.4049/jimmunol.146.1.244",
"article-title": "Moderate Zinc Deficiency in Rhesus Monkeys. An Intrinsic Defect of Neutrophil Chemotaxis Corrected by Zinc Repletion",
"author": "Vruwink",
"doi-asserted-by": "crossref",
"first-page": "244",
"journal-title": "J. Immunol.",
"key": "ref_75",
"volume": "146",
"year": "1991"
},
{
"DOI": "10.1186/1742-4933-6-9",
"article-title": "The Immune System and the Impact of Zinc during Aging",
"author": "Haase",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Immun. Ageing A",
"key": "ref_76",
"volume": "6",
"year": "2009"
},
{
"DOI": "10.1146/annurev.nu.10.070190.002215",
"article-title": "Zinc Deficiency and Immune Function",
"author": "Keen",
"doi-asserted-by": "crossref",
"first-page": "415",
"journal-title": "Annu. Rev. Nutr.",
"key": "ref_77",
"volume": "10",
"year": "1990"
},
{
"DOI": "10.1016/0008-8749(79)90318-6",
"article-title": "Circulating Thymic Hormone Levels in Zinc Deficiency",
"author": "Iwata",
"doi-asserted-by": "crossref",
"first-page": "100",
"journal-title": "Cell. Immunol.",
"key": "ref_78",
"volume": "47",
"year": "1979"
},
{
"DOI": "10.1086/528803",
"article-title": "Duration and Severity of Symptoms and Levels of Plasma Interleukin-1 Receptor Antagonist, Soluble Tumor Necrosis Factor Receptor, and Adhesion Molecules in Patients with Common Cold Treated with Zinc Acetate",
"author": "Prasad",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "J. Infect. Dis.",
"key": "ref_79",
"volume": "197",
"year": "2008"
},
{
"article-title": "Changes in Cytokine Production and T Cell Subpopulations in Experimentally Induced Zinc-Deficient Humans",
"author": "Beck",
"first-page": "E1002",
"journal-title": "Am. J. Physiol.",
"key": "ref_80",
"volume": "272",
"year": "1997"
},
{
"DOI": "10.1146/annurev.nutr.24.012003.132454",
"article-title": "Reprogramming of the Immune System during Zinc Deficiency",
"author": "Fraker",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Annu. Rev. Nutr.",
"key": "ref_81",
"volume": "24",
"year": "2004"
},
{
"DOI": "10.1093/ajcn/68.2.447S",
"article-title": "Zinc and Immune Function: The Biological Basis of Altered Resistance to Infection",
"author": "Shankar",
"doi-asserted-by": "crossref",
"first-page": "447S",
"journal-title": "Am. J. Clin. Nutr.",
"key": "ref_82",
"volume": "68",
"year": "1998"
},
{
"DOI": "10.3945/an.112.003038",
"article-title": "Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life",
"author": "Maret",
"doi-asserted-by": "crossref",
"first-page": "82",
"journal-title": "Adv. Nutr.",
"key": "ref_83",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1152/ajplung.00057.2008",
"article-title": "The Human Zinc Transporter SLC39A8 (Zip8) Is Critical in Zinc-Mediated Cytoprotection in Lung Epithelia",
"author": "Besecker",
"doi-asserted-by": "crossref",
"first-page": "L1127",
"journal-title": "Am. J. Physiol. Lung Cell. Mol. Physiol.",
"key": "ref_84",
"volume": "294",
"year": "2008"
},
{
"DOI": "10.1186/1741-7015-10-14",
"doi-asserted-by": "crossref",
"key": "ref_85",
"unstructured": "Srinivasan, M.G., Ndeezi, G., Mboijana, C.K., Kiguli, S., Bimenya, G.S., Nankabirwa, V., and Tumwine, J.K. (2012). Zinc Adjunct Therapy Reduces Case Fatality in Severe Childhood Pneumonia: A Randomized Double Blind Placebo-Controlled Trial. BMC Med., 10."
},
{
"DOI": "10.1002/biof.1829",
"article-title": "Zinc and Gut Microbiota in Health and Gastrointestinal Disease under the COVID-19 Suggestion",
"author": "Scarpellini",
"doi-asserted-by": "crossref",
"first-page": "294",
"journal-title": "BioFactors",
"key": "ref_86",
"volume": "48",
"year": "2022"
},
{
"DOI": "10.1002/advs.202205097",
"article-title": "A Zinc- and Calcium-Rich Lysosomal Nanoreactor Rescues Monocyte/Macrophage Dysfunction under Sepsis",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "e2205097",
"journal-title": "Adv. Sci.",
"key": "ref_87",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1080/15548627.2016.1226732",
"article-title": "Intracellular Staphylococcus Aureus Eludes Selective Autophagy by Activating a Host Cell Kinase",
"author": "Neumann",
"doi-asserted-by": "crossref",
"first-page": "2069",
"journal-title": "Autophagy",
"key": "ref_88",
"volume": "12",
"year": "2016"
},
{
"DOI": "10.1111/jcmm.15027",
"article-title": "Staphylococcus Aureus Facilitates Its Survival in Bovine Macrophages by Blocking Autophagic Flux",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "3460",
"journal-title": "J. Cell. Mol. Med.",
"key": "ref_89",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1128/AEM.00036-15",
"article-title": "Methicillin-Resistant Staphylococcus Aureus in Commercial Swine Herds Is Associated with Disinfectant and Zinc Usage",
"author": "Slifierz",
"doi-asserted-by": "crossref",
"first-page": "2690",
"journal-title": "Appl. Environ. Microbiol.",
"key": "ref_90",
"volume": "81",
"year": "2015"
},
{
"DOI": "10.1016/j.ijmm.2013.06.004",
"article-title": "The Broader Context of Antibiotic Resistance: Zinc Feed Supplementation of Piglets Increases the Proportion of Multi-Resistant Escherichia Coli in Vivo",
"author": "Bednorz",
"doi-asserted-by": "crossref",
"first-page": "396",
"journal-title": "Int. J. Med. Microbiol. IJMM",
"key": "ref_91",
"volume": "303",
"year": "2013"
},
{
"DOI": "10.3390/ijms222313074",
"doi-asserted-by": "crossref",
"key": "ref_92",
"unstructured": "Skalny, A.V., Aschner, M., Lei, X.G., Gritsenko, V.A., Santamaria, A., Alekseenko, S.I., Prakash, N.T., Chang, J.-S., Sizova, E.A., and Chao, J.C.J. (2021). Gut Microbiota as a Mediator of Essential and Toxic Effects of Zinc in the Intestines and Other Tissues. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.3233/JAD-2007-12110",
"article-title": "Zinc Dyshomeostasis, Ageing and Neurodegeneration: Implications of A2M and Inflammatory Gene Polymorphisms",
"author": "Mocchegiani",
"doi-asserted-by": "crossref",
"first-page": "101",
"journal-title": "J. Alzheimers Dis. JAD",
"key": "ref_93",
"volume": "12",
"year": "2007"
},
{
"DOI": "10.3389/fcimb.2021.768182",
"doi-asserted-by": "crossref",
"key": "ref_94",
"unstructured": "Vasquez, M., Zuniga, M., and Rodriguez, A. (2021). Oxidative Stress and Pathogenesis in Malaria. Front. Cell. Infect. Microbiol., 11."
