A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients
et al., Journal of Medical Virology, doi:10.1002/jmv.26895, Feb 2021
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000019 from 42 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
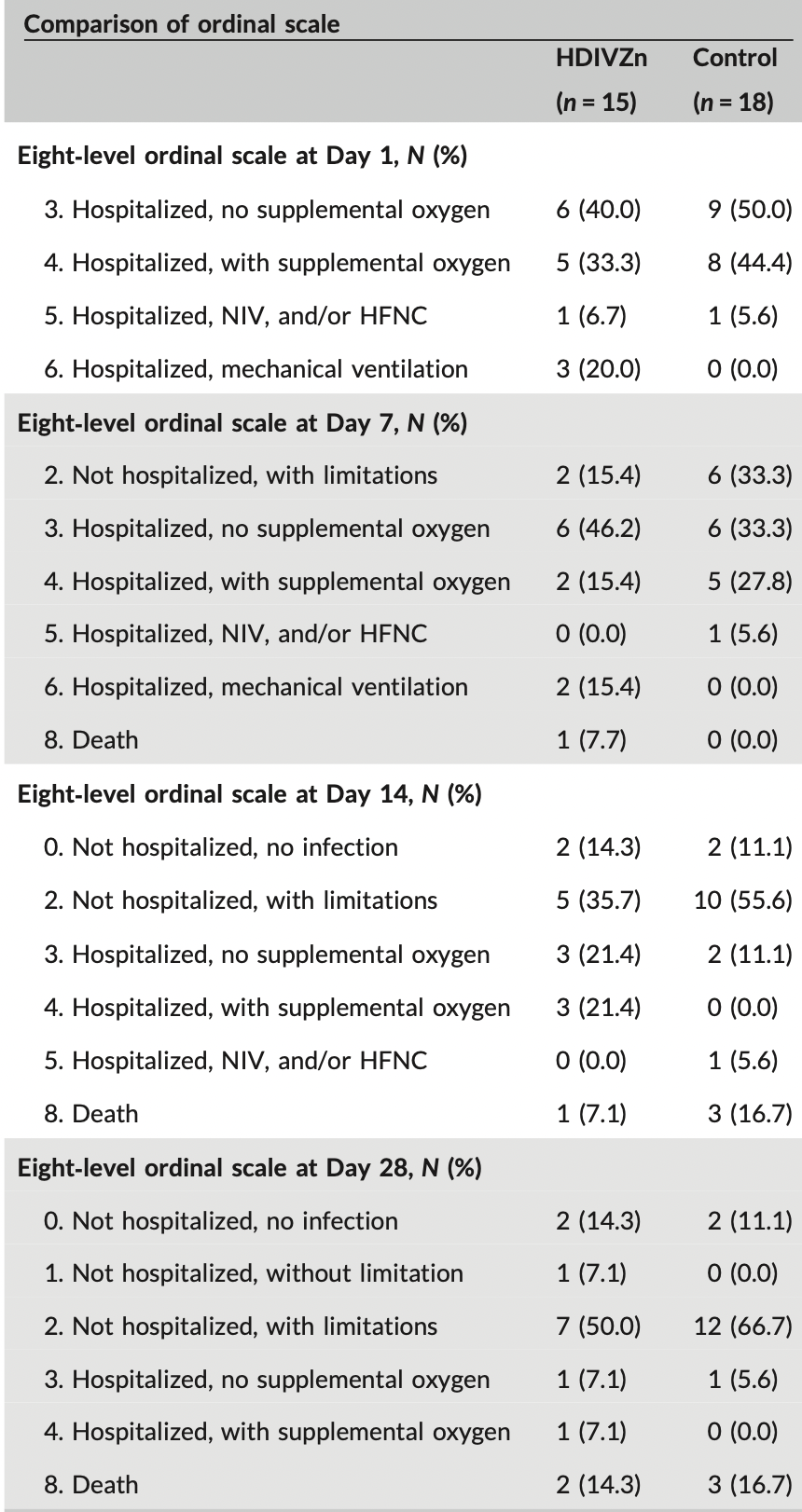
Small early terminated RCT with 33 hospitalized patients in Australia, 15 treated with zinc, showing no significant difference in clinical outcomes. Treatment increased zinc levels above the deficiency cutoff. Intravenous zinc 0.5mg/kg/day (elemental zinc concentration 0.24mg/kg/day) for up to 7 days. ACTRN12620000454976.
