Post-Exposure Prophylaxis with Favipiravir among Household Close Contacts to Confirmed COVID-19 Cases: A Cluster-Randomized Trial (PEPfavi)
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2026.103150, PEPfavi, TCTR20210909002, Jan 2026
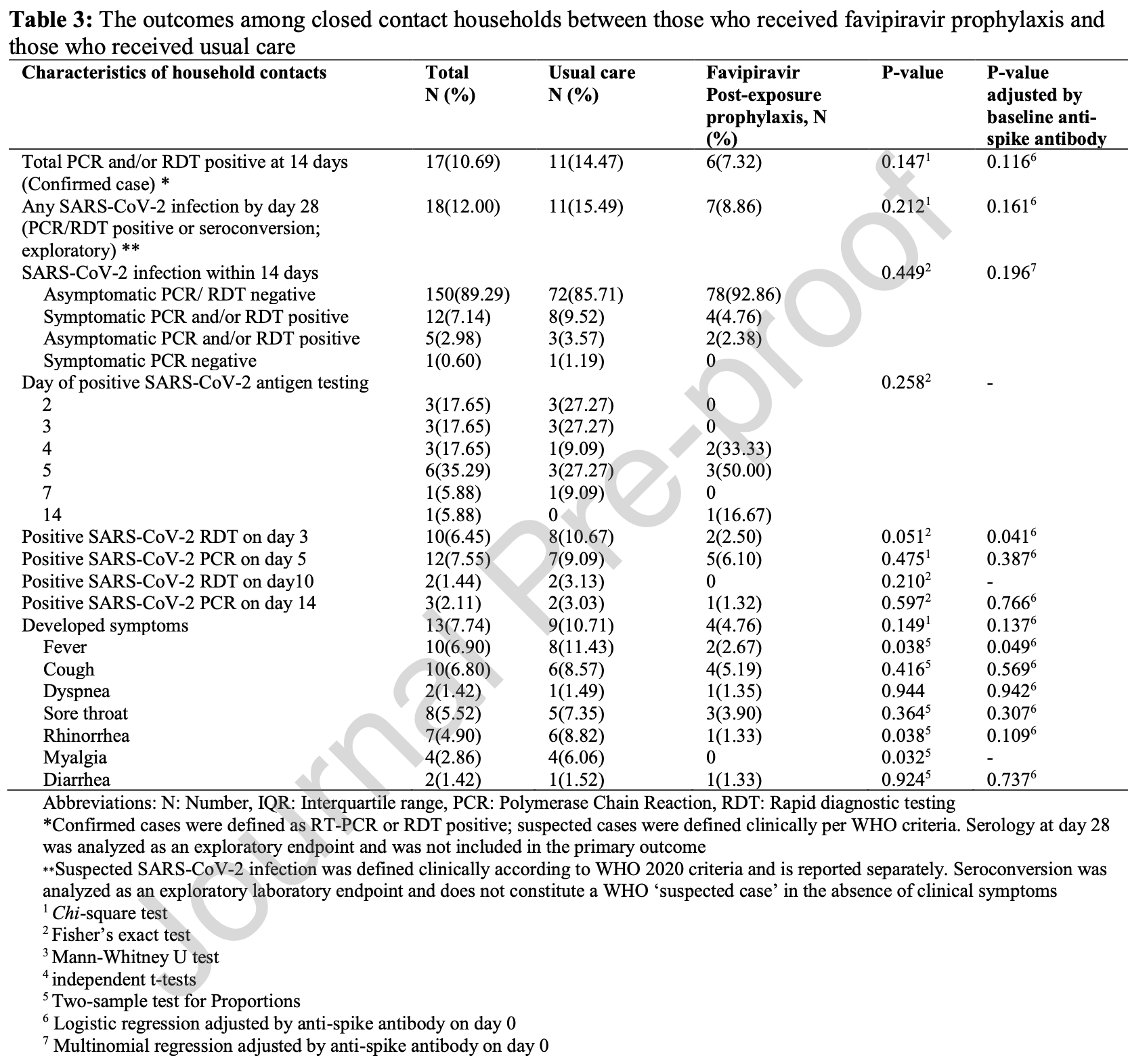
RCT 168 household close contacts showing no significant difference in SARS-CoV-2 infection with favipiravir post-exposure prophylaxis. The primary endpoint of laboratory-confirmed infection by day 14 occurred in 7.3% of the favipiravir group versus 14.5% of usual care (p=0.147).