},
{
"DOI": "10.1053/j.gastro.2014.06.024",
"article-title": "Activation of Pattern Recognition Receptors Up-Regulates Metallothioneins, Thereby Increasing Intracellular Accumulation of Zinc, Autophagy, and Bacterial Clearance by Macrophages",
"author": "Lahiri",
"doi-asserted-by": "crossref",
"first-page": "835",
"journal-title": "Gastroenterology",
"key": "ref_95",
"volume": "147",
"year": "2014"
},
{
"DOI": "10.18388/abp.2015_1038",
"article-title": "Metal Responsive Transcription Factor 1 (MTF-1) Regulates Zinc Dependent Cellular Processes at the Molecular Level",
"author": "Grzywacz",
"doi-asserted-by": "crossref",
"first-page": "491",
"journal-title": "Acta Biochim. Pol.",
"key": "ref_96",
"volume": "62",
"year": "2015"
},
{
"DOI": "10.1016/j.clim.2023.109698",
"article-title": "ATF4 Knockdown in Macrophage Impairs Glycolysis and Mediates Immune Tolerance by Targeting HK2 and HIF-1α Ubiquitination in Sepsis",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "109698",
"journal-title": "Clin. Immunol.",
"key": "ref_97",
"volume": "254",
"year": "2023"
},
{
"DOI": "10.1186/s13054-022-03893-6",
"article-title": "Immune Effects of PI3K/Akt/HIF-1α-Regulated Glycolysis in Polymorphonuclear Neutrophils during Sepsis",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Crit. Care",
"key": "ref_98",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1038/s41392-021-00726-w",
"article-title": "HIF-1α Promotes SARS-CoV-2 Infection and Aggravates Inflammatory Responses to COVID-19",
"author": "Tian",
"doi-asserted-by": "crossref",
"first-page": "308",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_99",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1007/s10753-022-01769-z",
"article-title": "Hypoxia-Inducible Factor 1α and Its Role in Lung Injury: Adaptive or Maladaptive",
"author": "Suresh",
"doi-asserted-by": "crossref",
"first-page": "491",
"journal-title": "Inflammation",
"key": "ref_100",
"volume": "46",
"year": "2023"
},
{
"DOI": "10.3389/fonc.2017.00286",
"article-title": "Non-Canonical Mechanisms Regulating Hypoxia-Inducible Factor 1 Alpha in Cancer",
"author": "Iommarini",
"doi-asserted-by": "crossref",
"first-page": "286",
"journal-title": "Front. Oncol.",
"key": "ref_101",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1111/cns.13918",
"article-title": "Zinc Improves Neurological Recovery by Promoting Angiogenesis via the Astrocyte-Mediated HIF-1α/VEGF Signaling Pathway in Experimental Stroke",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1790",
"journal-title": "CNS Neurosci. Ther.",
"key": "ref_102",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S0025-7125(16)31861-2",
"article-title": "Trace Metals in Endocrinology",
"author": "Ri",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "Med. Clin. N. Am.",
"key": "ref_103",
"volume": "60",
"year": "1976"
},
{
"DOI": "10.1079/BJN19910051",
"article-title": "Role of Insulin-like Growth Factor-1 and Growth Hormone in Growth Inhibition Induced by Magnesium and Zinc Deficiencies",
"author": "Flyvbjerg",
"doi-asserted-by": "crossref",
"first-page": "505",
"journal-title": "Br. J. Nutr.",
"key": "ref_104",
"volume": "66",
"year": "1991"
},
{
"DOI": "10.1016/0006-8993(84)90734-0",
"article-title": "Cholinergic Denervation-Induced Increase of Chelatable Zinc in Mossy-Fiber Region of the Hippocampal Formation",
"author": "Stewart",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Brain Res.",
"key": "ref_105",
"volume": "290",
"year": "1984"
},
{
"DOI": "10.3390/medicina57101087",
"doi-asserted-by": "crossref",
"key": "ref_106",
"unstructured": "Salzano, C., Saracino, G., and Cardillo, G. (2021). Possible Adrenal Involvement in Long COVID Syndrome. Med. Kaunas Lith., 57."
},
{
"DOI": "10.1111/pcn.13508",
"article-title": "Neuropsychiatric Aspects of Long COVID: A Comprehensive Review",
"author": "Kubota",
"doi-asserted-by": "crossref",
"first-page": "84",
"journal-title": "Psychiatry Clin. Neurosci.",
"key": "ref_107",
"volume": "77",
"year": "2023"
},
{
"DOI": "10.1177/00220345231182926",
"article-title": "The Pathogenesis of COVID-19-Related Taste Disorder and Treatments",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "J. Dent. Res.",
"key": "ref_108",
"volume": "102",
"year": "2023"
},
{
"DOI": "10.1007/s10787-023-01183-3",
"article-title": "Nutritional Deficiencies That May Predispose to Long COVID",
"author": "Schloss",
"doi-asserted-by": "crossref",
"first-page": "573",
"journal-title": "Inflammopharmacology",
"key": "ref_109",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1016/j.bbrc.2019.11.046",
"article-title": "Zinc Supplementation Protects against Diabetic Endothelial Dysfunction via GTP Cyclohydrolase 1 Restoration",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1049",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "ref_110",
"volume": "521",
"year": "2020"
},
{
"DOI": "10.1016/j.fertnstert.2020.04.025",
"article-title": "Prior and Novel Coronaviruses, Coronavirus Disease 2019 (COVID-19), and Human Reproduction: What Is Known?",
"author": "Segars",
"doi-asserted-by": "crossref",
"first-page": "1140",
"journal-title": "Fertil. Steril.",
"key": "ref_111",
"volume": "113",
"year": "2020"
},
{
"DOI": "10.1038/s41392-022-01110-y",
"article-title": "Regulated Cell Death (RCD) in Cancer: Key Pathways and Targeted Therapies",
"author": "Peng",
"doi-asserted-by": "crossref",
"first-page": "286",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_112",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41422-019-0164-5",
"article-title": "The Molecular Machinery of Regulated Cell Death",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "347",
"journal-title": "Cell Res.",
"key": "ref_113",
"volume": "29",
"year": "2019"
},
{
"DOI": "10.1080/15216540500264554",
"article-title": "Zinc Deficiency-Induced Cell Death",
"author": "Clegg",
"doi-asserted-by": "crossref",
"first-page": "661",
"journal-title": "IUBMB Life",
"key": "ref_114",
"volume": "57",
"year": "2005"
},
{
"DOI": "10.1038/s41467-017-00406-w",
"article-title": "Regulation of RIPK1 Activation by TAK1-Mediated Phosphorylation Dictates Apoptosis and Necroptosis",
"author": "Geng",
"doi-asserted-by": "crossref",
"first-page": "359",
"journal-title": "Nat. Commun.",
"key": "ref_115",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1097/SHK.0b013e31820b2db1",
"article-title": "The Dual Functions of Receptor Interacting Protein 1 in Fas-Induced Hepatocyte Death during Sepsis",
"author": "McNeal",
"doi-asserted-by": "crossref",
"first-page": "499",
"journal-title": "Shock",
"key": "ref_116",
"volume": "35",
"year": "2011"
},
{
"DOI": "10.1016/S0163-7258(97)00167-8",
"article-title": "The 2-5A System: Modulation of Viral and Cellular Processes through Acceleration of RNA Degradation",
"author": "Player",
"doi-asserted-by": "crossref",
"first-page": "55",
"journal-title": "Pharmacol. Ther.",
"key": "ref_117",
"volume": "78",
"year": "1998"
},
{
"DOI": "10.1089/107999000750053762",
"article-title": "Caspase-Dependent Apoptosis by 2′,5′-Oligoadenylate Activation of RNase L Is Enhanced by IFN-Beta",
"author": "Rusch",
"doi-asserted-by": "crossref",
"first-page": "1091",
"journal-title": "J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res.",
"key": "ref_118",
"volume": "20",
"year": "2000"
},
{
"DOI": "10.1038/s41598-020-78208-2",
"article-title": "Survivin and Caspases Serum Protein Levels and Survivin Variants mRNA Expression in Sepsis",
"author": "Miliaraki",
"doi-asserted-by": "crossref",
"first-page": "1049",
"journal-title": "Sci. Rep.",
"key": "ref_119",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.20944/preprints201705.0176.v1",
"doi-asserted-by": "crossref",
"key": "ref_120",
"unstructured": "Gammoh, N.Z., and Rink, L. (2017). Zinc in Infection and Inflammation. Nutrients, 9."
