Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study
et al., The Lancet, doi:10.1016/S0140-6736(22)01586-0, Oct 2022
PSM retrospective 1,074,856 outpatients in Hong Kong, showing lower mortality with molnupiravir.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments25.
Study covers molnupiravir and paxlovid.
|
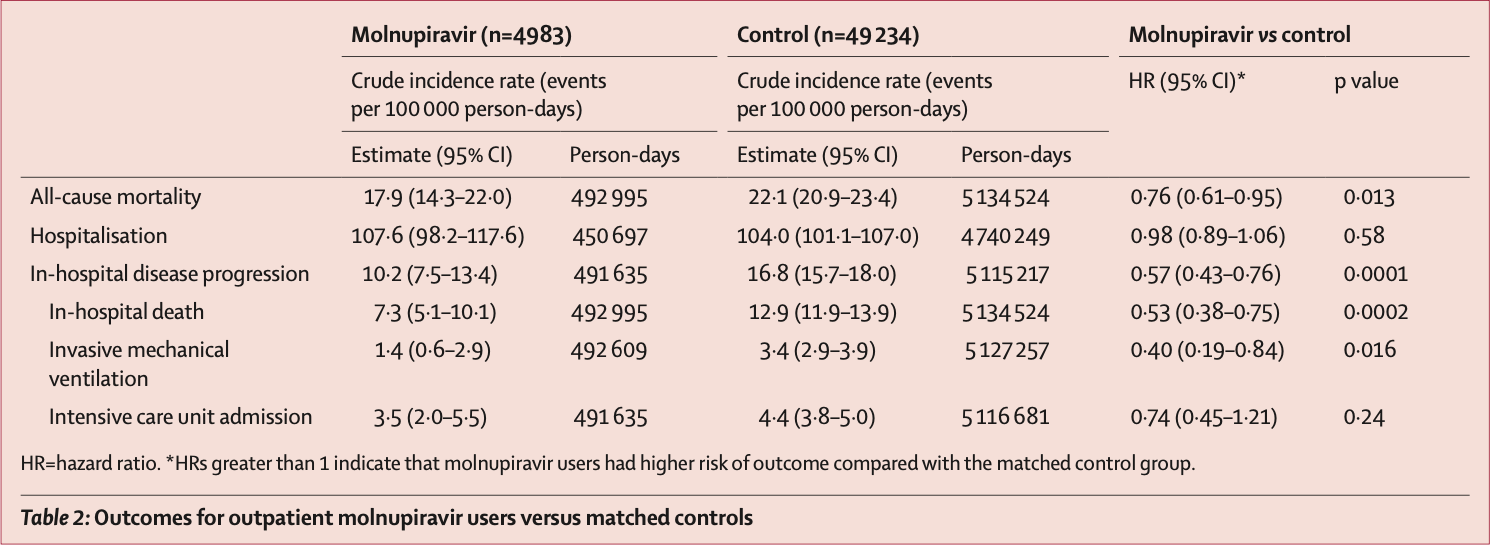
risk of death, 24.0% lower, HR 0.76, p = 0.01, treatment 4,983, control 49,234, propensity score matching.
|
|
risk of mechanical ventilation, 60.0% lower, HR 0.40, p = 0.02, treatment 4,983, control 49,234, propensity score matching.
|
|
risk of ICU admission, 26.0% lower, HR 0.74, p = 0.24, treatment 4,983, control 49,234, propensity score matching.
|
|
risk of hospitalization, 2.0% lower, HR 0.98, p = 0.58, treatment 4,983, control 49,234, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Wong et al., 8 Oct 2022, retrospective, China, peer-reviewed, 6 authors, study period 26 February, 2022 - 26 June, 2022.
Contact: carlosho@hku.hk, bcowling@hku.hk.
Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and inhospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study
Background Little is known about the real-world effectiveness of oral antivirals against the SARS-CoV-2 omicron (B.1.1.529) variant. We aimed to assess the clinical effectiveness of two oral antiviral drugs among communitydwelling COVID-19 outpatients in Hong Kong.
