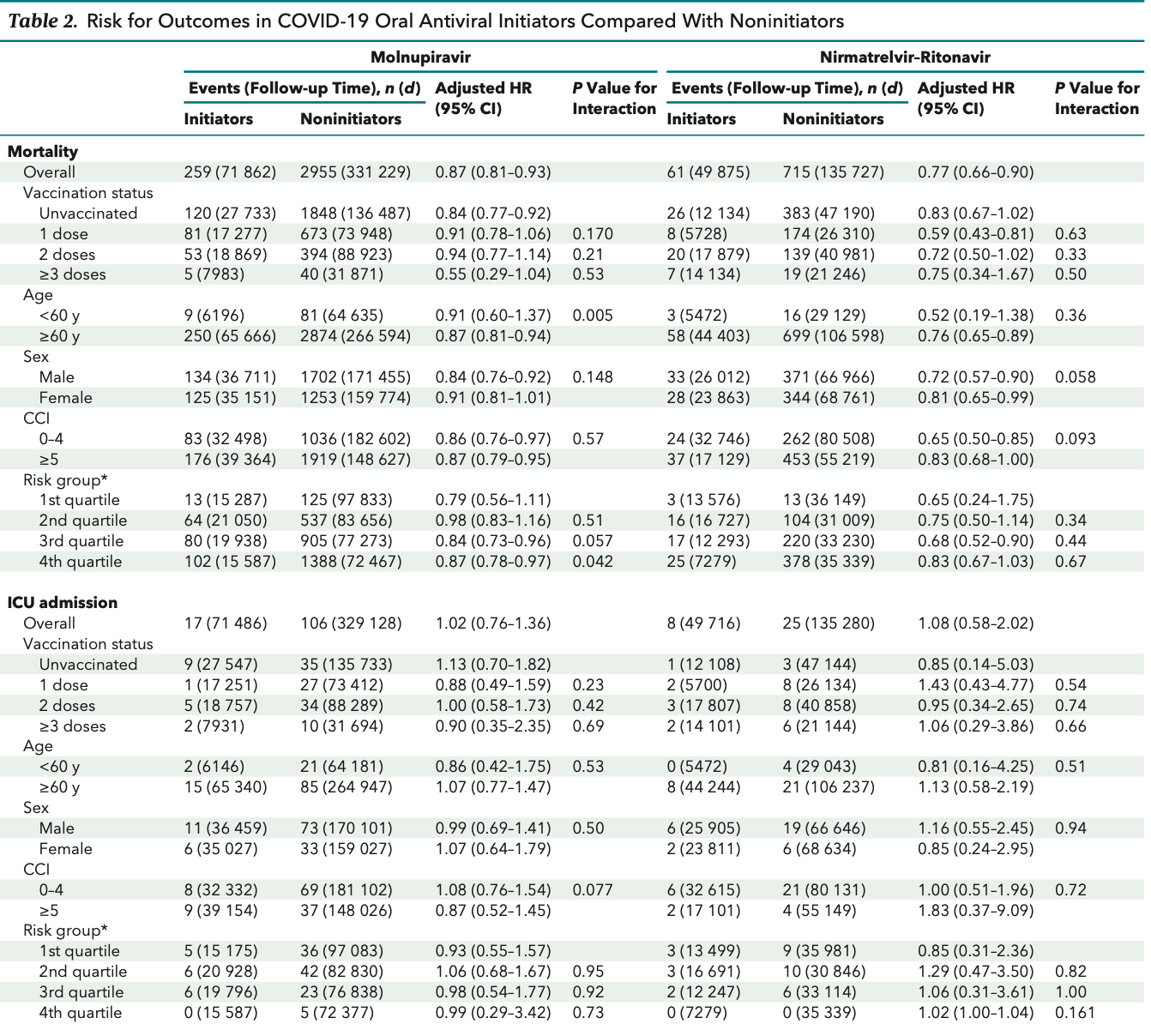
Effectiveness of Molnupiravir and Nirmatrelvir–Ritonavir in Hospitalized Patients With COVID-19
et al., Annals of Internal Medicine, doi:10.7326/M22-3057, Apr 2023
Target trial emulation retrospective with 16,495 patients in Hong Kong, showing lower mortality with molnupiravir, but no significant difference for ventilation and ICU admission.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending molnupiravir also recommended them, or
because the patient seeking out molnupiravir is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity6-20. Multiple analyses have identified variants potentially created by molnupiravir21-25. Studies show significantly increased risk of acute kidney injury26, cardiovascular toxocity27, and neurological symptoms26. Treatment may increase viral rebound28,29.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments30.