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of symptomatic case, 50.0% lower, RR 0.50, p = 0.37, treatment 4 of 84 (4.8%), control 8 of 84 (9.5%), NNT 21, day 14.
|
|
risk of case, 36.4% lower, RR 0.64, p = 0.46, treatment 7 of 84 (8.3%), control 11 of 84 (13.1%), NNT 21, day 28.
|
|
risk of case, 45.5% lower, RR 0.55, p = 0.31, treatment 6 of 84 (7.1%), control 11 of 84 (13.1%), NNT 17, day 14, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Siripongboonsitti et al., 14 Jan 2026, Randomized Controlled Trial, Thailand, peer-reviewed, median age 41.0, mean age 44.0, 11 authors, study period 17 October, 2021 - 5 May, 2022, trial TCTR20210909002 (PEPfavi).
Contact: taweegrit.sir@cra.ac.th.
Post-Exposure Prophylaxis with Favipiravir among Household Close Contacts to Confirmed COVID-19 Cases: A Cluster-Randomized Trial (PEPfavi)
Journal of Infection and Public Health, doi:10.1016/j.jiph.2026.103150
limited evidence supporting the efficacy of antiviral post-exposure prophylaxis (PEP) in this context.
Methods: The phase 2/3, open-label, (1:1) cluster-randomized controlled trial in Thailand, 168 household close contacts from 76 index cases were enrolled to receive either favipiravir-PEP (FPV-PEP) (1600-2000 mg/day for 7 days) or usual care. The efficacy of FPV-PEP was investigated in preventing SARS-CoV-2 infection after contact with index cases.
Results: The incidence of confirmed SARS-CoV-2 infection was lower in the FPV-PEP group than in the usual care group (7.32% vs. 14.47%), although the difference was not statistically significant. A trend toward fewer early positive rapid diagnostic test results on day 3 was observed in the FPV-PEP group. Symptom development was less frequent among FPV-PEP recipients, with fewer cases of fever, rhinorrhea, and myalgia. A significantly higher probability of remaining asymptomatic and delayed symptom onset was observed in the FPV-PEP group. No participants developed severe COVID-19 or required hospitalization.
Conclusion: FPV-PEP was associated with a lower incidence of fever, rhinorrhea, and myalgia among household contacts. While a reduction in secondary transmission was observed, it did not reach statistical significance. Further large-scale studies are warranted to clarify its role in preventing household transmission. Trial Registration: Thai clinical trials registry (TCTR) no.20210909002
Competing interests All authors do not have an association that might pose a conflict of interest.
Authors' Contributions T.S. had full access to all data in this study and was responsible for the data's integrity and accuracy. T.S., first authors and corresponding authors, Concept and design-T.S., T.U., N.M.; Investigation-T.S., T.U., K.T., K.N., K.C., W.S., N.W., M.M., S.W., K.S., N.M.; The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
J o u r n a l P r e -p r o o f
Authors' information
Not applicable
ABSTRACT J o u r n a l P r e -p r o o f
References
Alpizar, Accini, Anderson, Eysa, Medina-Piñón et al., Molnupiravir for intra-household prevention of COVID-19: The MOVe-AHEAD randomized, placebo-controlled trial, J Infect, doi:10.1016/j.jinf.2023.08.016
Christensen, Olsen, Long, Snehal, Davis et al., Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with Coronavirus Disease 2019 Caused by the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas, Am J Pathol, doi:10.1016/j.ajpath.2022.01.007
Feikin, Higdon, Abu-Raddad, Andrews, Araos et al., Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression, Lancet, doi:10.1016/s0140-6736(22)00152-0
Focosi, Mcconnell, Casadevall, Cappello, Valdiserra et al., Monoclonal antibody therapies against SARS-CoV-2, Lancet Infect Dis, doi:10.1016/s1473-3099(22)00311-5
Godoy, Martínez-Baz, Parron, García-Cenoz, Ferras et al., Vaccinated COVID-19 Index Cases Are Less Likely to Transmit SARS-CoV-2 to Their Household Contacts: A Cohort Study, Vaccines, doi:10.3390/vaccines12030240
Hall, Harris, Zaidi, Woodhall, Dabrera et al., HOSTED-England's Household Transmission Evaluation Dataset: preliminary findings from a novel passive surveillance system of COVID-19, Int J Epidemiol, doi:10.1093/ije/dyab057
Hammond, Yunis, Fountaine, Luscan, Burr et al., Oral Nirmatrelvir-Ritonavir as Postexposure Prophylaxis for Covid-19, N Engl J Med, doi:10.1056/NEJMoa2309002