},
{
"DOI": "10.1073/pnas.1323549111",
"article-title": "Zinc Transporter SLC39A10/ZIP10 Facilitates Antiapoptotic Signaling during Early B-Cell Development",
"author": "Miyai",
"doi-asserted-by": "crossref",
"first-page": "11780",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_121",
"volume": "111",
"year": "2014"
},
{
"article-title": "The Role of Zinc in Caspase Activation and Apoptotic Cell Death",
"author": "Carter",
"first-page": "315",
"journal-title": "Biometals Int. J. Role Met. Ions Biol. Biochem. Med.",
"key": "ref_122",
"volume": "14",
"year": "2001"
},
{
"DOI": "10.1074/jbc.272.41.25719",
"article-title": "Biochemical Characteristics of Caspases-3, -6, -7, and -8",
"author": "Stennicke",
"doi-asserted-by": "crossref",
"first-page": "25719",
"journal-title": "J. Biol. Chem.",
"key": "ref_123",
"volume": "272",
"year": "1997"
},
{
"DOI": "10.1152/ajpgi.00442.2014",
"article-title": "Zinc Deficiency Mediates Alcohol-Induced Apoptotic Cell Death in the Liver of Rats through Activating ER and Mitochondrial Cell Death Pathways",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "G757",
"journal-title": "Am. J. Physiol. Gastrointest. Liver Physiol.",
"key": "ref_124",
"volume": "308",
"year": "2015"
},
{
"DOI": "10.1016/j.cellsig.2013.02.003",
"article-title": "ZnT7 Can Protect MC3T3-E1 Cells from Oxidative Stress-Induced Apoptosis via PI3K/Akt and MAPK/ERK Signaling Pathways",
"author": "Liang",
"doi-asserted-by": "crossref",
"first-page": "1126",
"journal-title": "Cell. Signal.",
"key": "ref_125",
"volume": "25",
"year": "2013"
},
{
"DOI": "10.1146/annurev.physiol.60.1.601",
"article-title": "Cell Cycle Regulation and Apoptosis",
"author": "King",
"doi-asserted-by": "crossref",
"first-page": "601",
"journal-title": "Annu. Rev. Physiol.",
"key": "ref_126",
"volume": "60",
"year": "1998"
},
{
"DOI": "10.4103/2045-8932.105032",
"article-title": "Functional Role of Intracellular Labile Zinc in Pulmonary Endothelium",
"author": "Thambiayya",
"doi-asserted-by": "crossref",
"first-page": "443",
"journal-title": "Pulm. Circ.",
"key": "ref_127",
"volume": "2",
"year": "2012"
},
{
"DOI": "10.1016/j.micinf.2023.105179",
"doi-asserted-by": "crossref",
"key": "ref_128",
"unstructured": "Palacios, Y., Ramón-Luing, L.A., Ruiz, A., García-Martínez, A., Sánchez-Monciváis, A., Barreto-Rodríguez, O., Falfán-Valencia, R., Pérez-Rubio, G., Medina-Quero, K., and Buendia-Roldan, I. (2023). COVID-19 Patients with High TNF/IFN-γ Levels Show Hallmarks of PANoptosis, an Inflammatory Cell Death. Microbes Infect."
},
{
"DOI": "10.3390/nu10080976",
"doi-asserted-by": "crossref",
"key": "ref_129",
"unstructured": "Alker, W., and Haase, H. (2018). Zinc and Sepsis. Nutrients, 10."
},
{
"DOI": "10.1093/jn/132.5.974",
"article-title": "Apoptosis Plays a Distinct Role in the Loss of Precursor Lymphocytes during Zinc Deficiency in Mice",
"author": "King",
"doi-asserted-by": "crossref",
"first-page": "974",
"journal-title": "J. Nutr.",
"key": "ref_130",
"volume": "132",
"year": "2002"
},
{
"DOI": "10.1086/315916",
"article-title": "Effects of Zinc Deficiency on Th1 and Th2 Cytokine Shifts",
"author": "Prasad",
"doi-asserted-by": "crossref",
"first-page": "S62",
"journal-title": "J. Infect. Dis.",
"key": "ref_131",
"volume": "182",
"year": "2000"
},
{
"DOI": "10.1016/j.jphs.2017.11.003",
"article-title": "Effects of Histidine-Rich Glycoprotein on Erythrocyte Aggregation and Hemolysis: Implications for a Role under Septic Conditions",
"author": "Zhong",
"doi-asserted-by": "crossref",
"first-page": "97",
"journal-title": "J. Pharmacol. Sci.",
"key": "ref_132",
"volume": "136",
"year": "2018"
},
{
"DOI": "10.1016/j.jtemb.2023.127152",
"doi-asserted-by": "crossref",
"key": "ref_133",
"unstructured": "Guttek, K., Reinhold, A., Grüngreiff, K., Schraven, B., and Reinhold, D. (2023). Zinc Aspartate Induces Proliferation of Resting and Antigen-Stimulated Human PBMC under High-Density Cell Culture Condition. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS, 77."
},
{
"DOI": "10.1038/s41598-022-19614-6",
"article-title": "Toll-like Receptor 4-Mediated Endoplasmic Reticulum Stress Induces Intestinal Paneth Cell Damage in Mice Following CLP-Induced Sepsis",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "15256",
"journal-title": "Sci. Rep.",
"key": "ref_134",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.4110/in.2023.23.e26",
"article-title": "SARS-CoV-2 Infection Induces HMGB1 Secretion Through Post-Translational Modification and PANoptosis",
"author": "Kwak",
"doi-asserted-by": "crossref",
"first-page": "e26",
"journal-title": "Immune Netw.",
"key": "ref_135",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1038/s41420-023-01581-0",
"article-title": "Different Types of Cell Death and Their Shift in Shaping Disease",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "284",
"journal-title": "Cell Death Discov.",
"key": "ref_136",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1146/annurev-immunol-032414-112248",
"article-title": "Programmed Necrosis in the Cross Talk of Cell Death and Inflammation",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Annu. Rev. Immunol.",
"key": "ref_137",
"volume": "33",
"year": "2015"
},
{
"DOI": "10.14309/ajg.0000000000000620",
"article-title": "Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "766",
"journal-title": "Am. J. Gastroenterol.",
"key": "ref_138",
"volume": "115",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2021.640073",
"article-title": "New Insights Into the Physiopathology of COVID-19: SARS-CoV-2-Associated Gastrointestinal Illness",
"author": "Devaux",
"doi-asserted-by": "crossref",
"first-page": "640073",
"journal-title": "Front. Med.",
"key": "ref_139",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.3390/v10050225",
"doi-asserted-by": "crossref",
"key": "ref_140",
"unstructured": "Holly, M.K., and Smith, J.G. (2018). Paneth Cells during Viral Infection and Pathogenesis. Viruses, 10."