Study covers paxlovid and molnupiravir.
|
risk of death, 13.0% lower, HR 0.87, p < 0.001, treatment 2,700, control 13,795.
|
|
risk of mechanical ventilation, 7.0% higher, HR 1.07, p = 0.49, treatment 2,700, control 13,795.
|
|
risk of ICU admission, 2.0% higher, HR 1.02, p = 0.90, treatment 2,700, control 13,795.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
6.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
7.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
8.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
11.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
12.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
13.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
14.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
15.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
16.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
17.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
18.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
19.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
20.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
21.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
22.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
23.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
24.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
26.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
27.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
28.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Wan et al., 30 Apr 2023, retrospective, China, peer-reviewed, 16 authors, study period 26 February, 2022 - 18 July, 2022.
Effectiveness of Molnupiravir and Nirmatrelvir–Ritonavir in Hospitalized Patients With COVID-19
Annals of Internal Medicine, doi:10.7326/m22-3057
Background: Whether hospitalized patients benefit from COVID-19 oral antivirals is uncertain. Objective: To examine the real-world effectiveness of molnupiravir and nirmatrelvir-ritonavir in hospitalized patients with COVID-19 during the Omicron outbreak. Design: Target trial emulation study. Setting: Electronic health databases in Hong Kong.
Participants: The molnupiravir emulated trial included hospitalized patients with COVID-19 aged 18 years or older between 26 February and 18 July 2022 (n = 16 495). The nirmatrelvirritonavir emulated trial included hospitalized patients with COVID-19 aged 18 years or older between 16 March and 18 July 2022 (n = 7119). Intervention: Initiation of molnupiravir or nirmatrelvir-ritonavir within 5 days of hospitalization with COVID-19 versus no initiation of molnupiravir or nirmatrelvir-ritonavir. Measurements: Effectiveness against all-cause mortality, intensive care unit (ICU) admission, or use of ventilatory support within 28 days.
Results: The use of oral antivirals in hospitalized patients with COVID-19 was associated with a lower risk for all-cause mortality (molnupiravir: hazard ratio [HR], 0.87 [95% CI, 0.81 to 0.93]; nirmatrelvir-ritonavir: HR, 0.77 [CI, 0.66 to 0.90]) but no significant risk reduction in terms of ICU admission (molnupiravir: HR, 1.02 [CI, 0.76 to 1.36]; nirmatrelvir-ritonavir: HR, 1.08 [CI, 0.58 to 2.02]) or the need for ventilatory support (molnupiravir: HR, 1.07 [CI, 0.89 to 1.30]; nirmatrelvir-ritonavir: HR, 1.03 [CI, 0.70 to 1.52]). There was no significant interaction between drug treatment and the number of COVID-19 vaccine doses received, thereby supporting the effectiveness of oral antivirals regardless of vaccination status. No significant interaction between nirmatrelvir-ritonavir treatment and age, sex, or Charlson Comorbidity Index was observed, whereas molnupiravir tended to be more effective in older people.
Limitation: The outcome of ICU admission or need for ventilatory support may not capture all severe COVID-19 cases; unmeasured confounders, such as obesity and health behaviors, may exist.
Conclusion: Molnupiravir and nirmatrelvir-ritonavir reduced all-cause mortality in both vaccinated and unvaccinated hospitalized patients. No significant reduction in ICU admission or the need for ventilatory support was observed.
Disclaimer: The corresponding authors had full access to all of the data in the study and take final responsibility for the decision to submit it for publication.