Harris, Hall, Zaidi, Andrews, Dunbar et al., Effect of Vaccination on Household Transmission of SARS-CoV-2 in England, N Engl J Med, doi:10.1056/NEJMc2107717
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep, doi:10.1038/s41598-021-90551-6
Ikematsu, Hayden, Kawaguchi, Kinoshita, De Jong et al., Baloxavir Marboxil for Prophylaxis against Influenza in Household Contacts, N Engl J Med, doi:10.1056/NEJMoa1915341
Irie, Nakagawa, Fujita, Tamura, Eto et al., Population pharmacokinetics of favipiravir in patients with COVID-19, CPT Pharmacometrics Syst Pharmacol, doi:10.1186/s12879-021-06164-x
J O U R N A L P R E, None
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.10.069
Kim, Kim, Son, Heo, Shin, Vaccine Effect on Household Transmission of Omicron and Delta SARS-CoV-2 Variants, J Korean Med Sci, doi:10.3346/jkms.2023.38.e9
Liu, Rocklöv, The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta, J Travel Med, doi:10.1093/jtm/taac037
López-Muñoz, Torrella, Pérez-Quílez, Castillo-Zuza, Martró et al., SARS-CoV-2 Secondary Attack Rates in Vaccinated and Unvaccinated Household Contacts during Replacement of Delta with Omicron Variant, Spain, Emerg Infect Dis, doi:10.3201/eid2810.220494
Madewell, Yang, Longini, Halloran, Dean, Household Secondary Attack Rates of SARS-CoV-2 by Variant and Vaccination Status: An Updated Systematic Review and Meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.9317
Madewell, Yang, Longini, Halloran, Dean, Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.31756
Madewell, Yang, Longini, Jr, Halloran et al., Factors Associated With Household Transmission of SARS-CoV-2: An Updated Systematic Review and Meta-analysis, JAMA Network Open, doi:10.1001/jamanetworkopen.2021.22240
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis, doi:10.1186/s12879-021-06164-x
Muadchimkaew, Siripongboonsitti, Wongpatcharawarakul, Boonsankaew, Tawinprai et al., Effect of Inactivated SARS-CoV-2 Vaccines and ChAdOx1 nCoV-19 Vaccination to Prevent COVID-19 in Thai Households (VacPrevent trial), Int J Infect Dis, doi:10.1016/j.ijid.2022.09.032
O'brien, Forleo-Neto, Musser, Chan, Sarkar, Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19, N Engl J Med, doi:10.1056/NEJMoa2109682
Ogata, Tanaka, Tanaka, Osaki, Noguchi et al., Increased Secondary Attack Rates among the Household Contacts of Patients with the Omicron Variant of the Coronavirus Disease 2019 in Japan, Int J Environ Res Public Health, doi:10.3390/ijerph19138068
Relan, Motaze, Kothari, Askie, Polain et al., Severity and outcomes of Omicron variant of SARS-CoV-2 compared to Delta variant and severity of Omicron sublineages: a systematic review and metanalysis, BMJ Glob Health, doi:10.1136/bmjgh-2023-012328
Salvadori, Jourdain, Krittayaphong, Siripongboonsitti, Kongsaengdao et al., Molnupiravir versus favipiravir in at-risk outpatients with COVID-19: A randomized controlled trial in Thailand, Int J Infect Dis, doi:10.1016/j.ijid.2024.107021
Siripongboonsitti, Muadchimkaew, Tawinprai, Issaranon, Meepholkij et al., Assessing favipiravir impact on SARS-CoV-2 transmission within J o u r n a l P r e -p r o o f households: Insights from a multi-center study (FaviPrev), J Virus Erad, doi:10.1016/j.jve.2024.100576
Siripongboonsitti, Muadchimkaew, Tawinprai, Issaranon, Meepholkij et al., Favipiravir treatment in non-severe COVID-19: promising results from multicenter propensity score-matched study (FAVICOV), Sci Rep, doi:10.1038/s41598-023-42195-x
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Vogt, Rebuli, Cretikos, Liu, Macartney et al., Assessing the effects of SARS-CoV-2 vaccination on the risk of household transmission during delta variant circulation: a population-based data linkage cohort study, The Lancet Regional Health -Western Pacific, doi:10.1016/j.lanwpc.2023.100930
Watanapokasin, Siripongboonsitti, Ungtrakul, Muadchimkaew, Wongpatcharawarakul et al., Transmissibility of SARS-CoV-2 Variants as a Secondary Attack in Thai Households: a Retrospective Study, IJID Regions, doi:10.1016/j.ijregi.2021.09.001
Welliver, Monto, Carewicz, Schatteman, Hassman et al., Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial, Jama, doi:10.1001/jama.285.6.748
DOI record:
{