},
{
"DOI": "10.1016/j.jcmgh.2015.12.006",
"article-title": "ZnT2-Mediated Zinc Import Into Paneth Cell Granules Is Necessary for Coordinated Secretion and Paneth Cell Function in Mice",
"author": "Podany",
"doi-asserted-by": "crossref",
"first-page": "369",
"journal-title": "Cell. Mol. Gastroenterol. Hepatol.",
"key": "ref_141",
"volume": "2",
"year": "2016"
},
{
"DOI": "10.1007/BF01006887",
"article-title": "Paneth Cell Zinc: A Comparison of Histochemical and Microanalytical Techniques",
"author": "Elmes",
"doi-asserted-by": "crossref",
"first-page": "335",
"journal-title": "Histochem. J.",
"key": "ref_142",
"volume": "13",
"year": "1981"
},
{
"DOI": "10.1016/j.jnutbio.2012.06.020",
"article-title": "Intracellular Zinc Is Required for Intestinal Cell Survival Signals Triggered by the Inflammatory Cytokine TNFα",
"author": "Ranaldi",
"doi-asserted-by": "crossref",
"first-page": "967",
"journal-title": "J. Nutr. Biochem.",
"key": "ref_143",
"volume": "24",
"year": "2013"
},
{
"DOI": "10.1371/journal.pgen.1006349",
"doi-asserted-by": "crossref",
"key": "ref_144",
"unstructured": "Ohashi, W., Kimura, S., Iwanaga, T., Furusawa, Y., Irié, T., Izumi, H., Watanabe, T., Hijikata, A., Hara, T., and Ohara, O. (2016). Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress. PLoS Genet., 12."
},
{
"DOI": "10.1038/ncb2581",
"article-title": "Dll1+ Secretory Progenitor Cells Revert to Stem Cells upon Crypt Damage",
"author": "Sato",
"doi-asserted-by": "crossref",
"first-page": "1099",
"journal-title": "Nat. Cell Biol.",
"key": "ref_145",
"volume": "14",
"year": "2012"
},
{
"DOI": "10.1186/1746-6148-10-75",
"doi-asserted-by": "crossref",
"key": "ref_146",
"unstructured": "Chai, W., Zakrzewski, S.S., Günzel, D., Pieper, R., Wang, Z., Twardziok, S., Janczyk, P., Osterrieder, N., and Burwinkel, M. (2014). High-Dose Dietary Zinc Oxide Mitigates Infection with Transmissible Gastroenteritis Virus in Piglets. BMC Vet. Res., 10."
},
{
"DOI": "10.1172/jci.insight.128834",
"article-title": "Necroptosis: A Crucial Pathogenic Mediator of Human Disease",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "e128834",
"journal-title": "JCI Insight",
"key": "ref_147",
"volume": "4",
"year": "2019"
},
{
"DOI": "10.1038/s41598-021-88970-6",
"article-title": "Association of Plasma Level of High-Mobility Group Box-1 with Necroptosis and Sepsis Outcomes",
"author": "Yoo",
"doi-asserted-by": "crossref",
"first-page": "9512",
"journal-title": "Sci. Rep.",
"key": "ref_148",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.2217/imt-2022-0219",
"article-title": "A New Perspective on the Potential Application of RIPK1 in the Treatment of Sepsis",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Immunotherapy",
"key": "ref_149",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1038/s41418-018-0215-3",
"article-title": "ABIN-1 Heterozygosity Sensitizes to Innate Immune Response in Both RIPK1-Dependent and RIPK1-Independent Manner",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "1077",
"journal-title": "Cell Death Differ.",
"key": "ref_150",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.1016/j.immuni.2016.06.007",
"article-title": "RIPK1 and RIPK3 Kinases Promote Cell-Death-Independent Inflammation by Toll-like Receptor 4",
"author": "Najjar",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Immunity",
"key": "ref_151",
"volume": "45",
"year": "2016"
},
{
"DOI": "10.4049/jimmunol.1400590",
"article-title": "Cutting Edge: RIPK1 Kinase Inactive Mice Are Viable and Protected from TNF-Induced Necroptosis in Vivo",
"author": "Polykratis",
"doi-asserted-by": "crossref",
"first-page": "1539",
"journal-title": "J. Immunol.",
"key": "ref_152",
"volume": "193",
"year": "2014"
},
{
"DOI": "10.1038/s41418-019-0347-0",
"article-title": "RIP1 Inhibition Blocks Inflammatory Diseases but Not Tumor Growth or Metastases",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "161",
"journal-title": "Cell Death Differ.",
"key": "ref_153",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1172/JCI96147",
"article-title": "RIP Kinase 1-Dependent Endothelial Necroptosis Underlies Systemic Inflammatory Response Syndrome",
"author": "Zelic",
"doi-asserted-by": "crossref",
"first-page": "2064",
"journal-title": "J. Clin. Investig.",
"key": "ref_154",
"volume": "128",
"year": "2018"
},
{
"DOI": "10.1016/j.surg.2018.02.017",
"article-title": "Inhibition of Necroptosis Attenuates Lung Injury and Improves Survival in Neonatal Sepsis",
"author": "Bolognese",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "Surgery",
"key": "ref_155",
"volume": "164",
"year": "2018"
},
{
"DOI": "10.1038/s41419-020-03365-1",
"article-title": "Necroptosis Is Active and Contributes to Intestinal Injury in a Piglet Model with Lipopolysaccharide Challenge",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Cell Death Dis.",
"key": "ref_156",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.molcel.2020.11.008",
"article-title": "RIPK1 Promotes Energy Sensing by the mTORC1 Pathway",
"author": "Najafov",
"doi-asserted-by": "crossref",
"first-page": "370",
"journal-title": "Mol. Cell",
"key": "ref_157",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1186/s13075-021-02468-0",
"article-title": "A Randomized, Placebo-Controlled Experimental Medicine Study of RIPK1 Inhibitor GSK2982772 in Patients with Moderate to Severe Rheumatoid Arthritis",
"author": "Weisel",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Arthritis Res. Ther.",
"key": "ref_158",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.3390/biom11050646",
"doi-asserted-by": "crossref",
"key": "ref_159",
"unstructured": "Speir, M., Djajawi, T.M., Conos, S.A., Tye, H., and Lawlor, K.E. (2021). Targeting RIP Kinases in Chronic Inflammatory Disease. Biomolecules, 11."
},
{
"DOI": "10.1016/j.tips.2020.01.002",
"article-title": "Inhibitors Targeting RIPK1/RIPK3: Old and New Drugs",
"author": "Martens",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Trends Pharmacol. Sci.",
"key": "ref_160",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1016/j.isci.2022.105748",
"article-title": "Early Alveolar Epithelial Cell Necrosis Is a Potential Driver of COVID-19-Induced Acute Respiratory Distress Syndrome",
"author": "Tojo",
"doi-asserted-by": "crossref",
"first-page": "105748",
"journal-title": "iScience",
"key": "ref_161",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.21203/rs.3.rs-3059466/v1",
"doi-asserted-by": "crossref",
"key": "ref_162",
"unstructured": "Riegler, A., Benson, P., Long, K., and Leal, S. (2023). Differential Activation of Programmed Cell Death in Patients with Severe SARS-CoV-2 Infection. Res. Sq."
},
{
"DOI": "10.4103/abr.abr_232_22",
"doi-asserted-by": "crossref",
"key": "ref_163",
"unstructured": "Heidarvand, M., Hosseini, R., Kazemi, M., Andalib, A., Sami, R., Eskandari, N., and Ghezelbash, B. (2023). Differentially Expressed Inflammatory Cell Death-Related Genes and the Serum Levels of IL-6 Are Determinants for Severity of Coronaviruses Diseases-2019 (COVID-19). Adv. Biomed. Res., 12."
},
{
"DOI": "10.21203/rs.3.rs-2468706/v1",
"doi-asserted-by": "crossref",
"key": "ref_164",
"unstructured": "Wiscovitch-Russo, R., Ibáñez-Prada, E.D., Serrano-Mayorga, C.C., Sievers, B.L., Engelbride, M.A., Padmanabhan, S., Tan, G.S., Vashee, S., Bustos, I.G., and Pachecho, C. (2023). Necroptosis Drives Major Adverse Cardiovascular Events During Severe COVID-19. Res. Sq."
