Assessing Favipiravir's Impact on SARS-CoV-2 Transmission within Households: Insights from a Multi-center Study (FaviPrev)
et al., Journal of Virus Eradication, doi:10.1016/j.jve.2024.100576, FaviPrev, Dec 2024
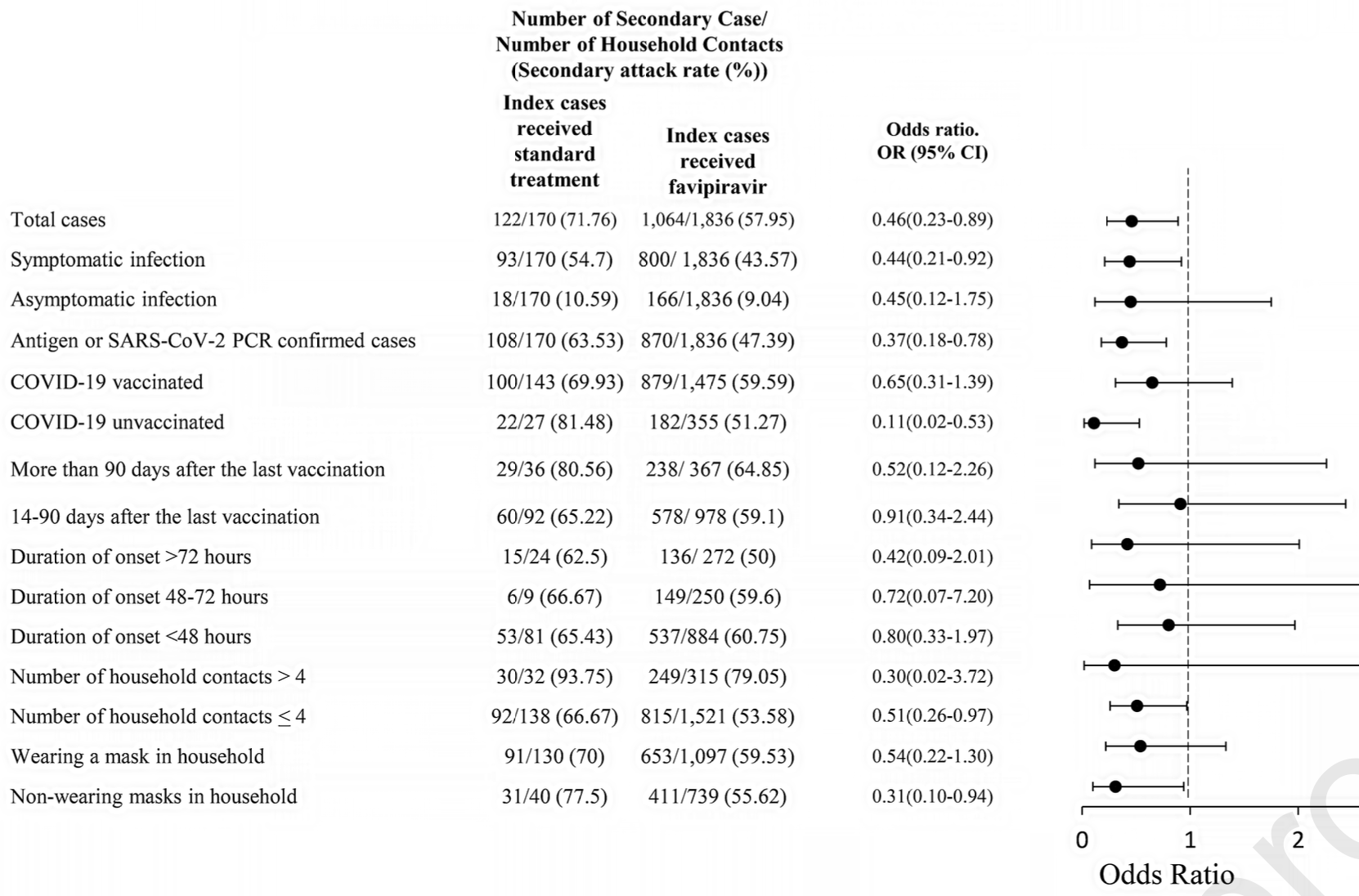
Retrospective 976 mild to moderate COVID-19 outpatients in Thailand showing significantly lower household transmission with favipiravir treatment of index cases.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of transmission, 24.9% lower, RR 0.75, p = 0.05, treatment 1,064 of 1,836 (58.0%), control 122 of 170 (71.8%), NNT 7.2, adjusted per study, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Siripongboonsitti et al., 12 Dec 2024, retrospective, Thailand, peer-reviewed, 11 authors, study period 1 April, 2021 - 31 May, 2022, FaviPrev trial.