Methods In this observational study, we used data from the Hong Kong Hospital Authority to identify an unselected, territory-wide cohort of non-hospitalised patients with an officially registered diagnosis of SARS-CoV-2 infection between Feb 26 and June 26, 2022, during the period in which the omicron subvariant BA.2.2 was dominant in Hong Kong. We used a retrospective cohort design as primary analysis, and a case-control design as sensitivity analysis. We identified patients with COVID-19 who received either molnupiravir (800 mg twice daily for 5 days) or nirmatrelvir plus ritonavir (nirmatrelvir 300 mg and ritonavir 100 mg twice daily for 5 days, or nirmatrelvir 150 mg and ritonavir 100 mg if estimated glomerular filtration rate was 30-59 mL/min per 1•73 m²). Outpatient oral antiviral users were matched with controls using propensity score (1:10) according to age, sex, date of SARS-CoV-2 infection diagnosis, Charlson Comorbidity Index score, and vaccination status. Study outcomes were death, COVID-19-related hospitalisation, and in-hospital disease progression (in-hospital death, invasive mechanical ventilation, or intensive care unit admission). Hazard ratios (HRs) were estimated by Cox regression for the primary analysis, and odds ratios in oral antiviral users compared with non-users by logistic regression for the sensitivity analysis. Findings Among 1 074 856 non-hospitalised patients with COVID-19, 5383 received molnupiravir and 6464 received nirmatrelvir plus ritonavir in the community setting. Patients were followed up for a median of 103 days in the molnupiravir group and 99 days in the nirmatrelvir plus ritonavir group. Compared with nirmatrelvir plus ritonavir users, those on molnupiravir were older (4758 [85•9%] vs 4418 [88.7%] aged >60 years) and less likely to have been fully vaccinated (1850 [33•4%] vs 800 [16•1%]). Molnupiravir use was associated with lower risks of death (HR 0•76 [95% CI 0•61-0•95]) and in-hospital disease progression (0•57 [0•43-0•76]) than non-use was, whereas risk of hospitalisation was similar in both groups (0•98 [0•89-1•06]). Nirmatrelvir plus ritonavir use was associated with lower risks of death (0•34 [0•22-0•52]), hospitalisation (0•76 [0•67-0•86]), and in-hospital disease progression (0•57 [0•38-0•87]) than non-use was. We consistently found reduced risks of mortality and hospitalisation associated with early oral antiviral use among older patients. The findings from the case-control analysis broadly supported those from the primary analysis. Interpretation During Hong Kong's wave of SARS-CoV-2 omicron subvariant BA.2.2, among non-hospitalised patients with COVID-19, early initiation of novel oral antivirals was associated with reduced..
Molnupiravir Control Not hospitalised Hospitalised with no oxygen therapy Supplemental oxygen without ventilation or mechanical ventilation Discharged In-hospital death
Nirmatrelvir plus ritonavir
Control disease burden and vaccine effectiveness. He has not provided scientific advice to either company related to COVID-19 antiviral effectiveness, and he has not received any funding from Pfizer or AstraZeneca for any research on antiviral effectiveness including the current work. All other authors declare no competing interests.
Data sharing The clinical outcome data and vaccination records were extracted from the Hospital Authority database in Hong Kong and data on confirmed cases of SARS-CoV-2 infection were extracted from the eSARS data provided by the Centre for Health Protection. Restrictions apply to the availability of these data, which were used under licence for this study.
References
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge, NEJM
Austin, Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations, Biom J
Chen, Abdullah, Chan, Contribution of low population immunity to the severe omicron BA.2 outbreak in Hong Kong, Nat Commun
Da Silva, Musungaie, vaccinated patients were defined as those with at least two doses of BNT162b2 (Fosun-BioNTech) or three doses of CoronaVac (Sinovac). Table 1: Baseline characteristics of non-hospitalised patients with COVID-19 after 1:10 propensity-score matching References 1 Jayk Bernal A, N Engl J Med
Dal-Ré, Becker, Bottieau, Holm, Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach, Lancet Infect Dis
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, medRxiv, doi:10.1101/2022.06.14.22276393
Dyer, Covid-19: FDA expert panel recommends authorising molnupiravir but also voices concerns, BMJ
Fischer Wa 2nd, Eron, Jr, Holman, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Githaka, Molnupiravir does not induce mutagenesis in host lung cells during SARS-CoV-2 treatment, Bioinform Biol Insights
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
Johnson, Puenpatom, Moncada, Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19 : a randomized, placebo-controlled trial, Ann Intern Med
Lee, Morris, Grover, Murthy, Mcdonald, Outpatient therapies for COVID-19: how do we choose?, Open Forum Infect Dis
Li, Wang, Lavrijsen, SARS-CoV-2 omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination, Cell Res