},
{
"DOI": "10.1093/infdis/jiad056",
"article-title": "The Defenders of the Alveolus Succumb in COVID-19 Pneumonia to SARS-CoV-2 and Necroptosis, Pyroptosis, and PANoptosis",
"author": "Schifanella",
"doi-asserted-by": "crossref",
"first-page": "1245",
"journal-title": "J. Infect. Dis.",
"key": "ref_165",
"volume": "227",
"year": "2023"
},
{
"DOI": "10.1186/s40001-022-00913-7",
"article-title": "Potential Therapeutic Value of Necroptosis Inhibitor for the Treatment of COVID-19",
"author": "Kang",
"doi-asserted-by": "crossref",
"first-page": "283",
"journal-title": "Eur. J. Med. Res.",
"key": "ref_166",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1016/j.toxlet.2021.12.015",
"article-title": "Zinc Finger Protein 91 Mediates Necroptosis by Initiating RIPK1-RIPK3-MLKL Signal Transduction in Response to TNF Receptor 1 Ligation",
"author": "Zhong",
"doi-asserted-by": "crossref",
"first-page": "75",
"journal-title": "Toxicol. Lett.",
"key": "ref_167",
"volume": "356",
"year": "2022"
},
{
"DOI": "10.1038/s41418-018-0192-6",
"article-title": "Systematic Genetic Mapping of Necroptosis Identifies SLC39A7 as Modulator of Death Receptor Trafficking",
"author": "Fauster",
"doi-asserted-by": "crossref",
"first-page": "1138",
"journal-title": "Cell Death Differ.",
"key": "ref_168",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.1042/bj20031377",
"article-title": "Zinc-Finger Protein A20, a Regulator of Inflammation and Cell Survival, Has de-Ubiquitinating Activity",
"author": "Evans",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "Biochem. J.",
"key": "ref_169",
"volume": "378",
"year": "2004"
},
{
"DOI": "10.1038/ni1110",
"article-title": "The Ubiquitin-Modifying Enzyme A20 Is Required for Termination of Toll-like Receptor Responses",
"author": "Boone",
"doi-asserted-by": "crossref",
"first-page": "1052",
"journal-title": "Nat. Immunol.",
"key": "ref_170",
"volume": "5",
"year": "2004"
},
{
"DOI": "10.3389/fnut.2022.1003340",
"article-title": "Crosstalk between Regulated Necrosis and Micronutrition, Bridged by Reactive Oxygen Species",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1003340",
"journal-title": "Front. Nutr.",
"key": "ref_171",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1038/s41556-019-0324-3",
"article-title": "A20 Prevents Inflammasome-Dependent Arthritis by Inhibiting Macrophage Necroptosis through Its ZnF7 Ubiquitin-Binding Domain",
"author": "Polykratis",
"doi-asserted-by": "crossref",
"first-page": "731",
"journal-title": "Nat. Cell Biol.",
"key": "ref_172",
"volume": "21",
"year": "2019"
},
{
"DOI": "10.1038/ni.3174",
"article-title": "A20 Is a Regulator of Necroptosis",
"author": "Gurung",
"doi-asserted-by": "crossref",
"first-page": "596",
"journal-title": "Nat. Immunol.",
"key": "ref_173",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1016/j.jid.2017.03.031",
"article-title": "Requirement of Zinc Transporter SLC39A7/ZIP7 for Dermal Development to Fine-Tune Endoplasmic Reticulum Function by Regulating Protein Disulfide Isomerase",
"author": "Bin",
"doi-asserted-by": "crossref",
"first-page": "1682",
"journal-title": "J. Investig. Dermatol.",
"key": "ref_174",
"volume": "137",
"year": "2017"
},
{
"DOI": "10.15252/emmm.201911917",
"article-title": "Zinc Inhibits Lethal Inflammatory Shock by Preventing Microbe-Induced Interferon Signature in Intestinal Epithelium",
"author": "Souffriau",
"doi-asserted-by": "crossref",
"first-page": "e11917",
"journal-title": "EMBO Mol. Med.",
"key": "ref_175",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1038/s41556-017-0003-1",
"article-title": "ABIN-1 Regulates RIPK1 Activation by Linking Met1 Ubiquitylation with Lys63 Deubiquitylation in TNF-RSC",
"author": "Dziedzic",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "Nat. Cell Biol.",
"key": "ref_176",
"volume": "20",
"year": "2018"
},
{
"DOI": "10.1038/nature07575",
"article-title": "ABIN-1 Is a Ubiquitin Sensor That Restricts Cell Death and Sustains Embryonic Development",
"author": "Oshima",
"doi-asserted-by": "crossref",
"first-page": "906",
"journal-title": "Nature",
"key": "ref_177",
"volume": "457",
"year": "2009"
},
{
"DOI": "10.1016/j.cell.2011.11.031",
"article-title": "Mixed Lineage Kinase Domain-like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "213",
"journal-title": "Cell",
"key": "ref_178",
"volume": "148",
"year": "2012"
},
{
"DOI": "10.1016/j.cell.2012.06.019",
"article-title": "The RIP1/RIP3 Necrosome Forms a Functional Amyloid Signaling Complex Required for Programmed Necrosis",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "339",
"journal-title": "Cell",
"key": "ref_179",
"volume": "150",
"year": "2012"
},
{
"DOI": "10.1101/gad.312561.118",
"article-title": "Necroptosis in Development and Diseases",
"author": "Shan",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Genes Dev.",
"key": "ref_180",
"volume": "32",
"year": "2018"
},
{
"DOI": "10.1038/s41586-019-1548-x",
"article-title": "Cleavage of RIPK1 by Caspase-8 Is Crucial for Limiting Apoptosis and Necroptosis",
"author": "Newton",
"doi-asserted-by": "crossref",
"first-page": "428",
"journal-title": "Nature",
"key": "ref_181",
"volume": "574",
"year": "2019"
},
{
"DOI": "10.3945/ajcn.2010.28674I",
"article-title": "Zinc Bioavailability and Homeostasis",
"author": "Hambidge",
"doi-asserted-by": "crossref",
"first-page": "1478S",
"journal-title": "Am. J. Clin. Nutr.",
"key": "ref_182",
"volume": "91",
"year": "2010"
},
{
"DOI": "10.1074/jbc.M109.003459",
"article-title": "Structure, Properties, and Engineering of the Major Zinc Binding Site on Human Albumin",
"author": "Blindauer",
"doi-asserted-by": "crossref",
"first-page": "23116",
"journal-title": "J. Biol. Chem.",
"key": "ref_183",
"volume": "284",
"year": "2009"
},
{
"DOI": "10.1111/bcp.14826",
"article-title": "Zinc Supplementation as an Adjunct Therapy for COVID-19: Challenges and Opportunities",
"author": "Chinni",
"doi-asserted-by": "crossref",
"first-page": "3737",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_184",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.3945/ajcn.2010.28674B",
"article-title": "Micronutrient Bioavailability: Dietary Reference Intakes and a Future Perspective",
"author": "Hambidge",
"doi-asserted-by": "crossref",
"first-page": "1430S",
"journal-title": "Am. J. Clin. Nutr.",
"key": "ref_185",
"volume": "91",
"year": "2010"
},
{
"DOI": "10.1024/0300-9831/a000030",
"article-title": "Gastrointestinal Factors Influencing Zinc Absorption and Homeostasis",
"author": "Cousins",
"doi-asserted-by": "crossref",
"first-page": "243",
"journal-title": "Int. J. Vitam. Nutr. Res. Int. Z. Vitam. Ernahrungsforschung J. Int. Vitaminol. Nutr.",
"key": "ref_186",
"volume": "80",
"year": "2010"
},
{
"article-title": "Zinc: Physiology, Deficiency, and Parenteral Nutrition",
"author": "Livingstone",
"first-page": "371",
"journal-title": "Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr.",
"key": "ref_187",
"volume": "30",
"year": "2015"
},
{
"article-title": "Cellular Zinc and Redox Buffering Capacity of Metallothionein/Thionein in Health and Disease",
"author": "Maret",
"first-page": "371",
"journal-title": "Mol. Med. Camb. Mass",
"key": "ref_188",
"volume": "13",
"year": "2007"
},
{
"DOI": "10.1152/ajpcell.00541.2007",
"article-title": "Insights into Zn2+ Homeostasis in Neurons from Experimental and Modeling Studies",
"author": "Colvin",
"doi-asserted-by": "crossref",
"first-page": "C726",
"journal-title": "Am. J. Physiol. Cell Physiol.",
"key": "ref_189",
"volume": "294",
"year": "2008"
},
{
"DOI": "10.1038/350564b0",
"article-title": "Homeostatic Muffling",
"author": "Thomas",
"doi-asserted-by": "crossref",
"first-page": "564",
"journal-title": "Nature",
"key": "ref_190",
"volume": "350",
"year": "1991"
},
{
"DOI": "10.3390/nu9121286",
"doi-asserted-by": "crossref",
"key": "ref_191",
"unstructured": "Wessels, I., Maywald, M., and Rink, L. (2017). Zinc as a Gatekeeper of Immune Function. Nutrients, 9."