References
Agostino, Lee, Belanger, Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study, Stat Med
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Arribas, Bhagani, Lobo, Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19, NEJM Evid, doi:10.1056/EVIDoa2100044
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Chua, Kwan, Chui, Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination, Clin Infect Dis, doi:10.1093/cid/ciab989
Emilsson, García-Alb Eniz, Logan, Examining bias in studies of statin treatment and survival in patients with cancer, JAMA Oncol, doi:10.1001/jamaoncol.2017.2752
Extance, Covid-19: What is the evidence for the antiviral Paxlovid, BMJ, doi:10.1136/bmj.o1037
Extance, Covid-19: What is the evidence for the antiviral molnupiravir, BMJ, doi:10.1136/bmj.o926
Fakhroo, Alkhatib, Thani, Reinfections in COVID-19 patients: impact of virus genetic variability and host immunity, Vaccines, doi:10.3390/vaccines9101168
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hernán, Brumback, Robins, Marginal structural models to estimate the causal effect of zidovudine on the survival of HIVpositive men, Epidemiology
Hernán, Logan, Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease, Epidemiology, doi:10.1097/EDE.0b013e3181875e61
Hernán, Methods of public health research -strengthening causal inference from observational data, N Engl J Med, doi:10.1056/NEJMp2113319
Hernán, Robins, Using big data to emulate a target trial when a randomized trial is not available, Am J Epidemiol, doi:10.1093/aje/kwv254
Ho, Hong, Kong's Hospital Authority expands use of Covid-19 oral drugs
Kent, Paulus, Van Klaveren, The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement, Ann Intern Med, doi:10.7326/M18-3667
Lai, Huang, Chui, Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong, Nat Commun, doi:10.1038/s41467-022-28068-3
Lai, Huang, Peng, Post-Covid-19-vaccination adverse events and healthcare utilization among individuals with or without previous SARS-CoV-2 infection, J Intern Med, doi:10.1111/joim.13453
Lai, Li, Peng, Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine. A case-control study, Ann Intern Med, doi:10.7326/M21-3700
Li, Tong, Wong, Lack of inflammatory bowel disease flare-up following two-dose BNT162b2 vaccine: a population-based cohort study, Gut, doi:10.1136/gutjnl-2021-326860
Li, Tong, Yeung, Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong, Ann Rheum Dis, doi:10.1136/annrheumdis-2021-221571
Mahase, Covid-19: results from India's 12 molnupiravir clinical trials remain unpublished, BMJ, doi:10.1136/bmj.o2063
Mensah, Lacy, Stowe, Disease severity during SARS-COV-2 reinfection: a nationwide study, J Infect, doi:10.1016/j.jinf.2022.01.012
Moderbacher, Ramirez, Dan, Antigenspecific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity, Cell, doi:10.1016/j.cell.2020.09.038
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Phizackerley, Three more points about Paxlovid for covid-19, BMJ, doi:10.1136/bmj.o1397
Rubin, From positive to negative to positive again-the mystery of why COVID-19 rebounds in some patients who take Paxlovid, JAMA, doi:10.1001/jama.2022.9925
Sadarangani, Marchant, Kollmann, Immunological mechanisms of vaccine-induced protection against COVID-19 in humans, Nat Rev Immunol, doi:10.1038/s41577-021-00578-z
Santos, Filho, Silva, Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers, J Infect, doi:10.1016/j.jinf.2021.01.020
Wai, Chan, Cheung, Association of molnupiravir and nirmatrelvir-ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among highrisk patients with mild to moderate COVID-19, Lancet Reg Health West Pac, doi:10.1016/j.lanwpc.2022.100602
Wan, Chui, Lai, Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00451-5
Wan, Chui, Wang, Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested casecontrol study, Lancet Reg Health West Pac, doi:10.1016/j.lanwpc.2022.100393
Wan, Mok, Yan, Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 Omicron BA.2 infection, hospitalisation, severe complications, cardiovascular disease and mortality in patients with diabetes mellitus: a case control study, J Infect, doi:10.1016/j.jinf.2022.08.008
Wong, Au, Lau, Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00507-2
Wong, Au, Lau, Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Xiong, Wong, Au, Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study, Thyroid, doi:10.1089/thy.2021.0684
Yan, Wan, Ye, Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: a case-control study, Emerg Microbes Infect, doi:10.1080/22221751.2022.2114854
Yip, Lui, Lai, Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciac687
DOI record:
{
"DOI": "10.7326/m22-3057",
"ISSN": [
"0003-4819",
"1539-3704"
],
"URL": "http://dx.doi.org/10.7326/M22-3057",
"alternative-id": [
"10.7326/M22-3057"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6275-1147",
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, and Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (E.Y.F.W., C.K.H.W.)"
}
],
"authenticated-orcid": false,
"family": "Wan",
"given": "Eric Yuk Fai",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-5225-3032",
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (V.K.C.Y., F.W.T.C.)"
}
],
"authenticated-orcid": false,
"family": "Yan",
"given": "Vincent Ka Chun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (A.H.Y.M., B.W., W.X.)"
}
],
"family": "Mok",
"given": "Anna Hoi Ying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (A.H.Y.M., B.W., W.X.)"