Contact: taweegrit.sir@cra.ac.th.
Assessing Favipiravir's Impact on SARS-CoV-2 Transmission within Households: Insights from a Multi-center Study (FaviPrev)
Journal of Virus Eradication, doi:10.1016/j.jve.2024.100576
Background: While certain studies have demonstrated that antiviral treatment administered to index patients with influenza can mitigate the transmission within households, the efficacy of anti-SARS-CoV-2 agents in curtailing household transmission remains to be conclusively established.
Methods : A retrospective study conducted from April 2021 to May 2022 across multiple centers in Thailand compared 892 patients treated with favipiravir to 84 who received standard treatment among mild to moderate COVID-19 index patients. The study focused on the impact of favipiravir treatment in reducing household SARS-CoV-2 transmission by examining the secondary attack rate. Results: Favipiravir significantly reduced household SARS-CoV-2 transmission, comparing 1,836 household contacts with favipiravir-treated index cases to 170 contacts whose index cases received standard care. Favipiravir led to a 58% secondary attack rate, substantially lower than the 71.8% observed with standard treatment, representing a 54% reduction in transmission likelihood, with an odds ratio of 0.46 (95% Confidence Interval [CI] [0.23-0.89]). Index cases treated with favipiravir also demonstrated a relative risk reduction of 0.19 in transmission (95% CI [0.11-0.27]). Remarkably, favipiravir's effectiveness was most notable in unvaccinated index cases, those with symptomatic infections, individuals living in shared spaces like dormitories, flats, or apartments, and those not adhering to mask-wearing within their households. Conclusions: Favipiravir has demonstrated a crucial indirect role in reducing household SARS-CoV-2 transmission, showing notable efficacy in symptomatic and unvaccinated index cases. This breakthrough highlights its potential in broader public health strategies. Exploring the roles and challenges of other anti-SARS-CoV-2 agents remains a vital frontier in ongoing research.
Competing interests All authors do not have an association that might pose a conflict of interest.
Authors' Contributions T.S. had full access to all of the data in this study and took responsibility for the data's integrity and accuracy. T.S.-first authors and Corresponding author, M.M., K.T., O.I., W.M., P.A., A.V., O.D., W.P., K.S., N.M. contributed equally to the study. Concept and design-T.S., N.M.; Investigation-T.S., M.M., K.T., O.I., W.M., P.A., A.V., O.D., W.P., K.S., N.M. Acquisition, analysis, or interpretation of data: T.S.; Critical revision of the manuscript for important intellectual content: T.S.; Statistical analysis: K.S.; Obtained funding T.S.; Administrative, technical, or material support: M.M., K.T., O.I., W.M., P.A., A.V., O.D., W.P.; Supervision: T.S., N.M. ☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of generative AI in scientific writing
J o u r n a l P r e -p r o o f
Authors' information
Not applicable ☐The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
Akaishi, Kushimoto, Katori, Kure, Igarashi et al., COVID-19 transmission in group living environments and households, Sci Rep, doi:10.1038/s41598-021-91220-4
Alpizar, Accini, Anderson, Eysa, Medina-Piñón et al., Molnupiravir for intra-household prevention of COVID-19: The MOVe-AHEAD randomized, placebo-controlled trial, J Infect, doi:10.1016/j.jinf.2023.08.016
Bender, Brandl, Höhle, Buchholz, Zeitlmann, Analysis of Asymptomatic and Presymptomatic Transmission in SARS-CoV-2 Outbreak, Germany, Emerg Infect Dis, doi:10.3201/eid2704.204576
Couch, Kasel, Glezen, Cate, Six et al., Influenza: its control in persons and populations, J Infect Dis, doi:10.1093/infdis/153.3.431
Cox, Lieber, Wolf, Karimi, Lieberman et al., Comparing molnupiravir and nirmatrelvir/ritonavir efficacy and the effects on SARS-CoV-2 transmission in animal models, Nat Commun, doi:10.1038/s41467-023-40556-8
Deng, Yang, Yang, Chen, Qiu et al., Evaluation of favipiravir in the treatment of COVID-19 based on the real-world, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2022.2012155