"DOI": "10.1016/j.jiph.2026.103150",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2026.103150",
"alternative-id": [
"S1876034126000225"
],
"article-number": "103150",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Post-Exposure Prophylaxis with Favipiravir among Household Close Contacts to Confirmed COVID-19 Cases: A Cluster-Randomized Trial (PEPfavi)"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2026.103150"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2026 The Author(s). Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-7256-9982",
"affiliation": [],
"authenticated-orcid": false,
"family": "Siripongboonsitti",
"given": "Taweegrit",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-0140-9866",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ungtrakul",
"given": "Teerapat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tawinprai",
"given": "Kriangkrai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Niemsorn",
"given": "Krongkwan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6777-2492",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cheirsilpa",
"given": "Kunsuda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sangwipasnapaporn",
"given": "Worrawat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wattanapokasilp",
"given": "Natcha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muadchimkaew",
"given": "Marisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wongpatcharawarakul",
"given": "Saowanee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soonklang",
"given": "Kamonwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahanonda",
"given": "Nithi",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.com",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.jp",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2026,
1,
14
]
],
"date-time": "2026-01-14T16:18:43Z",
"timestamp": 1768407523000
},
"deposited": {
"date-parts": [
[
2026,
1,
16
]
],
"date-time": "2026-01-16T15:50:08Z",
"timestamp": 1768578608000
},
"funder": [
{
"DOI": "10.13039/100016175",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100016175",
"id-type": "DOI"
}
],
"name": "Chulabhorn Royal Academy"
}
],
"indexed": {
"date-parts": [
[
2026,
1,
17
]
],
"date-time": "2026-01-17T15:46:09Z",
"timestamp": 1768664769187,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2026,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
1
]
],
"date-time": "2026-01-01T00:00:00Z",
"timestamp": 1767225600000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
1
]
],
"date-time": "2026-01-01T00:00:00Z",
"timestamp": 1767225600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 7,
"start": {
"date-parts": [
[
2026,
1,
8
]
],
"date-time": "2026-01-08T00:00:00Z",
"timestamp": 1767830400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034126000225?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034126000225?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "103150",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2026,
1
]
]
},
"published-print": {
"date-parts": [
[
2026,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1093/ije/dyab057",
"article-title": "HOSTED-England's Household Transmission Evaluation Dataset: preliminary findings from a novel passive surveillance system of COVID-19",
"author": "Hall",
"doi-asserted-by": "crossref",
"first-page": "743",
"issue": "3",
"journal-title": "Int J Epidemiol",
"key": "10.1016/j.jiph.2026.103150_bib1",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.22240",
"article-title": "Factors Associated With Household Transmission of SARS-CoV-2: An Updated Systematic Review and Meta-analysis",
"author": "Madewell",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "JAMA Network Open",
"key": "10.1016/j.jiph.2026.103150_bib2",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.9317",
"article-title": "Household Secondary Attack Rates of SARS-CoV-2 by Variant and Vaccination Status: An Updated Systematic Review and Meta-analysis",
"author": "Madewell",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jiph.2026.103150_bib3",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2020.31756",
"article-title": "Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis",