Mcmenamin, Lin, Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study, Lancet Infect Dis
Najjar-Debbiny, Gronich, Weber, Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Nyberg, Ferguson, Nash, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, Lancet
Pfizer, PAXLOVID supporting upcoming new drug application submission to US FDA
Sendi, Razonable, Nelson, Soriano, Gandhi, Firstgeneration oral antivirals against SARS-CoV-2, Clin Microbiol Infect
Singh, Singh, Singh, Misra, An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19, Diabetes Metab Syndr
Singh, Singh, Singh, Misra, Molnupiravir in COVID-19: a systematic review of literature, Diabetes Metab Syndr
Tian, Pang, Li, Molnupiravir and its antiviral activity against COVID-19, Front Immunol
Ulloa, Buchan, Daneman, Brown, Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada, JAMA
Vena, Traman, Bavastro, Early clinical experience with molnupiravir for mild to moderate breakthrough COVID-19 among fully vaccinated patients at risk for disease progression, Vaccines
Waters, Warren, Hughes, Lewis, Zhang, Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environ Mol Mutagen
Wen, Chen, Tang, Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-1: a meta-analysis, Ann Med
Who, Therapeutics and COVID-19: living guideline
Wong, Wan, Luo, Clinical outcomes of different therapeutic options for COVID-19 in two Chinese case cohorts: a propensity-score analysis, EClinicalMedicine
Zhou, Hill, Sarkar, β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells, J Infect Dis
DOI record:
{
"DOI": "10.1016/s0140-6736(22)01586-0",
"ISSN": [
"0140-6736"
],
"URL": "http://dx.doi.org/10.1016/S0140-6736(22)01586-0",
"alternative-id": [
"S0140673622015860"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S0140-6736(22)01586-0"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S0140-6736(22)01929-8"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Wong",
"given": "Carlos K H",
"sequence": "first"
},
{
"affiliation": [],
"family": "Au",
"given": "Ivan C H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lau",
"given": "Kristy T K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lau",
"given": "Eric H Y",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cowling",
"given": "Benjamin J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leung",
"given": "Gabriel M",
"sequence": "additional"
}
],
"container-title": "The Lancet",
"container-title-short": "The Lancet",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
7
]
],
"date-time": "2022-10-07T12:05:40Z",
"timestamp": 1665144340000
},
"deposited": {
"date-parts": [
[
2022,
10,
7
]
],
"date-time": "2022-10-07T15:45:51Z",
"timestamp": 1665157551000
},
"indexed": {
"date-parts": [
[
2022,
10,
7
]
],
"date-time": "2022-10-07T16:14:43Z",
"timestamp": 1665159283390
},
"is-referenced-by-count": 1,
"issue": "10359",
"issued": {
"date-parts": [
[
2022,
10
]
]
},
"journal-issue": {
"issue": "10359",
"published-print": {
"date-parts": [
[
2022,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
1
]
],
"date-time": "2022-10-01T00:00:00Z",
"timestamp": 1664582400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0140673622015860?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0140673622015860?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1213-1222",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/S0140-6736(22)01586-0_bib1",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.dsx.2021.102329",
"article-title": "Molnupiravir in COVID-19: a systematic review of literature",
"author": "Singh",
"doi-asserted-by": "crossref",
"journal-title": "Diabetes Metab Syndr",
"key": "10.1016/S0140-6736(22)01586-0_bib2",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"article-title": "Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-1: a meta-analysis",
"author": "Wen",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Ann Med",
"key": "10.1016/S0140-6736(22)01586-0_bib3",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"article-title": "A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus",
"author": "Fischer",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S0140-6736(22)01586-0_bib5",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/S0140-6736(22)01586-0_bib6",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge",
"author": "Arbel",
"doi-asserted-by": "crossref",
"first-page": "790",
"journal-title": "NEJM",
"key": "10.1016/S0140-6736(22)01586-0_bib7",
"volume": "387",
"year": "2022"
},
{
"article-title": "Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system",
"author": "Dryden-Peterson",
"journal-title": "medRxiv",
"key": "10.1016/S0140-6736(22)01586-0_bib8",
"year": "2022"
},
{
"article-title": "Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients",
"author": "Najjar-Debbiny",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S0140-6736(22)01586-0_bib9",
"year": "2022"
},
{
"DOI": "10.3390/vaccines10071141",
"article-title": "Early clinical experience with molnupiravir for mild to moderate breakthrough COVID-19 among fully vaccinated patients at risk for disease progression",
"author": "Vena",
"doi-asserted-by": "crossref",
"journal-title": "Vaccines",
