Molnupiravir for Intra-Household Prevention of COVID-19: the MOVe-AHEAD Randomized, Placebo-Controlled Trial
et al., Journal of Infection, doi:10.1016/j.jinf.2023.08.016, MOVe-AHEAD, NCT04939428, Sep 2023
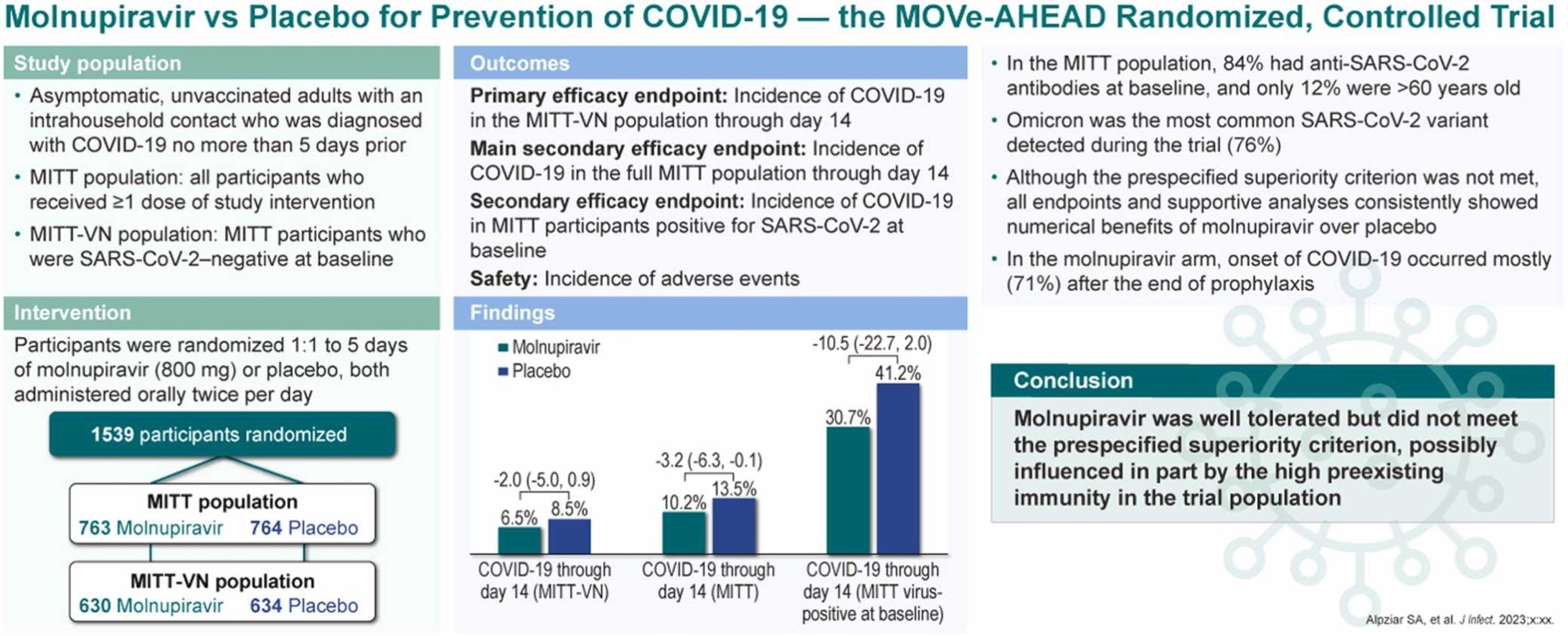
PEP RCT 1,527 patients showing lower COVID-19 cases with molnupiravir, without statistical significance.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
|
risk of symptomatic case, 24.2% lower, RR 0.76, p = 0.06, treatment 78 of 763 (10.2%), control 103 of 764 (13.5%), NNT 31, MITT.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Alpizar et al., 8 Sep 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 18 authors, trial NCT04939428 (history) (MOVe-AHEAD).
DOI record:
{
"DOI": "10.1016/j.jinf.2023.08.016",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2023.08.016",
"alternative-id": [
"S0163445323005005"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Molnupiravir for intra-household prevention of COVID-19: The MOVe-AHEAD randomized, placebo-controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2023.08.016"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and The Author(s). Published by Elsevier Ltd on behalf of The British Infection Association."