"author": "Madewell",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jiph.2026.103150_bib4",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.3201/eid2810.220494",
"article-title": "SARS-CoV-2 Secondary Attack Rates in Vaccinated and Unvaccinated Household Contacts during Replacement of Delta with Omicron Variant, Spain",
"author": "López-Muñoz",
"doi-asserted-by": "crossref",
"first-page": "1999",
"issue": "10",
"journal-title": "Emerg Infect Dis",
"key": "10.1016/j.jiph.2026.103150_bib5",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.3390/ijerph19138068",
"article-title": "Increased Secondary Attack Rates among the Household Contacts of Patients with the Omicron Variant of the Coronavirus Disease 2019 in Japan",
"author": "Ogata",
"doi-asserted-by": "crossref",
"issue": "13",
"journal-title": "Int J Environ Res Public Health",
"key": "10.1016/j.jiph.2026.103150_bib6",
"volume": "19",
"year": "2022"
},
{
"article-title": "Assessing favipiravir impact on SARS-CoV-2 transmission within households: Insights from a multi-center study (FaviPrev)",
"author": "Siripongboonsitti",
"issue": "4",
"journal-title": "J Virus Erad",
"key": "10.1016/j.jiph.2026.103150_bib7",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1016/j.ijid.2022.09.032",
"article-title": "Effect of Inactivated SARS-CoV-2 Vaccines and ChAdOx1 nCoV-19 Vaccination to Prevent COVID-19 in Thai Households (VacPrevent trial)",
"author": "Muadchimkaew",
"doi-asserted-by": "crossref",
"first-page": "190",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiph.2026.103150_bib8",
"volume": "124",
"year": "2022"
},
{
"DOI": "10.1016/j.ijregi.2021.09.001",
"article-title": "Transmissibility of SARS-CoV-2 Variants as a Secondary Attack in Thai Households: a Retrospective Study",
"author": "Watanapokasin",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "IJID Regions",
"key": "10.1016/j.jiph.2026.103150_bib9",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2107717",
"article-title": "Effect of Vaccination on Household Transmission of SARS-CoV-2 in England",
"author": "Harris",
"doi-asserted-by": "crossref",
"first-page": "759",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2026.103150_bib10",
"volume": "385",
"year": "2021"
},
{
"article-title": "Vaccinated COVID-19 Index Cases Are Less Likely to Transmit SARS-CoV-2 to Their Household Contacts: A Cohort Study",
"author": "Godoy",
"issue": "3",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.jiph.2026.103150_bib11",
"volume": "12",
"year": "2024"
},
{
"DOI": "10.3346/jkms.2023.38.e9",
"article-title": "Vaccine Effect on Household Transmission of Omicron and Delta SARS-CoV-2 Variants",
"author": "Kim",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "J Korean Med Sci",
"key": "10.1016/j.jiph.2026.103150_bib12",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1016/j.lanwpc.2023.100930",
"article-title": "Assessing the effects of SARS-CoV-2 vaccination on the risk of household transmission during delta variant circulation: a population-based data linkage cohort study",
"author": "Vogt",
"doi-asserted-by": "crossref",
"journal-title": "The Lancet Regional Health – Western Pacific",
"key": "10.1016/j.jiph.2026.103150_bib13",
"volume": "42",
"year": "2024"
},
{
"DOI": "10.1001/jama.285.6.748",
"article-title": "Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial",
"author": "Welliver",
"doi-asserted-by": "crossref",
"first-page": "748",
"issue": "6",
"journal-title": "Jama",
"key": "10.1016/j.jiph.2026.103150_bib14",
"volume": "285",
"year": "2001"
},
{
"DOI": "10.1056/NEJMoa1915341",
"article-title": "Baloxavir Marboxil for Prophylaxis against Influenza in Household Contacts",
"author": "Ikematsu",
"doi-asserted-by": "crossref",
"first-page": "309",
"issue": "4",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2026.103150_bib15",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2309002",
"article-title": "Oral Nirmatrelvir-Ritonavir as Postexposure Prophylaxis for Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "224",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2026.103150_bib16",
"volume": "391",
"year": "2024"
},
{
"DOI": "10.1016/j.jinf.2023.08.016",
"article-title": "Molnupiravir for intra-household prevention of COVID-19: The MOVe-AHEAD randomized, placebo-controlled trial",
"author": "Alpizar",
