Molnupiravir Use and Severe Covid-19 Outcomes During the Omicron Surge
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2204919, Sep 2022 (preprint)
Retrospective 19,868 patients eligible for molnupiravir treatment in Israel with 1,069 treated, showing lower mortality and hospitalization with treatment for the subgroup of patients ≥65, and higher mortality for patients 40-64. Authors only provide subgroup results.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending molnupiravir also recommended them, or
because the patient seeking out molnupiravir is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity4-18. Multiple analyses have identified variants potentially created by molnupiravir19-23. Studies show significantly increased risk of acute kidney injury24, cardiovascular toxocity25, and neurological symptoms24. Treatment may increase viral rebound26,27.
Study covers molnupiravir and paxlovid.
|
risk of death, 74.0% lower, HR 0.26, p = 0.008, treatment 4 of 845 (0.5%), control 137 of 12,724 (1.1%), adjusted per study, multivariable, Cox proportional hazards, age 65+.
|
|
risk of death, 1182.0% higher, HR 12.82, p < 0.001, treatment 4 of 224 (1.8%), control 7 of 6,075 (0.1%), adjusted per study, multivariable, Cox proportional hazards, age 40-64.
|
|
risk of hospitalization, 45.0% lower, HR 0.55, p = 0.01, treatment 18 of 845 (2.1%), control 513 of 12,724 (4.0%), NNT 53, adjusted per study, multivariable, Cox proportional hazards, age 65+.
|
|
risk of hospitalization, 80.0% higher, HR 1.80, p = 0.12, treatment 8 of 224 (3.6%), control 97 of 6,075 (1.6%), adjusted per study, multivariable, Cox proportional hazards, age 40-64.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
5.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
6.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
7.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
8.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
9.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
10.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
11.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
12.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
13.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
14.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
15.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
16.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
17.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
18.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
19.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
20.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
21.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
22.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
24.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
25.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Arbel et al., 29 Sep 2022, retrospective, Israel, preprint, 16 authors, study period 16 January, 2022 - 31 March, 2022.
Contact: ronen.arbel@gmail.com.
Molnupiravir Use and Severe Covid-19 Outcomes During the Omicron Surge
doi:10.21203/rs.3.rs-2115769/v1
Background The oral antiviral molnupiravir is moderately effective in high-risk, unvaccinated non-hospitalized patients infected with early variants of SARS-CoV-2. Data regarding the effectiveness of molnupiravir against the B.1.1.529 (omicron) variant and in vaccinated populations are limited.
Methods We obtained data for all members of Clalit Health Services, 40 years of age and older, eligible for molnupiravir therapy during the omicron surge. A Cox proportional-hazards regression model with timedependent covariates was used to estimate the association between molnupiravir treatment and hospitalizations and deaths due to Covid-19, with adjustment for sociodemographic factors, coexisting conditions, and prior Covid-19 immunity status.
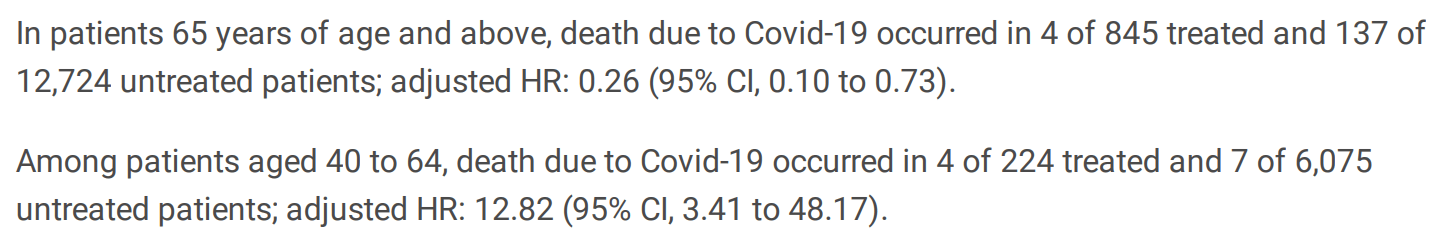
Results A total of 19,868 participants met the eligibility criteria, of whom 1,069 (5%) received molnupiravir during the study period. In patients 65 years and above, the rate of hospitalizations related to Covid-19 in treated compared to untreated patients was 74.6 versus 127.6 per 100,000 person-days; adjusted hazard ratio (HR) 0.55 (95% CI, 0.34 to 0.88). The adjusted HR for death due to Covid-19 was 0.26 (95% CI, 0.10 to 0.73). Among patients aged 40 to 64, the hospitalizations rate in treated compared to untreated patients was 125.8 versus 49.1 per 100,000 person-days; adjusted HR 1.80 (95% CI, 0.86 to 3.77). The adjusted HR for death was 12.8 (95% CI, 3.41 to 48.2).