},
{
"article-title": "Intracellular Zinc Distribution in Mitochondria, ER and the Golgi Apparatus",
"author": "Lu",
"first-page": "35",
"journal-title": "Int. J. Physiol. Pathophysiol. Pharmacol.",
"key": "ref_192",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1039/C6SC02267G",
"article-title": "Circulatory Zinc Transport Is Controlled by Distinct Interdomain Sites on Mammalian Albumins",
"author": "Handing",
"doi-asserted-by": "crossref",
"first-page": "6635",
"journal-title": "Chem. Sci.",
"key": "ref_193",
"volume": "7",
"year": "2016"
},
{
"article-title": "Albumin Facilitates Zinc Acquisition by Endothelial Cells",
"author": "Rowe",
"first-page": "178",
"journal-title": "Proc. Soc. Exp. Biol. Med.",
"key": "ref_194",
"volume": "224",
"year": "2000"
},
{
"DOI": "10.1016/j.bbagen.2013.05.028",
"article-title": "Allosteric Modulation of Zinc Speciation by Fatty Acids",
"author": "Barnett",
"doi-asserted-by": "crossref",
"first-page": "5456",
"journal-title": "Biochim. Biophys. Acta",
"key": "ref_195",
"volume": "1830",
"year": "2013"
},
{
"DOI": "10.1007/s12011-019-01671-0",
"article-title": "Comparative Response of Cardiomyocyte ZIPs and ZnTs to Extracellular Zinc and TPEN",
"author": "Thokala",
"doi-asserted-by": "crossref",
"first-page": "297",
"journal-title": "Biol. Trace Elem. Res.",
"key": "ref_196",
"volume": "192",
"year": "2019"
},
{
"DOI": "10.1155/2018/9365747",
"article-title": "Function, Structure, and Transport Aspects of ZIP and ZnT Zinc Transporters in Immune Cells",
"author": "Bin",
"doi-asserted-by": "crossref",
"first-page": "9365747",
"journal-title": "J. Immunol. Res.",
"key": "ref_197",
"volume": "2018",
"year": "2018"
},
{
"DOI": "10.1189/jlb.1208759",
"article-title": "Zinc Transporter ZIP8 (SLC39A8) and Zinc Influence IFN-Gamma Expression in Activated Human T Cells",
"author": "Aydemir",
"doi-asserted-by": "crossref",
"first-page": "337",
"journal-title": "J. Leukoc. Biol.",
"key": "ref_198",
"volume": "86",
"year": "2009"
},
{
"article-title": "Mechanism and Regulation of Cellular Zinc Transport",
"author": "Sekler",
"first-page": "337",
"journal-title": "Mol. Med. Camb. Mass",
"key": "ref_199",
"volume": "13",
"year": "2007"
},
{
"DOI": "10.1039/b926662c",
"article-title": "Cytosolic Zinc Buffering and Muffling: Their Role in Intracellular Zinc Homeostasis",
"author": "Colvin",
"doi-asserted-by": "crossref",
"first-page": "306",
"journal-title": "Met. Integr. Biometal Sci.",
"key": "ref_200",
"volume": "2",
"year": "2010"
},
{
"DOI": "10.1152/ajpgi.00179.2015",
"article-title": "Zinc Dyshomeostasis during Polymicrobial Sepsis in Mice Involves Zinc Transporter Zip14 and Can Be Overcome by Zinc Supplementation",
"author": "Wessels",
"doi-asserted-by": "crossref",
"first-page": "G768",
"journal-title": "Am. J. Physiol. Gastrointest. Liver Physiol.",
"key": "ref_201",
"volume": "309",
"year": "2015"
},
{
"DOI": "10.1007/s12011-023-03824-8",
"doi-asserted-by": "crossref",
"key": "ref_202",
"unstructured": "Gumus, M., Gulbahce-Mutlu, E., Unal, O., Baltaci, S.B., Unlukal, N., Mogulkoc, R., and Baltaci, A.K. (2023). Marginal Maternal Zinc Deficiency Produces Liver Damage and Altered Zinc Transporter Expression in Offspring Male Rats. Biol. Trace Elem. Res."
},
{
"DOI": "10.1177/153537020623100509",
"article-title": "Marginal Zinc Deficiency Increased the Susceptibility to Acute Lipopolysaccharide-Induced Liver Injury in Rats",
"author": "Dojka",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Exp. Biol. Med.",
"key": "ref_203",
"volume": "231",
"year": "2006"
},
{
"DOI": "10.1097/MCC.0000000000001062",
"article-title": "An Update on Essential Micronutrients in Critical Illness",
"author": "Koekkoek",
"doi-asserted-by": "crossref",
"first-page": "315",
"journal-title": "Curr. Opin. Crit. Care",
"key": "ref_204",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1177/0148607108322402",
"article-title": "Zinc Supplementation in Critically Ill Patients: A Key Pharmaconutrient?",
"author": "Heyland",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "JPEN J. Parenter. Enteral Nutr.",
"key": "ref_205",
"volume": "32",
"year": "2008"
},
{
"DOI": "10.1177/0148607115572193",
"article-title": "Safety and Dose Escalation Study of Intravenous Zinc Supplementation in Pediatric Critical Illness",
"author": "Cvijanovich",
"doi-asserted-by": "crossref",
"first-page": "860",
"journal-title": "JPEN J. Parenter. Enteral Nutr.",
"key": "ref_206",
"volume": "40",
"year": "2016"
},
{
"DOI": "10.3945/ajcn.111.023812",
"article-title": "Quantitative Data on the Magnitude of the Systemic Inflammatory Response and Its Effect on Micronutrient Status Based on Plasma Measurements",
"author": "Duncan",
"doi-asserted-by": "crossref",
"first-page": "64",
"journal-title": "Am. J. Clin. Nutr.",
"key": "ref_207",
"volume": "95",
"year": "2012"
},
{
"DOI": "10.1093/jn/133.5.1485S",
"article-title": "Zinc Deficiency, Infectious Disease and Mortality in the Developing World",
"author": "Black",
"doi-asserted-by": "crossref",
"first-page": "1485S",
"journal-title": "J. Nutr.",
"key": "ref_208",
"volume": "133",
"year": "2003"
},
{
"DOI": "10.3945/ajcn.110.008417",
"article-title": "A Comparison of Zinc Metabolism, Inflammation, and Disease Severity in Critically Ill Infected and Noninfected Adults Early after Intensive Care Unit Admission",
"author": "Besecker",
"doi-asserted-by": "crossref",
"first-page": "1356",
"journal-title": "Am. J. Clin. Nutr.",
"key": "ref_209",
"volume": "93",
"year": "2011"
},
{
"DOI": "10.3390/nu15020340",
"doi-asserted-by": "crossref",
"key": "ref_210",
"unstructured": "Almasaud, A.S., Chalabi, J., Arfaj, A.A., Qarni, A.A., Alkroud, A., Nagoor, Z., Akhtar, S., and Iqbal, J. (2023). Association of Serum Zinc and Inflammatory Markers with the Severity of COVID-19 Infection in Adult Patients. Nutrients, 15."