In Table 1, lactate for the HDIVZn group is reported as 0.661 +/- 2.1 with a range of 0.8-2.9. The mean (0.661) cannot logically be lower than the minimum value of the range (0.8). Furthermore, the maximum possible SD for a range of 0.8-2.9 is approximately 1.05, making an SD of 2.1 impossible. A similar error exists for the control group. The mean and standard deviation values may have been swapped.
|
risk of death, 20.0% lower, RR 0.80, p = 1.00, treatment 2 of 15 (13.3%), control 3 of 18 (16.7%), NNT 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Patel et al., 25 Feb 2021, Double Blind Randomized Controlled Trial, Australia, peer-reviewed, 12 authors.
A pilot double‐blind safety and feasibility randomized controlled trial of high‐dose intravenous zinc in hospitalized COVID‐19 patients
Journal of Medical Virology, doi:10.1002/jmv.26895
Zinc inhibits replication of the SARS-CoV virus. We aimed to evaluate the safety, feasibility, and biological effect of administering high-dose intravenous zinc (HDIVZn) to patients with COVID-19. We performed a Phase IIa doubleblind, randomized controlled trial to compare HDIVZn to placebo in hospitalized patients with COVID-19. We administered trial treatment per day for a maximum of 7 days until either death or hospital discharge. We measured zinc concentration at baseline and during treatment and observed patients for any significant side effects. For eligible patients, we randomized and administered treatment to 33 adult participants to either HDIVZn (n = 15) or placebo (n = 18). We observed no serious adverse events throughout the study for a total of 94 HDIVZn administrations. However, three participants in the HDIVZn group reported infusion site irritation. Mean serum zinc on Day 1 in the placebo, and the HDIVZn group was 6.9 ± 1.1 and 7.7 ± 1.6 µmol/l, respectively, consistent with zinc deficiency. HDIVZn, but not placebo, increased serum zinc levels above the deficiency cutoff of 10.7 µmol/l (p < .001) on Day 6. Our study did not reach its target enrollment because stringent public health measures markedly reduced patient hospitalizations. Hospitalized COVID-19 patients demonstrated zinc deficiency. This can be corrected with HDIVZn. Such treatment appears safe, feasible, and only associated with minimal peripheral infusion site irritation. This pilot study justifies further investigation of this treatment in COVID-19 patients.
PEER REVIEW The peer review history for this article is available at https://publons. com/publon/10.1002/jmv.26895.
References
Arnold, Ghosh, Cameron, Poliovirus RNA-dependent RNA polymerase (3D(pol)). Divalent cation modulation of primer, template, and nucleotide selection, J Biol Chem
Beckett, Ball, Zinc status of northern Tasmanian adults, J Nutr Sci
Boulware, Pullen, Bangdiwala, A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19, N Engl J Med
Brown, Rivera, International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control, Food Nutr Bull
Butterworth, Korant, Characterization of the large picornaviral polypeptides produced in the presence of zinc ion, J Virol
Carlucci, Ahuja, Petrilli, Rajagopalan, Jones et al., Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients, J Med Microbiol
Derwand, Scholz, Zelenko, COVID-19 outpatients: early riskstratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study, Int J Antimicrob Agents
Duggan, Macleod, Krebs, Plasma zinc concentrations are depressed during the acute phase response in children with falciparum malaria, J Nutr
Femiano, Gombos, Scully, Recurrent herpes labialis: a pilot study of the efficacy of zinc therapy, J Oral Pathol Med
Finzi, Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients, Int J Infect Dis
Hambidge, Miller, Westcott, Sheng, Krebs et al., A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients, Am J Clin Nutr
Heller, Sun, Hackler, Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker, Redox Biol
Hotz, Peerson, Brown, Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980), Am J Clin Nutr
Hulisz, Efficacy of zinc against common cold viruses: an overview, J Am Pharm Assoc
Ischia, Bolton, Patel, Why is it worth testing the ability of zinc to protect against ischaemia reperfusion injury for human application, Metallomics
Jayawardena, Sooriyaarachchi, Chourdakis, Jeewandara, Ranasinghe, Enhancing immunity in viral infections, with special emphasis on COVID-19: a review, Diabetes Metab Syndr
Jothimani, Kailasam, Danielraj, COVID-19: poor outcomes in patients with zinc deficiency, Int J Infect Dis
Korant, Kauer, Butterworth, Zinc ions inhibit replication of rhinoviruses, Nature
Lanke, Krenn, Melchers, Seipelt, Van Kuppeveld, PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells, J Gen Virol
Li, Wen, Yu, Observation on clinical efficacy of combined therapy of zinc supplement and jinye baidu granule in treating human cytomegalovirus infection, Zhongguo Zhong Xi Yi Jie He Za Zhi
O'kane, Gibson, May, Zinc preconditioning protects against renal ischaemia reperfusion injury in a preclinical sheep large animal model, BioMetals