}
],
"family": "Wang",
"given": "Boyuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (A.H.Y.M., B.W., W.X.)"
}
],
"family": "Xu",
"given": "Wanchun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7818-1575",
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (V.K.C.Y., F.W.T.C.)"
}
],
"authenticated-orcid": false,
"family": "Cheng",
"given": "Franco Wing Tak",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9121-1959",
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, and Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, Hong Kong, China (F.T.T.L.)"
}
],
"authenticated-orcid": false,
"family": "Lai",
"given": "Francisco Tsz Tsun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1513-8726",
"affiliation": [
{
"name": "Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, School of Nursing, Li Ka Shing Faculty of Medicine, The University of Hong Kong, and School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (C.S.L.C.)"
}
],
"authenticated-orcid": false,
"family": "Chui",
"given": "Celine Sze Ling",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4836-7808",
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, and Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (X.L.)"
}
],
"authenticated-orcid": false,
"family": "Li",
"given": "Xue",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6895-6071",
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, and Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (E.Y.F.W., C.K.H.W.)"
}
],
"authenticated-orcid": false,
"family": "Wong",
"given": "Carlos King Ho",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9155-9162",
"affiliation": [
{
"name": "Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (P.H.L., I.F.N.H., C.S.L.)"
}
],
"authenticated-orcid": false,
"family": "Li",
"given": "Philip Hei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6297-7154",
"affiliation": [
{
"name": "Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, and WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (B.J.C.)"
}
],
"authenticated-orcid": false,
"family": "Cowling",
"given": "Benjamin John",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1556-2538",
"affiliation": [
{
"name": "Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (P.H.L., I.F.N.H., C.S.L.)"
}
],
"authenticated-orcid": false,
"family": "Hung",
"given": "Ivan Fan Ngai",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6698-8355",
"affiliation": [
{
"name": "Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China (P.H.L., I.F.N.H., C.S.L.)"
}
],
"authenticated-orcid": false,
"family": "Lau",
"given": "Chak Sing",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8242-0014",
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, and Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, Hong Kong, China, Research Department of Practice and Policy, School of Pharmacy, University College London, London, United Kingdom, and Aston Pharmacy School, Aston University, Birmingham, United Kingdom (I.C.K.W.)"
}
],
"authenticated-orcid": false,
"family": "Wong",
"given": "Ian Chi Kei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7602-9470",
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, and Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, Hong Kong, China, and Department of Pharmacy, The University of Hong Kong-Shenzhen Hospital, and The University of Hong Kong Shenzhen Institute of Research and Innovation, Shenzhen, China (E.W.Y.C.)."
}
],
"authenticated-orcid": false,
"family": "Chan",
"given": "Esther Wai Yin",
"sequence": "additional"
}
],
"container-title": "Annals of Internal Medicine",
"container-title-short": "Ann Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
3,
13
]
],
"date-time": "2023-03-13T21:00:21Z",
"timestamp": 1678741221000
},
"deposited": {
"date-parts": [
[
2023,
4,
17
]
],
"date-time": "2023-04-17T21:03:12Z",
"timestamp": 1681765392000
},
"indexed": {
"date-parts": [
[
2023,
10,
17
]
],
"date-time": "2023-10-17T22:36:27Z",
"timestamp": 1697582187081
},
"is-referenced-by-count": 4,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
4
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
4
]
]
}
},
"language": "en",
"member": "4285",
"original-title": [],