},
{
"DOI": "10.1111/j.1399-6576.2011.02425.x",
"article-title": "Serum Zinc in Critically Ill Adult Patients with Acute Respiratory Failure",
"author": "Linko",
"doi-asserted-by": "crossref",
"first-page": "615",
"journal-title": "Acta Anaesthesiol. Scand.",
"key": "ref_211",
"volume": "55",
"year": "2011"
},
{
"DOI": "10.1007/s12011-018-1429-4",
"article-title": "Serum Concentrations of Trace Elements Zinc, Copper, Selenium, and Manganese in Critically Ill Patients",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "316",
"journal-title": "Biol. Trace Elem. Res.",
"key": "ref_212",
"volume": "188",
"year": "2019"
},
{
"article-title": "Antioxidant Vitamins and Trace Elements in Critical Illness",
"author": "Koekkoek",
"first-page": "457",
"journal-title": "Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr.",
"key": "ref_213",
"volume": "31",
"year": "2016"
},
{
"DOI": "10.1016/j.jcrc.2010.06.002",
"article-title": "Prognostic Value of Serum Zinc Levels in Critically Ill Patients",
"author": "Cander",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "J. Crit. Care",
"key": "ref_214",
"volume": "26",
"year": "2011"
},
{
"article-title": "Tissue-Specific Regulation of Zinc Metabolism and Metallothionein Genes by Interleukin 1",
"author": "Cousins",
"first-page": "2884",
"journal-title": "FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol.",
"key": "ref_215",
"volume": "2",
"year": "1988"
},
{
"DOI": "10.1073/pnas.0502257102",
"article-title": "Interleukin-6 Regulates the Zinc Transporter Zip14 in Liver and Contributes to the Hypozincemia of the Acute-Phase Response",
"author": "Liuzzi",
"doi-asserted-by": "crossref",
"first-page": "6843",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_216",
"volume": "102",
"year": "2005"
},
{
"article-title": "Pitfalls in the Interpretation of Blood Tests Used to Assess and Monitor Micronutrient Nutrition Status",
"author": "Berger",
"first-page": "56",
"journal-title": "Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr.",
"key": "ref_217",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1016/j.clnu.2022.09.015",
"article-title": "Micronutrients: A Low Blood Concentration Is Not Equivalent to Deficiency",
"author": "Shenkin",
"doi-asserted-by": "crossref",
"first-page": "2562",
"journal-title": "Clin. Nutr.",
"key": "ref_218",
"volume": "41",
"year": "2022"
},
{
"DOI": "10.1016/j.clnu.2020.06.005",
"article-title": "Monitoring and Parenteral Administration of Micronutrients, Phosphate and Magnesium in Critically Ill Patients: The VITA-TRACE Survey",
"author": "Vankrunkelsven",
"doi-asserted-by": "crossref",
"first-page": "590",
"journal-title": "Clin. Nutr.",
"key": "ref_219",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1080/10408398.2022.2132212",
"doi-asserted-by": "crossref",
"key": "ref_220",
"unstructured": "Kadac-Czapska, K., Knez, E., and Grembecka, M. (2022). Food and Human Safety: The Impact of Microplastics. Crit. Rev. Food Sci. Nutr."
},
{
"DOI": "10.3389/fnut.2022.1057156",
"article-title": "Existing Knowledge on Zn Status Biomarkers (1963–2021) with a Particular Focus on FADS1 and FADS2 Diagnostic Performance and Recommendations for Further Research",
"author": "Knez",
"doi-asserted-by": "crossref",
"first-page": "1057156",
"journal-title": "Front. Nutr.",
"key": "ref_221",
"volume": "9",
"year": "2022"
},
{
"article-title": "Zinc-Rich Inhibitor of Apoptosis Proteins (IAPs) as Regulatory Factors in the Epithelium of Normal and Inflamed Airways",
"author": "Roscioli",
"first-page": "205",
"journal-title": "Biometals Int. J. Role Met. Ions Biol. Biochem. Med.",
"key": "ref_222",
"volume": "26",
"year": "2013"
},
{
"DOI": "10.1172/jci.insight.86507",
"article-title": "Zinc Deficiency Primes the Lung for Ventilator-Induced Injury",
"author": "Boudreault",
"doi-asserted-by": "crossref",
"first-page": "e86507",
"journal-title": "JCI Insight",
"key": "ref_223",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1371/journal.pone.0176069",
"doi-asserted-by": "crossref",
"key": "ref_224",
"unstructured": "Hoeger, J., Simon, T.-P., Beeker, T., Marx, G., Haase, H., and Schuerholz, T. (2017). Persistent Low Serum Zinc Is Associated with Recurrent Sepsis in Critically Ill Patients—A Pilot Study. PLoS ONE, 12."
},
{
"DOI": "10.1016/j.redox.2020.101764",
"doi-asserted-by": "crossref",
"key": "ref_225",
"unstructured": "Heller, R.A., Sun, Q., Hackler, J., Seelig, J., Seibert, L., Cherkezov, A., Minich, W.B., Seemann, P., Diegmann, J., and Pilz, M. (2021). Prediction of Survival Odds in COVID-19 by Zinc, Age and Selenoprotein P as Composite Biomarker. Redox Biol., 38."
},
{
"article-title": "Analysis of the Predictive Factors for a Critical Illness of COVID-19 during Treatment—Relationship between Serum Zinc Level and Critical Illness of COVID-19",
"author": "Yasui",
"first-page": "230",
"journal-title": "Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis.",
"key": "ref_226",
"volume": "100",
"year": "2020"
},
{
"article-title": "COVID-19: Poor Outcomes in Patients with Zinc Deficiency",
"author": "Jothimani",
"first-page": "343",
"journal-title": "Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis.",
"key": "ref_227",
"volume": "100",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26895",
"article-title": "A Pilot Double-Blind Safety and Feasibility Randomized Controlled Trial of High-Dose Intravenous Zinc in Hospitalized COVID-19 Patients",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "3261",
"journal-title": "J. Med. Virol.",
"key": "ref_228",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.3390/nu14020233",
"doi-asserted-by": "crossref",
"key": "ref_229",
"unstructured": "Sobczyk, M.K., and Gaunt, T.R. (2022). The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study. Nutrients, 14."
},
{
"DOI": "10.1371/journal.ppat.1001176",
"doi-asserted-by": "crossref",
"key": "ref_230",
"unstructured": "te Velthuis, A.J.W., van den Worm, S.H.E., Sims, A.C., Baric, R.S., Snijder, E.J., and van Hemert, M.J. (2010). Zn(2+) Inhibits Coronavirus and Arterivirus RNA Polymerase Activity in Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog., 6."
},
{
"DOI": "10.3390/metabo11090565",
"doi-asserted-by": "crossref",
"key": "ref_231",
"unstructured": "Tomasa-Irriguible, T.-M., Bielsa-Berrocal, L., Bordejé-Laguna, L., Tural-Llàcher, C., Barallat, J., Manresa-Domínguez, J.-M., and Torán-Monserrat, P. (2021). Low Levels of Few Micronutrients May Impact COVID-19 Disease Progression: An Observational Study on the First Wave. Metabolites, 11."