Doi, Ishihara, Banno, Ando, Kondo, Favipiravir for symptomatic COVID-19: A nationwide observational cohort study, J Infect Chemother, doi:10.1016/j.jiac.2022.10.008
Fry, Goswami, Nahar, Sharmin, Rahman et al., Effects of oseltamivir treatment of index patients with influenza on secondary household illness in an urban setting in Bangladesh: secondary analysis of a randomised, placebo-controlled trial, Lancet Infect Dis, doi:10.1016/s1473-3099(15)70041-1
Fry, Goswami, Nahar, Sharmin, Rahman et al., Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial, Lancet Infect Dis, doi:10.1016/s1473-3099(13)70267-6
Gisaid, hCoV-19 Variants Dashboard 2023
Hall, Harris, Zaidi, Woodhall, Dabrera et al., HOSTED-England's Household Transmission Evaluation Dataset: preliminary findings from a novel passive surveillance system of COVID-19, Int J Epidemiol, doi:10.1093/ije/dyab057.JournalPre-proof
Harris, Hall, Zaidi, Andrews, Dunbar et al., Effect of Vaccination on Household Transmission of SARS-CoV-2 in England, N Engl J Med, doi:10.1056/NEJMc2107717.JournalPre-proof
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and metaanalysis of clinical trials, Sci Rep, doi:10.1038/s41598-021-90551-6
Hayden, Asher, Cowling, Hurt, Ikematsu et al., Reducing Influenza Virus Transmission: The Potential Value of Antiviral Treatment, Clin Infect Dis, doi:10.1093/cid/ciab625
Hayden, Belshe, Clover, Hay, Oakes et al., Emergence and apparent transmission of rimantadine-resistant influenza A virus in families, N Engl J Med, doi:10.1056/nejm198912213212502.JournalPre-proof
Hayward, Does treatment with oseltamivir prevent transmission of influenza to household contacts?, Clin Infect Dis, doi:10.1086/650459
Irie, Nakagawa, Fujita, Tamura, Eto et al., Population pharmacokinetics of favipiravir in patients with COVID-19, CPT Pharmacometrics Syst Pharmacol, doi:10.1002/psp4.12685
J O U R N A L P R E, None
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.10.069
Komeda, Takazono, Hosogaya, Ogura, Fujiwara et al., Comparison of Household Transmission of Influenza Virus From Index Patients Treated With Baloxavir Marboxil or Neuraminidase Inhibitors: A Health Insurance Claims Database Study, Clin Infect Dis, doi:10.1093/cid/ciaa1622
Korula, Alexander, John, Kirubakaran, Singh et al., Favipiravir for treating COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858.CD015219.pub2
Madewell, Yang, Longini, Halloran, Dean, Household Secondary Attack Rates of SARS-CoV-2 by Variant and Vaccination Status: An Updated Systematic Review and Meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.9317
Madewell, Yang, Longini, Halloran, Dean, Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.31756
Madewell, Yang, Longini, Jr, Halloran et al., Factors Associated With Household Transmission of SARS-CoV-2: An Updated Systematic Review and Meta-analysis, JAMA Network Open, doi:10.1001/jamanetworkopen.2021.22240
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis, doi:10.1186/s12879-021-06164-x
Muadchimkaew, Siripongboonsitti, Wongpatcharawarakul, Boonsankaew, Tawinprai et al., Effect of Inactivated SARS-CoV-2 Vaccines and ChAdOx1 nCoV-19 Vaccination to Prevent COVID-19 in Thai Households (VacPrevent trial), Int J Infect Dis, doi:10.1016/j.ijid.2022.09.032
Nakano, Shiosakai, Spread of viral infection to family members from influenza patients treated with a neuraminidase inhibitor, J Infect Chemother, doi:10.1016/j.jiac.2014.01.012
Pollán, Pérez-Gómez, Pastor-Barriuso, Oteo, Hernán et al., Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study, Lancet, doi:10.1016/s0140-6736(20)31483-5
Salvadori, Jourdain, Krittayaphong, Siripongboonsitti, Kongsaengdao et al., Molnupiravir versus favipiravir in at-risk outpatients with COVID-19: A randomized controlled trial in Thailand, Int J Infect Dis, doi:10.1016/j.ijid.2024.107021
Sirijatuphat, Manosuthi, Niyomnaitham, Owen, Copeland et al., Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study, Emerg Microbes Infect, doi:10.1080/22221751.2022.2117092.JournalPre-proof
Siripongboonsitti, Muadchimkaew, Tawinprai, Issaranon, Meepholkij et al., Favipiravir treatment in non-severe COVID-19: promising results from multicenter propensity score-matched study (FAVICOV), Sci Rep, doi:10.1038/s41598-023-42195-x