Pal, Squitti, Picozza, Zinc and COVID-19: basis of current clinical trials, Biol Trace Elem Res
Perera, El-Khoury, Chinni, Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol, BMJ Open
Rahman, Idid, Can Zn be a critical element in COVID-19 treatment?, Biol Trace Elem Res
Rao, Sethi, Ischia, Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent, PLOS One
Rembach, Hare, Doecke, Decreased serum zinc is an effect of ageing and not Alzheimer's disease, Metallomics
Si, Mcmanus, Zhang, Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway, J Virol
Skalny, Rink, Ajsuvakova, Zinc and respiratory tract infections: perspectives for COVID-19 (Review), Int J Mol Med
Suara, Crowe, Effect of zinc salts on respiratory syncytial virus replication, Antimicrob Agents Chemother
Tevelthuis, Van Den Worm, Sims, Baric, Snijder et al., Zn 2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture, PLOS Pathog
Wessels, Rolles, Rink, The potential impact of zinc supplementation on COVID-19 pathogenesis, Front Immunol
Yasui, Yasui, Suzuki, Analysis of the predictive factors for a critical illness of COVID-19 during treatment relationship between serum zinc level and critical illness of COVID-19, Int J Infect Dis
DOI record:
{
"DOI": "10.1002/jmv.26895",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.26895",
"alternative-id": [
"10.1002/jmv.26895"
],
"archive": [
"Portico"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-12-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-01-20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-03-09"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-5628-7205",
"affiliation": [
{
"name": "Department of Surgery The University of Melbourne, Austin Health Heidelberg Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Patel",
"given": "Oneel",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5615-1815",
"affiliation": [
{
"name": "Department of Surgery The University of Melbourne, Austin Health Heidelberg Victoria Australia"
},
{
"name": "Department of Urology Austin Health Heidelberg Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Chinni",
"given": "Vidyasagar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery The University of Melbourne, Austin Health Heidelberg Victoria Australia"
},
{
"name": "Department of Urology Austin Health Heidelberg Victoria Australia"
}
],
"family": "El‐Khoury",
"given": "John",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1138-6389",
"affiliation": [
{
"name": "Department of Surgery The University of Melbourne, Austin Health Heidelberg Victoria Australia"
},
{
"name": "Department of Urology Austin Health Heidelberg Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Perera",
"given": "Marlon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1520-9387",
"affiliation": [
{
"name": "Australian and New Zealand Intensive Care‐Research Centre Monash University Melbourne Victoria Australia"
},
{
"name": "Centre for Integrated Critical Care The University of Melbourne Parkville Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Neto",
"given": "Ary S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Sleep Medicine Austin Health Heidelberg Victoria Australia"
}
],
"family": "McDonald",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Austin Hospital Heidelberg Victoria Australia"
}
],
"family": "See",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery The University of Melbourne, Austin Health Heidelberg Victoria Australia"
},
{
"name": "Department of Intensive Care Austin Hospital Heidelberg Victoria Australia"
}
],
"family": "Jones",
"given": "Daryl",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5145-6783",
"affiliation": [
{
"name": "Department of Surgery The University of Melbourne, Austin Health Heidelberg Victoria Australia"
},
{
"name": "Department of Urology Austin Health Heidelberg Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Bolton",
"given": "Damien",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1650-8939",
"affiliation": [
{
"name": "Centre for Integrated Critical Care The University of Melbourne Parkville Victoria Australia"
},
{
"name": "Department of Intensive Care Austin Hospital Heidelberg Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Bellomo",
"given": "Rinaldo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5111-6367",
"affiliation": [
{
"name": "Department of Infectious Disease The University of Melbourne, Austin Health Heidelberg Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Trubiano",
"given": "Jason",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7177-3631",
"affiliation": [
{
"name": "Department of Surgery The University of Melbourne, Austin Health Heidelberg Victoria Australia"
},
{
"name": "Department of Urology Austin Health Heidelberg Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Ischia",
"given": "Joseph",
"sequence": "additional"
}
],
"container-title": [
"Journal of Medical Virology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
25
]
],
"date-time": "2021-02-25T09:56:02Z",
"timestamp": 1614246962000
},
"deposited": {
"date-parts": [
[