Conclusions In a cohort of non-hospitalized, omicron-infected high-risk patients, molnupiravir therapy was associated with a signi cant reduction in hospitalizations and mortality due to Covid-19 in patients 65 years and above. However, no evidence of bene t was found in younger adults.
This work did not receive any nancial or in-kind support.
Con icts of Interest All authors report no Con icts of interest
References
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Non-hospitalized Patients, N Engl J Med
Li, Wang, Lavrijsen, SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination, Cell Res, doi:10.1038/s41422-022-00618-w
Lévesque, Hanley, Kezouh, Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes, BMJ, doi:10.1136/bmj.b5087
Takashita, Kinoshita, Yamayoshi, E cacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant, N Engl J Med, doi:10.1056/NEJMc2119407
Takashita, Kinoshita, Yamayoshi, E cacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2, N Engl J Med, doi:10.1056/NEJMc2201933
Yip, Lui, Lai, Wong, Tse et al., Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients, Clin Infect Dis, doi:10.1093/cid/ciac687
DOI record:
{
"DOI": "10.1056/nejmoa2204919",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2204919",
"alternative-id": [
"10.1056/NEJMoa2204919"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6058-8665",
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"authenticated-orcid": false,
"family": "Arbel",
"given": "Ronen",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Wolff Sagy",
"given": "Yael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Hoshen",
"given": "Moshe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Battat",
"given": "Erez",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Lavie",
"given": "Gil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Sergienko",
"given": "Ruslan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Friger",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Waxman",
"given": "Jacob G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8811-7825",
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"authenticated-orcid": false,
"family": "Dagan",
"given": "Noa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Balicer",
"given": "Ran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Ben-Shlomo",
"given": "Yatir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Peretz",
"given": "Alon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Yaron",
"given": "Shlomit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Serby",
"given": "Danielle",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4396-5246",
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"authenticated-orcid": false,
"family": "Hammerman",
"given": "Ariel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Division of Community Medical Services (R.A., A.P., S.Y., D.S., A.H., D.N.), the Branch of Planning and Strategy (Y.W.S., M.H., E.B., G.L.), and the Clalit Research Institute, Division of Innovation (J.G.W., N.D., R.B., Y.B.-S.), Clalit Health Services, Tel Aviv, the Maximizing Health Outcomes Research Lab, Sapir College, Sderot (R.A.), the Department of Bioinformatics, Jerusalem College of Technology, Jerusalem (M.H.), the Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute..."
}
],
"family": "Netzer",
"given": "Doron",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T21:00:10Z",
"timestamp": 1661374810000
},
"deposited": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T21:00:12Z",
"timestamp": 1661374812000
},
"indexed": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T21:41:53Z",
"timestamp": 1661377313466
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
24
]
]
},
"language": "en",
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2204919",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"prefix": "10.1056",
"published": {
"date-parts": [
[
2022,
8,
24
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
24
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1056/NEJMe2202699",
"doi-asserted-by": "publisher",
"key": "r1"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1056/NEJMe2202160",
"doi-asserted-by": "publisher",
"key": "r5"
},
{
"DOI": "10.1056/NEJMoa2101765",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.1056/NEJMoa2115624",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.1136/bmj.b5087",
"doi-asserted-by": "publisher",
"key": "r8"
},
{
"DOI": "10.1038/s41591-022-01832-0",
"doi-asserted-by": "publisher",
"key": "r9"
},
{
"DOI": "10.1056/NEJMc2119407",
"doi-asserted-by": "publisher",
"key": "r12"
},
{
"DOI": "10.1056/NEJMc2201933",
"doi-asserted-by": "publisher",
"key": "r13"
},
{
"DOI": "10.1038/s41422-022-00618-w",
"doi-asserted-by": "publisher",
"key": "r14"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"doi-asserted-by": "publisher",
"key": "r15"
},
{
"DOI": "10.1038/d41586-021-03614-z",
"doi-asserted-by": "publisher",
"key": "r16"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2204919"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge",
"type": "journal-article"
}