Siripongboonsitti, Nontawong, Tawinprai, Suptawiwat, Soonklang et al., Efficacy of combined COVID-19 convalescent plasma with oral RNAdependent RNA polymerase inhibitor treatment versus neutralizing monoclonal antibody therapy in COVID-19 outpatients: a multi-center, non-inferiority, open-label randomized controlled trial (PlasMab), Microbiol Spectr, doi:10.1128/spectrum.03257-23
Siripongboonsitti, Ungtrakul, Tawinprai, Auewarakul, Chartisathian et al., Efficacy of Andrographis paniculata extract treatment in mild to moderate COVID-19 patients being treated with favipiravir: A double-blind, randomized, placebo-controlled study (APFaVi trial), Phytomedicine, doi:10.1016/j.phymed.2023.155018
Siripongboonsitti, Ungtrakul, Tawinprai, Nimmol, Buttakosa et al., Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study), Int J Infect Dis, doi:10.1016/j.ijid.2023.06.018
Tan, Ge, Martinez, Sun, Li et al., Transmission roles of symptomatic and asymptomatic COVID-19 cases: a modelling study, Epidemiol Infect, doi:10.1017/s0950268822001467
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Watanapokasin, Siripongboonsitti, Ungtrakul, Muadchimkaew, Wongpatcharawarakul et al., Transmissibility of SARS-CoV-2 Variants as a Secondary Attack in Thai Households: a Retrospective Study, IJID Regions, doi:10.1016/j.ijregi.2021.09.001
DOI record:
{
"DOI": "10.1016/j.jve.2024.100576",
"ISSN": [
"2055-6640"
],
"URL": "http://dx.doi.org/10.1016/j.jve.2024.100576",
"alternative-id": [
"S2055664024001912"
],
"article-number": "100576",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Assessing Favipiravir's Impact on SARS-CoV-2 Transmission within Households: Insights from a Multi-center Study (FaviPrev)"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Virus Eradication"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jve.2024.100576"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-7256-9982",
"affiliation": [],
"authenticated-orcid": false,
"family": "Siripongboonsitti",
"given": "Taweegrit",
"sequence": "first"
},
{
"affiliation": [],
"family": "Muadchimkaew",
"given": "Marisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tawinprai",
"given": "Kriangkrai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Issaranon",
"given": "Ornisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meepholkij",
"given": "Wichuda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arttawejkul",
"given": "Pureepat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vararungzarit",
"given": "Apiradee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhissayakamol",
"given": "Onwalee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Preeyachit",
"given": "Wilaiporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soonklang",
"given": "Kamonwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahanonda",
"given": "Nithi",
"sequence": "additional"
}
],
"container-title": "Journal of Virus Eradication",
"container-title-short": "Journal of Virus Eradication",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
12,
12
]
],
"date-time": "2024-12-12T17:30:41Z",
"timestamp": 1734024641000
},
"deposited": {
"date-parts": [
[
2024,
12,
13
]
],
"date-time": "2024-12-13T00:24:28Z",
"timestamp": 1734049468000
},
"indexed": {
"date-parts": [
[
2024,
12,
13
]
],
"date-time": "2024-12-13T05:33:29Z",
"timestamp": 1734068009079,
"version": "3.30.2"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
1
]
],
"date-time": "2024-12-01T00:00:00Z",
"timestamp": 1733011200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
1
]
],
"date-time": "2024-12-01T00:00:00Z",
"timestamp": 1733011200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 8,
"start": {
"date-parts": [
[
2024,
12,
9
]
],
"date-time": "2024-12-09T00:00:00Z",
"timestamp": 1733702400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2055664024001912?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2055664024001912?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100576",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
12
]
]
},
"published-print": {