}
],
"author": [
{
"affiliation": [],
"family": "Alpizar",
"given": "Sady A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Accini",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anderson",
"given": "Duane C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eysa",
"given": "Basem",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3585-9475",
"affiliation": [],
"authenticated-orcid": false,
"family": "Medina-Piñón",
"given": "Isaí",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ohmagari",
"given": "Norio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ostrovskyy",
"given": "Mykola M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aggrey-Amable",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beck",
"given": "Karen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3947-6546",
"affiliation": [],
"authenticated-orcid": false,
"family": "Byrne",
"given": "Dana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0007-6755-4058",
"affiliation": [],
"authenticated-orcid": false,
"family": "Grayson",
"given": "Staci",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hwang",
"given": "Peggy M.T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lonchar",
"given": "Julia D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Strizki",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Yayun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paschke",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Anda",
"given": "Carisa S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sears",
"given": "Pamela S.",
"sequence": "additional"
}
],
"container-title": "Journal of Infection",
"container-title-short": "Journal of Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"journalofinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
9,
9
]
],
"date-time": "2023-09-09T22:16:31Z",
"timestamp": 1694297791000
},
"deposited": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T23:06:49Z",
"timestamp": 1697756809000
},
"funder": [
{
"DOI": "10.13039/100009947",
"doi-asserted-by": "publisher",
"name": "Merck Sharp and Dohme"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T10:54:22Z",
"timestamp": 1712055262731
},
"is-referenced-by-count": 2,
"issue": "5",
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
18
]
],
"date-time": "2023-09-18T00:00:00Z",
"timestamp": 1694995200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445323005005?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445323005005?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "392-402",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jinf.2023.08.016_bib1",
"unstructured": "World Health Organization. WHO Coronavirus (COVID-19) Dashboard (Updated September 05, 2023). World Health Organization (WHO); 2023. Available at: https://covid19.who.int. [Accessed on September 12, 2023]."
},
{
"DOI": "10.1016/j.euroneuro.2021.10.004",
"article-title": "Results of the COVID-19 mental health international for the general population (COMET-G) study",
"author": "Fountoulakis",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Eur Neuropsychopharmacol",
"key": "10.1016/j.jinf.2023.08.016_bib2",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.18931",
"article-title": "Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021",
"author": "Wulf Hanson",
"doi-asserted-by": "crossref",
"first-page": "1604",
"issue": "16",
"journal-title": "JAMA",
"key": "10.1016/j.jinf.2023.08.016_bib3",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)02796-3",
"article-title": "Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21",
"doi-asserted-by": "crossref",
"first-page": "1513",
"issue": "10334",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.08.016_bib4",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)02143-7",
"article-title": "Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic",
"doi-asserted-by": "crossref",
"first-page": "1700",
"issue": "10312",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.08.016_bib5",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.3389/fpubh.2023.1112383",
"article-title": "Post-acute COVID-19 symptom risk in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis",
"author": "Yuan",
"doi-asserted-by": "crossref",
"journal-title": "Front Public Health",
"key": "10.1016/j.jinf.2023.08.016_bib6",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2021.101019",
"article-title": "Characterizing long COVID in an international cohort: 7 months of symptoms and their impact",
"author": "Davis",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.jinf.2023.08.016_bib7",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.7326/M22-0729",
"article-title": "Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: A randomized, placebo-controlled trial",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "1126",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.jinf.2023.08.016_bib8",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1007/s40273-022-01168-0",
"article-title": "Cost-effectiveness analysis of molnupiravir versus best supportive care for the treatment of outpatient COVID-19 in adults in the US",
"author": "Goswami",
"doi-asserted-by": "crossref",
"first-page": "699",
"issue": "7",
"journal-title": "Pharmacoeconomics",
"key": "10.1016/j.jinf.2023.08.016_bib9",
"volume": "40",
"year": "2022"
},
{
"author": "Aleem",
"key": "10.1016/j.jinf.2023.08.016_bib10",
"series-title": "Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19)",