"key": "10.1016/S0140-6736(22)01586-0_bib10",
"volume": "10",
"year": "2022"
},
{
"article-title": "Contribution of low population immunity to the severe omicron BA.2 outbreak in Hong Kong",
"author": "Chen",
"journal-title": "Nat Commun",
"key": "10.1016/S0140-6736(22)01586-0_bib11",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.100743",
"article-title": "Clinical outcomes of different therapeutic options for COVID-19 in two Chinese case cohorts: a propensity-score analysis",
"author": "Wong",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/S0140-6736(22)01586-0_bib12",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(22)00345-0",
"article-title": "Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study",
"author": "McMenamin",
"doi-asserted-by": "crossref",
"first-page": "1435",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S0140-6736(22)01586-0_bib13",
"volume": "22",
"year": "2022"
},
{
"key": "10.1016/S0140-6736(22)01586-0_bib14",
"series-title": "Interim recommendation on clinical management of adult cases with COVID-19. Version 1.12",
"year": "2022"
},
{
"DOI": "10.1002/bimj.200810488",
"article-title": "Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "171",
"journal-title": "Biom J",
"key": "10.1016/S0140-6736(22)01586-0_bib16",
"volume": "51",
"year": "2009"
},
{
"DOI": "10.1016/S1473-3099(22)00119-0",
"article-title": "Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach",
"author": "Dal-Ré",
"doi-asserted-by": "crossref",
"first-page": "e231",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S0140-6736(22)01586-0_bib18",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofac008",
"article-title": "Outpatient therapies for COVID-19: how do we choose?",
"author": "Lee",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/S0140-6736(22)01586-0_bib19",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/j.dsx.2022.102396",
"article-title": "An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19",
"author": "Singh",
"doi-asserted-by": "crossref",
"journal-title": "Diabetes Metab Syndr",
"key": "10.1016/S0140-6736(22)01586-0_bib20",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"article-title": "Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study",
"author": "Nyberg",
"doi-asserted-by": "crossref",
"first-page": "1303",
"journal-title": "Lancet",
"key": "10.1016/S0140-6736(22)01586-0_bib21",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.2274",
"article-title": "Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada",
"author": "Ulloa",
"doi-asserted-by": "crossref",
"first-page": "1286",
"journal-title": "JAMA",
"key": "10.1016/S0140-6736(22)01586-0_bib22",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.04.015",
"article-title": "First-generation oral antivirals against SARS-CoV-2",
"author": "Sendi",
"doi-asserted-by": "crossref",
"first-page": "1230",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/S0140-6736(22)01586-0_bib23",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.7326/M22-0729",
"article-title": "Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19 : a randomized, placebo-controlled trial",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "1126",
"journal-title": "Ann Intern Med",
"key": "10.1016/S0140-6736(22)01586-0_bib24",
"volume": "175",
"year": "2022"
},
{
"key": "10.1016/S0140-6736(22)01586-0_bib27",
"series-title": "Therapeutics and COVID-19: living guideline, July 14, 2022",
"year": "2022"
},
{
"article-title": "Covid-19: FDA expert panel recommends authorising molnupiravir but also voices concerns",
"author": "Dyer",
"journal-title": "BMJ",
"key": "10.1016/S0140-6736(22)01586-0_bib28",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1177/11779322221085077",
"article-title": "Molnupiravir does not induce mutagenesis in host lung cells during SARS-CoV-2 treatment",
"author": "Githaka",
"doi-asserted-by": "crossref",
"journal-title": "Bioinform Biol Insights",
"key": "10.1016/S0140-6736(22)01586-0_bib29",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.855496",
"article-title": "Molnupiravir and its antiviral activity against COVID-19",
"author": "Tian",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "10.1016/S0140-6736(22)01586-0_bib30",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1002/em.22471",
"article-title": "Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir",
"author": "Waters",
"doi-asserted-by": "crossref",
"first-page": "37",
"journal-title": "Environ Mol Mutagen",
"key": "10.1016/S0140-6736(22)01586-0_bib31",
"volume": "63",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiab247",
"article-title": "β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "415",
"journal-title": "J Infect Dis",
"key": "10.1016/S0140-6736(22)01586-0_bib32",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1038/s41422-022-00618-w",
"article-title": "SARS-CoV-2 omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "322",
"journal-title": "Cell Res",
"key": "10.1016/S0140-6736(22)01586-0_bib33",
"volume": "32",
"year": "2022"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0140673622015860"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "400"
}
wong5