"date-parts": [
[
2024,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)31483-5",
"article-title": "Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study",
"author": "Pollán",
"doi-asserted-by": "crossref",
"first-page": "535",
"issue": "10250",
"journal-title": "Lancet",
"key": "10.1016/j.jve.2024.100576_bib1",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1093/ije/dyab057",
"article-title": "HOSTED-England's Household Transmission Evaluation Dataset: preliminary findings from a novel passive surveillance system of COVID-19",
"author": "Hall",
"doi-asserted-by": "crossref",
"first-page": "743",
"issue": "3",
"journal-title": "Int J Epidemiol",
"key": "10.1016/j.jve.2024.100576_bib2",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.31756",
"article-title": "Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis",
"author": "Madewell",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jve.2024.100576_bib3",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.22240",
"article-title": "Factors Associated With Household Transmission of SARS-CoV-2: An Updated Systematic Review and Meta-analysis",
"author": "Madewell",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "JAMA Network Open",
"key": "10.1016/j.jve.2024.100576_bib4",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.9317",
"article-title": "Household Secondary Attack Rates of SARS-CoV-2 by Variant and Vaccination Status: An Updated Systematic Review and Meta-analysis",
"author": "Madewell",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jve.2024.100576_bib5",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/j.ijregi.2021.09.001",
"article-title": "Transmissibility of SARS-CoV-2 Variants as a Secondary Attack in Thai Households: a Retrospective Study",
"author": "Watanapokasin",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "IJID Regions",
"key": "10.1016/j.jve.2024.100576_bib6",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2022.09.032",
"article-title": "Effect of Inactivated SARS-CoV-2 Vaccines and ChAdOx1 nCoV-19 Vaccination to Prevent COVID-19 in Thai Households (VacPrevent trial)",
"author": "Muadchimkaew",
"doi-asserted-by": "crossref",
"first-page": "190",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib7",
"volume": "124",
"year": "2022"
},
{
"DOI": "10.1038/s41467-023-40556-8",
"article-title": "Comparing molnupiravir and nirmatrelvir/ritonavir efficacy and the effects on SARS-CoV-2 transmission in animal models",
"author": "Cox",
"doi-asserted-by": "crossref",
"first-page": "4731",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.jve.2024.100576_bib8",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2023.08.016",
"article-title": "Molnupiravir for intra-household prevention of COVID-19: The MOVe-AHEAD randomized, placebo-controlled trial",
"author": "Alpizar",
"doi-asserted-by": "crossref",
"first-page": "392",
"issue": "5",
"journal-title": "J Infect",
"key": "10.1016/j.jve.2024.100576_bib9",
"volume": "87",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciab625",
"article-title": "Reducing Influenza Virus Transmission: The Potential Value of Antiviral Treatment",
"author": "Hayden",
"doi-asserted-by": "crossref",
"first-page": "532",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib10",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1056/NEJM198912213212502",
"article-title": "Emergence and apparent transmission of rimantadine-resistant influenza A virus in families",
"author": "Hayden",
"doi-asserted-by": "crossref",
"first-page": "1696",
"issue": "25",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jve.2024.100576_bib11",
"volume": "321",
"year": "1989"
},
{
"DOI": "10.1093/infdis/153.3.431",
"article-title": "Influenza: its control in persons and populations",
"author": "Couch",
"doi-asserted-by": "crossref",
"first-page": "431",
"issue": "3",
"journal-title": "J Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib12",
"volume": "153",
"year": "1986"
},
{
"DOI": "10.1086/650459",
"article-title": "Does treatment with oseltamivir prevent transmission of influenza to household contacts?",
"author": "Hayward",
"doi-asserted-by": "crossref",