"year": "2023"
},
{
"author": "Cascella",
"key": "10.1016/j.jinf.2023.08.016_bib11",
"series-title": "Features, evaluation, and treatment of coronavirus (COVID-19)",
"year": "2022"
},
{
"article-title": "The challenge of asymptomatic carriers of COVID-19: a rapid review of literature",
"author": "Albavera-Hernandez",
"first-page": "649",
"issue": "6",
"journal-title": "Rev Salud Publica",
"key": "10.1016/j.jinf.2023.08.016_bib12",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2023.03.025",
"article-title": "Impact of public health and social measures on contact dynamics during a SARS-CoV-2 Omicron variant outbreak in Quanzhou, China, March to April 2022",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jinf.2023.08.016_bib13",
"volume": "131",
"year": "2023"
},
{
"DOI": "10.1136/bmjpo-2022-001718",
"article-title": "Systematic review of outbreaks of COVID-19 within households in the European region when the child is the index case",
"author": "Vardavas",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "BMJ Paediatr Open",
"key": "10.1016/j.jinf.2023.08.016_bib14",
"volume": "7",
"year": "2023"
},
{
"article-title": "Household transmission of SARS-CoV-2 during the Omicron wave in Shanghai, China: a case-ascertained study",
"author": "Wei",
"issue": "2",
"journal-title": "Influenza Other Respir Virus",
"key": "10.1016/j.jinf.2023.08.016_bib15",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2021.10.015",
"article-title": "Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) from pre and asymptomatic infected individuals: a systematic review",
"author": "Jefferson",
"doi-asserted-by": "crossref",
"first-page": "178",
"issue": "2",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/j.jinf.2023.08.016_bib16",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2022.10.027",
"article-title": "Viral dynamics of the SARS-CoV-2 Omicron variant among household contacts with 2 or 3 COVID-19 vaccine doses",
"author": "Kandel",
"doi-asserted-by": "crossref",
"first-page": "666",
"issue": "6",
"journal-title": "J Infect",
"key": "10.1016/j.jinf.2023.08.016_bib17",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1111/irv.13002",
"article-title": "Transmission of SARS-CoV-2 in standardised first few X cases and household transmission investigations: a systematic review and meta-analysis",
"author": "Lewis",
"doi-asserted-by": "crossref",
"first-page": "803",
"issue": "5",
"journal-title": "Influenza Other Respir Virus",
"key": "10.1016/j.jinf.2023.08.016_bib18",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciad074",
"article-title": "Infection-induced immunity is associated with protection against SARS-CoV-2 infection and decreased infectivity",
"author": "Frutos",
"doi-asserted-by": "crossref",
"first-page": "2126",
"issue": "12",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jinf.2023.08.016_bib19",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofac676",
"article-title": "Association between population-level factors and household secondary attack rate of SARS-CoV-2: a systematic review and meta-analysis",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "ofac676",
"issue": "1",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.jinf.2023.08.016_bib20",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1038/s41467-022-31441-x",
"article-title": "Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021",
"author": "Lazarus",
"doi-asserted-by": "crossref",
"first-page": "3801",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.jinf.2023.08.016_bib21",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)00306-8",
"article-title": "Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment",
"author": "Wouters",
"doi-asserted-by": "crossref",
"first-page": "1023",
"issue": "10278",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.08.016_bib22",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.3389/fpubh.2023.1078009",
"article-title": "COVID-19 vaccine hesitancy among parents in Low- and Middle-Income Countries: a meta-analysis",
"author": "Abu El Kheir-Mataria",
"doi-asserted-by": "crossref",
"first-page": "1078009",
"journal-title": "Front Public Health",
"key": "10.1016/j.jinf.2023.08.016_bib23",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)00152-0",
"article-title": "Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression",
"author": "Feikin",
"doi-asserted-by": "crossref",
"first-page": "924",
"issue": "10328",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.08.016_bib24",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/j.ajpath.2022.01.007",
"article-title": "Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the Omicron variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas",
"author": "Christensen",
"doi-asserted-by": "crossref",
"first-page": "642",
"issue": "4",
"journal-title": "Am J Pathol",
"key": "10.1016/j.jinf.2023.08.016_bib25",
"volume": "192",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27871",
"article-title": "Clinical manifestations of COVID-19 breakthrough infections: a systematic review and meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "4234",
"issue": "9",
"journal-title": "J Med Virol",
"key": "10.1016/j.jinf.2023.08.016_bib26",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1017/S0950268823000249",
"article-title": "Characteristics of patients with SARS-COV-2 PCR re-positivity after recovering from COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"journal-title": "Epidemiol Infect",