"doi-asserted-by": "crossref",
"first-page": "392",
"issue": "5",
"journal-title": "J Infect",
"key": "10.1016/j.jiph.2026.103150_bib17",
"volume": "87",
"year": "2023"
},
{
"article-title": "Role of favipiravir in the treatment of COVID-19",
"author": "Joshi",
"journal-title": "International Journal of Infectious Diseases",
"key": "10.1016/j.jiph.2026.103150_bib18",
"year": "2020"
},
{
"DOI": "10.1038/s41598-023-42195-x",
"article-title": "Favipiravir treatment in non-severe COVID-19: promising results from multicenter propensity score-matched study (FAVICOV)",
"author": "Siripongboonsitti",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jiph.2026.103150_bib19",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiph.2026.103150_bib20",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jiph.2026.103150_bib21",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06164-x",
"article-title": "Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis",
"author": "Manabe",
"doi-asserted-by": "crossref",
"first-page": "489",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jiph.2026.103150_bib22",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2024.107021",
"article-title": "Molnupiravir versus favipiravir in at-risk outpatients with COVID-19: A randomized controlled trial in Thailand",
"author": "Salvadori",
"doi-asserted-by": "crossref",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiph.2026.103150_bib23",
"volume": "143",
"year": "2024"
},
{
"DOI": "10.1002/psp4.12685",
"article-title": "Population pharmacokinetics of favipiravir in patients with COVID-19",
"author": "Irie",
"doi-asserted-by": "crossref",
"first-page": "1161",
"issue": "10",
"journal-title": "CPT Pharmacometrics Syst Pharmacol",
"key": "10.1016/j.jiph.2026.103150_bib24",
"volume": "10",
"year": "2021"
},
{
"key": "10.1016/j.jiph.2026.103150_bib25",
"unstructured": "World Health Organization. Household transmission investigation protocol for 2019-novel coronavirus (COVID-19) infection. Epidemiological protocol. 2020;28."
},
{
"DOI": "10.1136/bmjgh-2023-012328",
"article-title": "Severity and outcomes of Omicron variant of SARS-CoV-2 compared to Delta variant and severity of Omicron sublineages: a systematic review and metanalysis",
"author": "Relan",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "BMJ Glob Health",
"key": "10.1016/j.jiph.2026.103150_bib26",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1093/jtm/taac037",
"article-title": "The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta",
"author": "Liu",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "J Travel Med",
"key": "10.1016/j.jiph.2026.103150_bib27",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1016/j.ajpath.2022.01.007",
"article-title": "Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with Coronavirus Disease 2019 Caused by the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas",
"author": "Christensen",
"doi-asserted-by": "crossref",
"first-page": "642",
"issue": "4",
"journal-title": "Am J Pathol",
"key": "10.1016/j.jiph.2026.103150_bib28",
"volume": "192",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00152-0",
"article-title": "Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression",
"author": "Feikin",
"doi-asserted-by": "crossref",
"first-page": "924",
"issue": "10328",
"journal-title": "Lancet",
"key": "10.1016/j.jiph.2026.103150_bib29",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00311-5",
"article-title": "Monoclonal antibody therapies against SARS-CoV-2",
"author": "Focosi",
"doi-asserted-by": "crossref",
"first-page": "e311",
"issue": "11",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jiph.2026.103150_bib30",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2109682",
"article-title": "Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "1184",
"issue": "13",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2026.103150_bib31",
"volume": "385",
"year": "2021"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034126000225"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Post-Exposure Prophylaxis with Favipiravir among Household Close Contacts to Confirmed COVID-19 Cases: A Cluster-Randomized Trial (PEPfavi)",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy"
}