"first-page": "715",
"issue": "5",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib13",
"volume": "50",
"year": "2010"
},
{
"DOI": "10.1016/S1473-3099(15)70041-1",
"article-title": "Effects of oseltamivir treatment of index patients with influenza on secondary household illness in an urban setting in Bangladesh: secondary analysis of a randomised, placebo-controlled trial",
"author": "Fry",
"doi-asserted-by": "crossref",
"first-page": "654",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib14",
"volume": "15",
"year": "2015"
},
{
"DOI": "10.1016/S1473-3099(13)70267-6",
"article-title": "Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial",
"author": "Fry",
"doi-asserted-by": "crossref",
"first-page": "109",
"issue": "2",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib15",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1016/j.jiac.2014.01.012",
"article-title": "Spread of viral infection to family members from influenza patients treated with a neuraminidase inhibitor",
"author": "Nakano",
"doi-asserted-by": "crossref",
"first-page": "401",
"issue": "7",
"journal-title": "J Infect Chemother",
"key": "10.1016/j.jve.2024.100576_bib16",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1093/cid/ciaa1622",
"article-title": "Comparison of Household Transmission of Influenza Virus From Index Patients Treated With Baloxavir Marboxil or Neuraminidase Inhibitors: A Health Insurance Claims Database Study",
"author": "Komeda",
"doi-asserted-by": "crossref",
"first-page": "e859",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib17",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2024.107021",
"article-title": "Molnupiravir versus favipiravir in at-risk outpatients with COVID-19: A randomized controlled trial in Thailand",
"author": "Salvadori",
"doi-asserted-by": "crossref",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib18",
"volume": "143",
"year": "2024"
},
{
"DOI": "10.1038/s41598-023-42195-x",
"article-title": "Favipiravir treatment in non-severe COVID-19: promising results from multicenter propensity score-matched study (FAVICOV)",
"author": "Siripongboonsitti",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jve.2024.100576_bib19",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1186/s12879-021-06164-x",
"article-title": "Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis",
"author": "Manabe",
"doi-asserted-by": "crossref",
"first-page": "489",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib20",
"volume": "21",
"year": "2021"
},
{
"article-title": "Role of favipiravir in the treatment of COVID-19",
"author": "Joshi",
"journal-title": "International Journal of Infectious Diseases",
"key": "10.1016/j.jve.2024.100576_bib21",
"year": "2020"
},
{
"DOI": "10.1016/j.jiac.2022.10.008",
"article-title": "Favipiravir for symptomatic COVID-19: A nationwide observational cohort study",
"author": "Doi",
"doi-asserted-by": "crossref",
"first-page": "150",
"issue": "2",
"journal-title": "J Infect Chemother",
"key": "10.1016/j.jve.2024.100576_bib22",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1080/14787210.2022.2012155",
"article-title": "Evaluation of favipiravir in the treatment of COVID-19 based on the real-world",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "555",
"issue": "4",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "10.1016/j.jve.2024.100576_bib23",
"volume": "20",
"year": "2022"
},
{
"article-title": "Favipiravir for treating COVID-19",
"author": "Korula",
"issue": "2",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/j.jve.2024.100576_bib24",
"volume": "2",
"year": "2024"
},
{
"DOI": "10.1016/j.phymed.2023.155018",
"article-title": "Efficacy of Andrographis paniculata extract treatment in mild to moderate COVID-19 patients being treated with favipiravir: A double-blind, randomized, placebo-controlled study (APFaVi trial)",
"author": "Siripongboonsitti",
"doi-asserted-by": "crossref",
"journal-title": "Phytomedicine",
"key": "10.1016/j.jve.2024.100576_bib25",
"volume": "119",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2023.06.018",
"article-title": "Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study)",