"key": "10.1016/j.jinf.2023.08.016_bib27",
"volume": "151",
"year": "2023"
},
{
"DOI": "10.3390/ijerph20043335",
"article-title": "Severity and outcomes of SARS-CoV-2 reinfection compared with primary infection: A systematic review and meta-analysis",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Int J Environ Res Public Health",
"key": "10.1016/j.jinf.2023.08.016_bib28",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1007/s40121-022-00753-2",
"article-title": "Durability of vaccine-induced and natural immunity against COVID-19: a narrative review",
"author": "Pooley",
"doi-asserted-by": "crossref",
"first-page": "367",
"issue": "2",
"journal-title": "Infect Dis Ther",
"key": "10.1016/j.jinf.2023.08.016_bib29",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1086/381128",
"article-title": "Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis",
"author": "Hayden",
"doi-asserted-by": "crossref",
"first-page": "440",
"issue": "3",
"journal-title": "J Infect Dis",
"key": "10.1016/j.jinf.2023.08.016_bib30",
"volume": "189",
"year": "2004"
},
{
"DOI": "10.1056/NEJMoa1915341",
"article-title": "Baloxavir marboxil for prophylaxis against influenza in household contacts",
"author": "Ikematsu",
"doi-asserted-by": "crossref",
"first-page": "309",
"issue": "4",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.08.016_bib31",
"volume": "383",
"year": "2020"
},
{
"article-title": "Pre-exposure prophylaxis for viral infections other than HIV",
"author": "Soriano",
"first-page": "362",
"issue": "3",
"journal-title": "Infez Med",
"key": "10.1016/j.jinf.2023.08.016_bib32",
"volume": "30",
"year": "2022"
},
{
"key": "10.1016/j.jinf.2023.08.016_bib33",
"unstructured": "United States Food and Drug Administration. FDA authorizes bamlanivimab and etesevimab monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19. U.S. Food & Drug Administration (FDA); 2021 (updated September 16, 2021). Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-bamlanivimab-and-etesevimab-monoclonal-antibody-therapy-post-exposure-prophylaxis. [Accessed on: September 13, 2023]."
},
{
"DOI": "10.1056/NEJMoa2109682",
"article-title": "Subcutaneous REGEN-COV antibody combination to prevent Covid-19",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "1184",
"issue": "13",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.08.016_bib34",
"volume": "385",
"year": "2021"
},
{
"key": "10.1016/j.jinf.2023.08.016_bib35",
"unstructured": "National Institues of Health. Coronavirus Disease 2019 (COVID-19) treatment guidelines (Updated August 22, 2023). National Institues of Health (NIH); 2023. Available at https://www.covid19treatmentguidelines.nih.gov. [Accessed on: September 13, 2023]."
},
{
"DOI": "10.1128/AAC.02428-20",
"article-title": "Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2",
"author": "Painter",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.jinf.2023.08.016_bib36",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"article-title": "An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice",
"author": "Sheahan",
"doi-asserted-by": "crossref",
"issue": "541",
"journal-title": "Sci Transl Med",
"key": "10.1016/j.jinf.2023.08.016_bib37",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"article-title": "SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801",
"author": "Wahl",
"doi-asserted-by": "crossref",
"first-page": "451",
"journal-title": "Nature",
"key": "10.1016/j.jinf.2023.08.016_bib38",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1128/AAC.00766-18",
"article-title": "Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses",
"author": "Yoon",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.jinf.2023.08.016_bib39",
"volume": "62",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.08.016_bib40",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1172/jci.insight.160108",
"article-title": "Molnupiravir inhibits SARS-CoV-2 variants including Omicron in the hamster model",
"author": "Rosenke",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "JCI Insight",
"key": "10.1016/j.jinf.2023.08.016_bib41",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiab361",
"article-title": "Molnupiravir inhibits the replication of the emerging SARS-CoV-2 variants of concern (VoCs) in a hamster infection model",
"author": "Abdelnabi",
"doi-asserted-by": "crossref",
"first-page": "749",
"issue": "5",
"journal-title": "J Infect Dis",
"key": "10.1016/j.jinf.2023.08.016_bib42",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-32045-1",
"article-title": "SARS-CoV-2 VOC type and biological sex affect molnupiravir efficacy in severe COVID-19 dwarf hamster model",
"author": "Lieber",
"doi-asserted-by": "crossref",
"first-page": "4416",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.jinf.2023.08.016_bib43",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"issue": "10",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.08.016_bib44",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1128/JVI.01348-19",
"article-title": "Small-molecule antiviral beta-d-N (4)-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance",
"author": "Agostini",
"doi-asserted-by": "crossref",
"issue": "24",
"journal-title": "J Virol",
"key": "10.1016/j.jinf.2023.08.016_bib45",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1128/JVI.01965-17",
"article-title": "β-d-N4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome",