},
{
"DOI": "10.1016/j.clnesp.2021.12.015",
"article-title": "Antioxidant Micronutrient Supplements for Adult Critically Ill Patients: A Bayesian Multiple Treatment Comparisons Meta-Analysis",
"author": "Gudivada",
"doi-asserted-by": "crossref",
"first-page": "78",
"journal-title": "Clin. Nutr. ESPEN",
"key": "ref_232",
"volume": "47",
"year": "2022"
},
{
"DOI": "10.1097/CCM.0000000000002122",
"article-title": "Vitamin D Deficiency in Sepsis: “Body Humors” Imbalance or Sepsis “Epiphenomenon”?",
"author": "Briassoulis",
"doi-asserted-by": "crossref",
"first-page": "376",
"journal-title": "Crit. Care Med.",
"key": "ref_233",
"volume": "45",
"year": "2017"
},
{
"article-title": "Nutrition Is More Than the Sum of Its Parts",
"author": "Briassoulis",
"first-page": "1087",
"journal-title": "Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc.",
"key": "ref_234",
"volume": "19",
"year": "2018"
},
{
"DOI": "10.1016/S0140-6736(12)60477-2",
"article-title": "Zinc as Adjunct Treatment in Infants Aged between 7 and 120 Days with Probable Serious Bacterial Infection: A Randomised, Double-Blind, Placebo-Controlled Trial",
"author": "Bhatnagar",
"doi-asserted-by": "crossref",
"first-page": "2072",
"journal-title": "Lancet",
"key": "ref_235",
"volume": "379",
"year": "2012"
},
{
"key": "ref_236",
"unstructured": "Osman, A. (2023, September 18). Zinc Supplementation in Pediatric Sepsis; a Randomized Controlled Trial; clinicaltrials.gov, 2023, Available online: https://clinicaltrials.gov/study/NCT05366595."
},
{
"article-title": "The Randomized Comparative Pediatric Critical Illness Stress-Induced Immune Suppression (CRISIS) Prevention Trial",
"author": "Carcillo",
"first-page": "165",
"journal-title": "Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc.",
"key": "ref_237",
"volume": "13",
"year": "2012"
},
{
"DOI": "10.1007/s12011-020-02194-9",
"article-title": "Can Zn Be a Critical Element in COVID-19 Treatment?",
"author": "Rahman",
"doi-asserted-by": "crossref",
"first-page": "550",
"journal-title": "Biol. Trace Elem. Res.",
"key": "ref_238",
"volume": "199",
"year": "2021"
},
{
"DOI": "10.1039/c9mt00079h",
"article-title": "Why Is It Worth Testing the Ability of Zinc to Protect against Ischaemia Reperfusion Injury for Human Application",
"author": "Ischia",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "Met. Integr. Biometal Sci.",
"key": "ref_239",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1093/cid/ciac849",
"article-title": "A Potential Role for Zinc to Enhance Treatment for Coronavirus Disease 2019 (COVID-19)?",
"author": "Vinceti",
"doi-asserted-by": "crossref",
"first-page": "192",
"journal-title": "Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am.",
"key": "ref_240",
"volume": "76",
"year": "2023"
},
{
"article-title": "COVID-19 Prophylaxis with Doxycycline and Zinc in Health Care Workers: A Prospective, Randomized, Double-Blind Clinical Trial",
"author": "Stambouli",
"first-page": "553",
"journal-title": "Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis.",
"key": "ref_241",
"volume": "122",
"year": "2022"
},
{
"DOI": "10.3390/nu14061227",
"doi-asserted-by": "crossref",
"key": "ref_242",
"unstructured": "Imran, M., Fatima, W., Alzahrani, A.K., Suhail, N., Alshammari, M.K., Alghitran, A.A., Alshammari, F.N., Ghoneim, M.M., Alshehri, S., and Shakeel, F. (2022). Development of Therapeutic and Prophylactic Zinc Compositions for Use against COVID-19: A Glimpse of the Trends, Inventions, and Patents. Nutrients, 14."
},
{
"DOI": "10.1093/cid/ciac807",
"article-title": "Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial",
"author": "Mhalla",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am.",
"key": "ref_243",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/j.cdnut.2023.101971",
"article-title": "Vitamin D and Zinc Supplementation to Improve Treatment Outcomes among COVID-19 Patients in India: Results from a Double-Blind Randomized Placebo-Controlled Trial",
"author": "Partap",
"doi-asserted-by": "crossref",
"first-page": "101971",
"journal-title": "Curr. Dev. Nutr.",
"key": "ref_244",
"volume": "7",
"year": "2023"
},
{
"key": "ref_245",
"unstructured": "Nouira, P.S. (2023, September 18). Evaluation of the Efficacy and Safety of Zinc in Viral Infections; clinicaltrials.gov, Available online: https://clinicaltrials.gov/study/NCT05212480."
},
{
"key": "ref_246",
"unstructured": "(2023, September 18). Parc de Salut Mar Zinc-Based Nutritional Immunity to Lower Inflammation, Viral Load and COVID-19 Mortality during SARS-CoV-2 Infection.; clinicaltrials.gov, Available online: https://clinicaltrials.gov/study/NCT05778383."
},
{
"key": "ref_247",
"unstructured": "(2023, September 18). St. Francis Hospital, New York A Randomized, Placebo-Controlled Study Evaluating the Efficacy of Zinc for the Treatment of COVID-19 in the Outpatient Setting; clinicaltrials.gov, Available online: https://clinicaltrials.gov/study/NCT04621461."
},
{
"key": "ref_248",
"unstructured": "Suarez, J.D. (2023, September 18). A Randomized, Double-Blinded, Placebo-Controlled, Phase 2 Study to Evaluate the Safety and Efficacy of Sesderma LACTYFERRINTM Forte and Sesderma ZINC DefenseTM (Liposomal Bovine Lactoferrin (LbLf) and Liposomal Zn (LZn)) and Standard of Care (SOC) vs. SOC in the Treatment of Non-Hospitalized Patients with COVID-19; clinicaltrials.gov, Available online: https://clinicaltrials.gov/study/NCT05783180."
},
{
"DOI": "10.1016/j.clnu.2020.10.007",
"article-title": "Considerations for Nutrition Support in Critically Ill Children with COVID-19 and Paediatric Inflammatory Multisystem Syndrome Temporally Associated with COVID-19",
"author": "Marino",
"doi-asserted-by": "crossref",
"first-page": "895",
"journal-title": "Clin. Nutr.",
"key": "ref_249",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1007/s00281-017-0639-8",
"article-title": "Cytokine Storm and Sepsis Disease Pathogenesis",
"author": "Chousterman",
"doi-asserted-by": "crossref",
"first-page": "517",
"journal-title": "Semin. Immunopathol.",
"key": "ref_250",
"volume": "39",
"year": "2017"
},
{
"article-title": "If You Get Good Nutrition, You Will Become Happy; If You Get a Bad One, You Will Become an ICU Philosopher",
"author": "Briassoulis",
"first-page": "89",
"journal-title": "Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc.",
"key": "ref_251",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.3389/fimmu.2022.1022673",
"article-title": "Association of COVID-19 Mortality with Serum Selenium, Zinc and Copper: Six Observational Studies across Europe",
"author": "Demircan",
"doi-asserted-by": "crossref",
"first-page": "1022673",
"journal-title": "Front. Immunol.",
"key": "ref_252",
"volume": "13",
"year": "2022"
}
],
"reference-count": 252,
"references-count": 252,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-3921/12/11/1942"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cell Biology",
"Clinical Biochemistry",
"Molecular Biology",
"Biochemistry",
"Physiology"
],
"subtitle": [],
"title": "The Anti-Oxidative, Anti-Inflammatory, Anti-Apoptotic, and Anti-Necroptotic Role of Zinc in COVID-19 and Sepsis",
"type": "journal-article",
"volume": "12"
}