"author": "Siripongboonsitti",
"doi-asserted-by": "crossref",
"first-page": "211",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib26",
"volume": "134",
"year": "2023"
},
{
"DOI": "10.1128/spectrum.03257-23",
"author": "Siripongboonsitti",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Microbiol Spectr",
"key": "10.1016/j.jve.2024.100576_bib27",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1080/22221751.2022.2117092",
"article-title": "Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study",
"author": "Sirijatuphat",
"doi-asserted-by": "crossref",
"first-page": "2197",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "10.1016/j.jve.2024.100576_bib28",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib29",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1002/psp4.12685",
"article-title": "Population pharmacokinetics of favipiravir in patients with COVID-19",
"author": "Irie",
"doi-asserted-by": "crossref",
"first-page": "1161",
"issue": "10",
"journal-title": "CPT Pharmacometrics Syst Pharmacol",
"key": "10.1016/j.jve.2024.100576_bib30",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jve.2024.100576_bib31",
"volume": "11",
"year": "2021"
},
{
"key": "10.1016/j.jve.2024.100576_bib32",
"unstructured": "World Health Organization, Household transmission investigation protocol for coronavirus disease 2019 (COVID-19) 2020 [updated 23 March 2020. 2.2:[Available from: https://www.who.int/publications/i/item/household-transmission-investigation-protocol-for-2019-novel-coronavirus-(2019-ncov)-infection."
},
{
"key": "10.1016/j.jve.2024.100576_bib33",
"unstructured": "Department of Medical Service. Clinical Practice Guideline to Diagnosis, Treatment and Prevention COVID-19 for Physicain and Health Care Provider, 21 July 2021 2021 [Available from: https://covid19.dms.go.th/Content/Select_Landding_page?contentId=139."
},
{
"key": "10.1016/j.jve.2024.100576_bib34",
"unstructured": "GISAID. hCoV-19 Variants Dashboard 2023 [cited 2023 14 October 2023]. Available from: https://gisaid.org/hcov-19-variants-dashboard/."
},
{
"DOI": "10.1017/S0950268822001467",
"article-title": "Transmission roles of symptomatic and asymptomatic COVID-19 cases: a modelling study",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "e171",
"journal-title": "Epidemiol Infect",
"key": "10.1016/j.jve.2024.100576_bib35",
"volume": "150",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-91220-4",
"article-title": "COVID-19 transmission in group living environments and households",
"author": "Akaishi",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jve.2024.100576_bib36",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3201/eid2704.204576",
"article-title": "Analysis of Asymptomatic and Presymptomatic Transmission in SARS-CoV-2 Outbreak, Germany, 2020",
"author": "Bender",
"doi-asserted-by": "crossref",
"first-page": "1159",
"issue": "4",
"journal-title": "Emerg Infect Dis",
"key": "10.1016/j.jve.2024.100576_bib37",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2107717",
"article-title": "Effect of Vaccination on Household Transmission of SARS-CoV-2 in England",
"author": "Harris",
"doi-asserted-by": "crossref",
"first-page": "759",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jve.2024.100576_bib38",
"volume": "385",
"year": "2021"
},
{
"article-title": "The indirect effect of mRNA-based Covid-19 vaccination on unvaccinated household members",
"author": "Salo",
"journal-title": "medRxiv",
"key": "10.1016/j.jve.2024.100576_bib39",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1038/d41586-021-00728-2",
"article-title": "Five reasons why COVID herd immunity is probably impossible",
"author": "Aschwanden",
"doi-asserted-by": "crossref",
"first-page": "520",
"issue": "7851",
"journal-title": "Nature",
"key": "10.1016/j.jve.2024.100576_bib40",
"volume": "591",
"year": "2021"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2055664024001912"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Assessing Favipiravir's Impact on SARS-CoV-2 Transmission within Households: Insights from a Multi-center Study (FaviPrev)",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy"
}