"page": "505-514",
"prefix": "10.7326",
"published": {
"date-parts": [
[
2023,
4
]
]
},
"published-print": {
"date-parts": [
[
2023,
4
]
]
},
"publisher": "American College of Physicians",
"reference": [
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "r3-M223057"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "r4-M223057"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"doi-asserted-by": "publisher",
"key": "r5-M223057"
},
{
"DOI": "10.1001/jama.2022.9925",
"doi-asserted-by": "publisher",
"key": "r6-M223057"
},
{
"DOI": "10.1136/bmj.o1397",
"doi-asserted-by": "publisher",
"key": "r7-M223057"
},
{
"DOI": "10.1016/j.lanwpc.2022.100602",
"doi-asserted-by": "publisher",
"key": "r8-M223057"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"doi-asserted-by": "publisher",
"key": "r9-M223057"
},
{
"DOI": "10.1056/NEJMp2113319",
"doi-asserted-by": "publisher",
"key": "r10-M223057"
},
{
"DOI": "10.1097/EDE.0b013e3181875e61",
"doi-asserted-by": "publisher",
"key": "r11-M223057"
},
{
"DOI": "10.1093/aje/kwv254",
"doi-asserted-by": "publisher",
"key": "r12-M223057"
},
{
"DOI": "10.1016/S1473-3099(21)00451-5",
"doi-asserted-by": "publisher",
"key": "r15-M223057"
},
{
"DOI": "10.1093/cid/ciab989",
"doi-asserted-by": "publisher",
"key": "r16-M223057"
},
{
"DOI": "10.1136/annrheumdis-2021-221571",
"doi-asserted-by": "publisher",
"key": "r17-M223057"
},
{
"DOI": "10.1038/s41467-022-28068-3",
"doi-asserted-by": "publisher",
"key": "r18-M223057"
},
{
"DOI": "10.7326/M21-3700",
"doi-asserted-by": "publisher",
"key": "r19-M223057"
},
{
"DOI": "10.1111/joim.13453",
"doi-asserted-by": "publisher",
"key": "r20-M223057"
},
{
"DOI": "10.1136/gutjnl-2021-326860",
"doi-asserted-by": "publisher",
"key": "r21-M223057"
},
{
"DOI": "10.1016/j.lanwpc.2022.100393",
"doi-asserted-by": "publisher",
"key": "r22-M223057"
},
{
"DOI": "10.1089/thy.2021.0684",
"doi-asserted-by": "publisher",
"key": "r23-M223057"
},
{
"DOI": "10.1002/sim.4780091214",
"doi-asserted-by": "publisher",
"key": "r28-M223057"
},
{
"DOI": "10.1097/00001648-200009000-00012",
"doi-asserted-by": "publisher",
"key": "r29-M223057"
},
{
"DOI": "10.7326/M18-3667",
"doi-asserted-by": "publisher",
"key": "r30-M223057"
},
{
"DOI": "10.1136/bmj.o2063",
"doi-asserted-by": "publisher",
"key": "r31-M223057"
},
{
"DOI": "10.1056/EVIDoa2100044",
"doi-asserted-by": "crossref",
"key": "r32-M223057",
"unstructured": "Arribas JR, Bhagani S, Lobo SM, et al. Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19. NEJM Evid. 2022;1:EVIDoa2100044. doi:10.1056/EVIDoa2100044"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"doi-asserted-by": "publisher",
"key": "r33-M223057"
},
{
"DOI": "10.1093/cid/ciac687",
"doi-asserted-by": "publisher",
"key": "r34-M223057"
},
{
"DOI": "10.1001/jamaoncol.2017.2752",
"doi-asserted-by": "publisher",
"key": "r35-M223057"
},
{
"DOI": "10.1093/cid/ciac443",
"doi-asserted-by": "publisher",
"key": "r36-M223057"
},
{
"DOI": "10.1136/bmj.o1037",
"doi-asserted-by": "publisher",
"key": "r37-M223057"
},
{
"DOI": "10.1136/bmj.o926",
"doi-asserted-by": "publisher",
"key": "r38-M223057"
},
{
"DOI": "10.1038/s41577-021-00578-z",
"doi-asserted-by": "publisher",
"key": "r39-M223057"
},
{
"DOI": "10.1016/j.cell.2020.09.038",
"doi-asserted-by": "publisher",
"key": "r40-M223057"
},
{
"DOI": "10.1056/NEJMoa2204919",
"doi-asserted-by": "publisher",
"key": "r41-M223057"
},
{
"DOI": "10.1016/j.jinf.2022.08.008",
"doi-asserted-by": "publisher",
"key": "r42-M223057"
},
{
"DOI": "10.1080/22221751.2022.2114854",
"doi-asserted-by": "publisher",
"key": "r43-M223057"
},
{
"DOI": "10.3390/vaccines9101168",
"doi-asserted-by": "publisher",
"key": "r44-M223057"
},
{
"DOI": "10.1016/j.jinf.2022.01.012",
"doi-asserted-by": "publisher",
"key": "r45-M223057"
},
{
"DOI": "10.1016/j.jinf.2021.01.020",
"doi-asserted-by": "publisher",
"key": "r46-M223057"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.acpjournals.org/doi/10.7326/M22-3057"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Internal Medicine"
],
"subtitle": [
"A Target Trial Emulation Study"
],
"title": "Effectiveness of Molnupiravir and Nirmatrelvir–Ritonavir in Hospitalized Patients With COVID-19",
"type": "journal-article",
"volume": "176"
}