"author": "Urakova",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "J Virol",
"key": "10.1016/j.jinf.2023.08.016_bib46",
"volume": "92",
"year": "2018"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"issue": "10373",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.08.016_bib47",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2023.02.012",
"article-title": "Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study",
"author": "Evans",
"doi-asserted-by": "crossref",
"first-page": "352",
"issue": "4",
"journal-title": "J Infect",
"key": "10.1016/j.jinf.2023.08.016_bib48",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1038/s41596-021-00536-y",
"article-title": "Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays",
"author": "Bewley",
"doi-asserted-by": "crossref",
"first-page": "3114",
"issue": "6",
"journal-title": "Nat Protoc",
"key": "10.1016/j.jinf.2023.08.016_bib49",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1259/0007-1285-44-526-793",
"article-title": "Repeated assessment of results in clinical trials of cancer treatment",
"author": "Haybittle",
"doi-asserted-by": "crossref",
"first-page": "793",
"issue": "526",
"journal-title": "Br J Radio",
"key": "10.1016/j.jinf.2023.08.016_bib50",
"volume": "44",
"year": "1971"
},
{
"DOI": "10.1038/bjc.1976.220",
"article-title": "Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design",
"author": "Peto",
"doi-asserted-by": "crossref",
"first-page": "585",
"issue": "6",
"journal-title": "Br J Cancer",
"key": "10.1016/j.jinf.2023.08.016_bib51",
"volume": "34",
"year": "1976"
},
{
"DOI": "10.1093/cid/ciaa1558",
"article-title": "The household secondary attack rate of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): a rapid review",
"author": "Fung",
"doi-asserted-by": "crossref",
"first-page": "S138",
"issue": "Suppl.2",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jinf.2023.08.016_bib52",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0240205",
"article-title": "What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors",
"author": "Koh",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "PLoS One",
"key": "10.1016/j.jinf.2023.08.016_bib53",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.31756",
"article-title": "Household transmission of SARS-CoV-2: a systematic review and meta-analysis",
"author": "Madewell",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jinf.2023.08.016_bib54",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.7326/M20-2671",
"article-title": "Contact settings and risk for transmission in 3410 close contacts of patients With COVID-19 in Guangzhou, China: a Prospective Cohort Study",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "879",
"issue": "11",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.jinf.2023.08.016_bib55",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1128/mbio.02916-22",
"article-title": "Why molnupiravir fails in hospitalized patients",
"author": "Brown",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "mBio",
"key": "10.1016/j.jinf.2023.08.016_bib56",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.resinv.2023.01.002",
"article-title": "COVID-19 symptom-onset to diagnosis and diagnosis to treatment intervals are significant predictors of disease progression and hospitalization in high-risk patients: a real world analysis",
"author": "Shimizu",
"doi-asserted-by": "crossref",
"first-page": "220",
"issue": "2",
"journal-title": "Respir Invest",
"key": "10.1016/j.jinf.2023.08.016_bib57",
"volume": "61",
"year": "2023"
},
{
"article-title": "Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study",
"author": "Xie",
"journal-title": "BMJ",
"key": "10.1016/j.jinf.2023.08.016_bib58",
"volume": "381",
"year": "2023"
},
{
"key": "10.1016/j.jinf.2023.08.016_bib59",
"unstructured": "Pfizer, Inc. FDA Briefing Document. NDA# 217188. Drug name: nirmatrelvir tablets and ritonavir tablets copackaged for oral use. Pfizer, Inc.; March 16, 2023."
},
{
"key": "10.1016/j.jinf.2023.08.016_bib60",
"unstructured": "Pfizer, Inc. PAXLOVID (nirmaltrevir [PF-07321332] tablets; ritonavir tablets). NDA 217188. Advisory committee briefing materials. Pfizer, Inc.; March 16, 2023."
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"article-title": "A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus",
"author": "Fischer",
"doi-asserted-by": "crossref",
"first-page": "eabl7430",
"issue": "628",
"journal-title": "Sci Transl Med",
"key": "10.1016/j.jinf.2023.08.016_bib61",
"volume": "14",
"year": "2022"
},
{
"article-title": "SARS-CoV-2 viral load and shedding kinetics",
"author": "Puhach",
"first-page": "147",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "10.1016/j.jinf.2023.08.016_bib62",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1086/345722",
"article-title": "Zanamivir prophylaxis: an effective strategy for the prevention of influenza types A and B within households",
"author": "Monto",
"doi-asserted-by": "crossref",
"first-page": "1582",
"issue": "11",
"journal-title": "J Infect Dis",
"key": "10.1016/j.jinf.2023.08.016_bib63",
"volume": "186",
"year": "2002"
}
],
"reference-count": 63,
"references-count": 63,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0163445323005005"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Molnupiravir for intra-household prevention of COVID-19: The MOVe-AHEAD randomized, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "87"
}