2021,
9,
6
]
],
"date-time": "2021-09-06T14:28:23Z",
"timestamp": 1630938503000
},
"indexed": {
"date-parts": [
[
2021,
12,
21
]
],
"date-time": "2021-12-21T15:23:06Z",
"timestamp": 1640100186972
},
"is-referenced-by-count": 10,
"issn-type": [
{
"type": "print",
"value": "0146-6615"
},
{
"type": "electronic",
"value": "1096-9071"
}
],
"issue": "5",
"issued": {
"date-parts": [
[
2021,
3,
9
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
9
]
],
"date-time": "2021-03-09T00:00:00Z",
"timestamp": 1615248000000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
9
]
],
"date-time": "2021-03-09T00:00:00Z",
"timestamp": 1615248000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.26895",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/jmv.26895",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.26895",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "3261-3267",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
3,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
3,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1331/1544-3191.44.5.594.Hulisz",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_2_1"
},
{
"DOI": "10.1128/AAC.48.3.783-790.2004",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"article-title": "Observation on clinical efficacy of combined therapy of zinc supplement and jinye baidu granule in treating human cytomegalovirus infection",
"author": "Li D",
"first-page": "449",
"issue": "5",
"journal-title": "Zhongguo Zhong Xi Yi Jie He Za Zhi",
"key": "e_1_2_9_4_1",
"volume": "25",
"year": "2005"
},
{
"DOI": "10.1111/j.1600-0714.2005.00327.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1038/248588a0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"DOI": "10.1099/vir.0.82634-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1128/JVI.79.13.8014-8023.2005",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"DOI": "10.1371/journal.ppat.1001176",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_9_1"
},
{
"DOI": "10.1074/jbc.274.52.37060",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"DOI": "10.1128/JVI.14.2.282-291.1974",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_11_1"
},
{
"DOI": "10.3389/fimmu.2020.01712",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_12_1"
},
{
"article-title": "Zinc and respiratory tract infections: perspectives for COVID‐19 (Review)",
"author": "Skalny A",
"first-page": "17",
"issue": "1",
"journal-title": "Int J Mol Med",
"key": "e_1_2_9_13_1",
"volume": "46",
"year": "2020"
},
{
"article-title": "Can Zn be a critical element in COVID‐19 treatment?",
"author": "Rahman MT",
"first-page": "1",
"journal-title": "Biol Trace Elem Res",
"key": "e_1_2_9_14_1",
"year": "2020"
},
{
"DOI": "10.1016/j.dsx.2020.04.015",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.1039/C9MT00079H",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.1007/s10534-018-0125-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.1371/journal.pone.0180028",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
},
{
"article-title": "Zinc and COVID‐19: basis of current clinical trials",
"author": "Pal A",
"first-page": "1",
"journal-title": "Biol Trace Elem Res",
"key": "e_1_2_9_19_1",
"year": "2020"
},
{
"DOI": "10.1136/bmjopen-2020-040580",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_20_1"
},
{
"DOI": "10.1016/j.ijid.2020.09.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.1016/j.ijid.2020.09.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
},
{
"DOI": "10.1093/ajcn/78.4.756",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_23_1"
},
{
"article-title": "International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control",
"author": "International Zinc Nutrition Consultative Group",
"first-page": "99",
"issue": "1",
"journal-title": "Food Nutr Bull",
"key": "e_1_2_9_24_1",
"volume": "25",
"year": "2004"
},
{
"DOI": "10.1017/jns.2015.12",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_25_1"
},
{
"DOI": "10.1039/C4MT00060A",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_26_1"
},
{
"DOI": "10.1093/jn/135.4.802",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_27_1"
},
{
"DOI": "10.1016/j.redox.2020.101764",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_28_1"
},
{
"DOI": "10.1016/j.ijid.2020.06.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_29_1"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_30_1"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106214",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_31_1"
},
{
"DOI": "10.1099/jmm.0.001250",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_32_1"
},
{
"DOI": "10.3945/ajcn.2010.28674I",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_33_1"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"score": 1,
"short-container-title": [
"J Med Virol"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": [
"A pilot double‐blind safety and feasibility randomized controlled trial of high‐dose intravenous zinc in hospitalized COVID‐19 patients"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "93"
}
