Methodological Analysis of Bias Risks in Adaptive Multi-Arm Platform Trials: A Case-Series from Three COVID-19 Studies
, S., Center for Open Science, doi:10.31222/osf.io/h5kc8_v1, Jan 2026
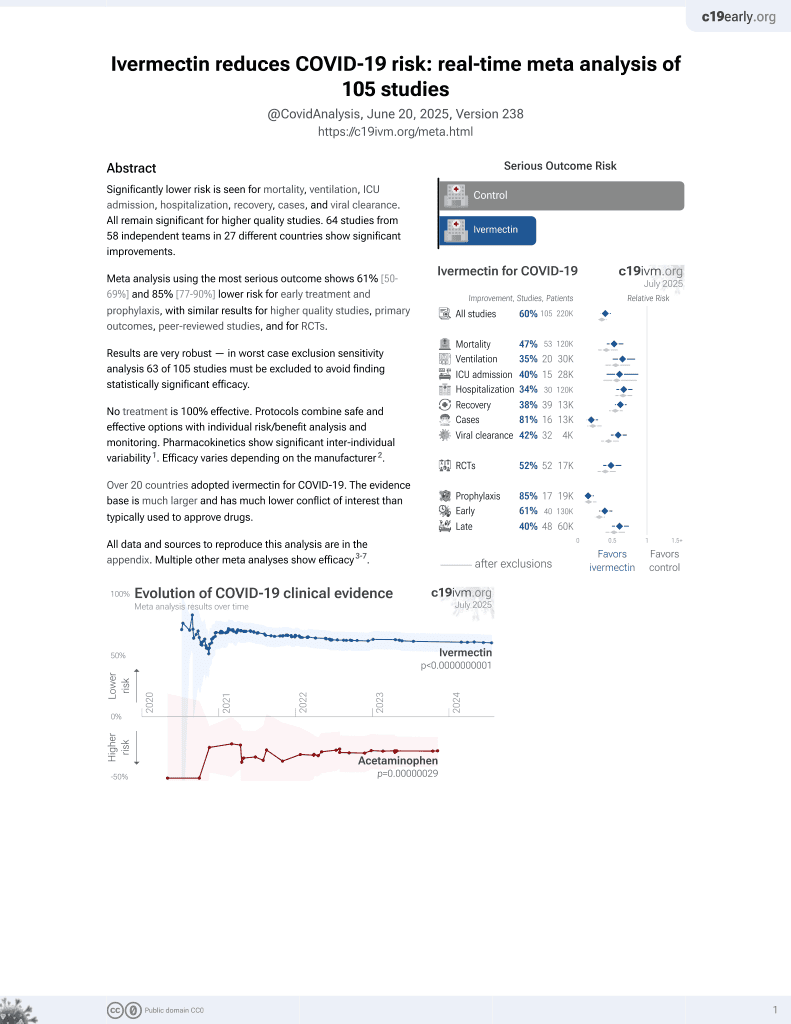
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
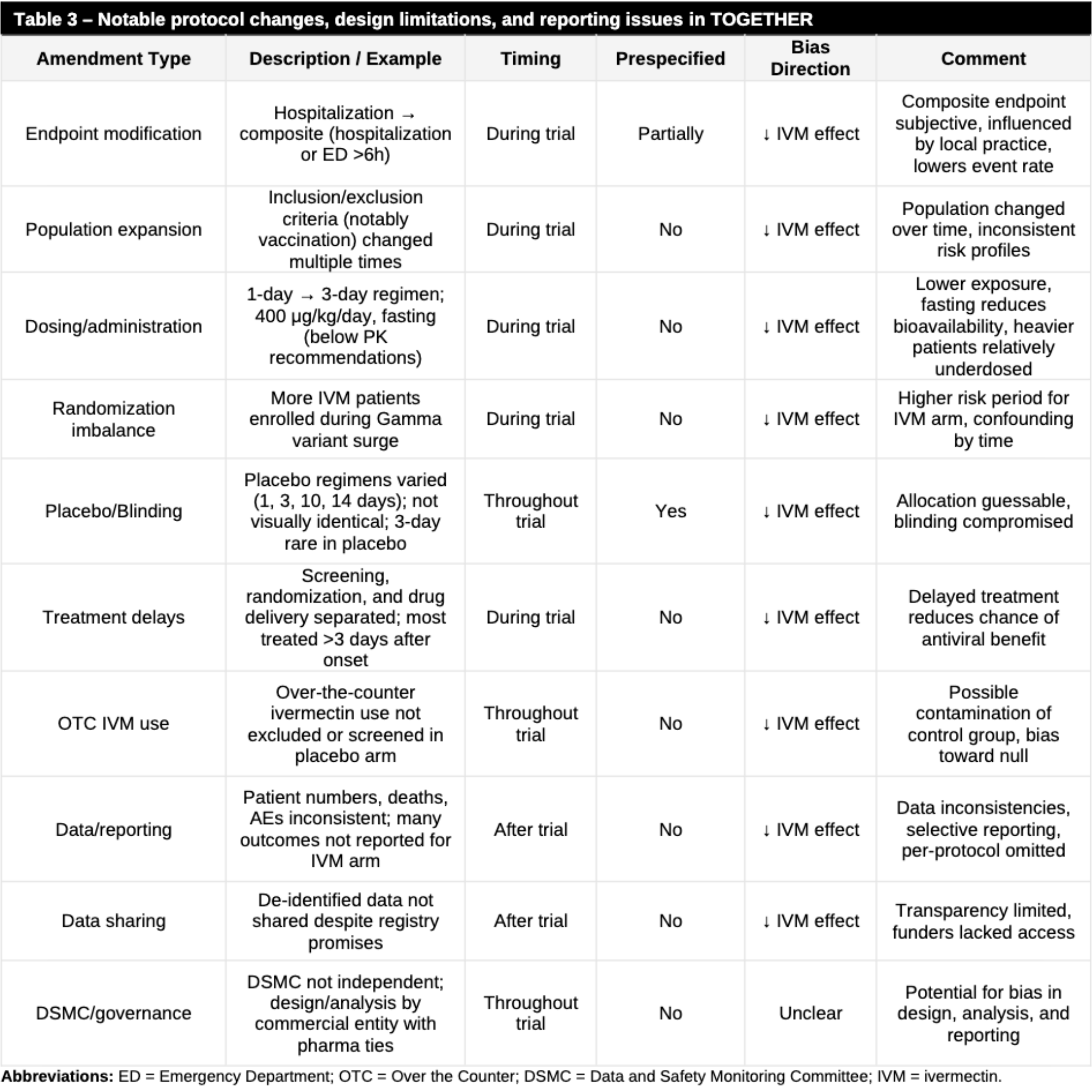
Review of methodological biases in three major adaptive platform trials (ACTIV-6, PRINCIPLE, and TOGETHER) evaluating ivermectin for COVID-19. Author finds that these influential studies were compromised by extensive post-enrollment protocol amendments, suboptimal dosing, and reporting inconsistencies that systematically biased results toward underestimating efficacy. Author details how primary endpoints were frequently modified after enrollment had begun, while eligibility criteria were expanded to include lower-risk populations, effectively reducing the power to detect differences in severe outcomes. Author also highlights that the dosing regimens were often below pharmacokinetic recommendations and, crucially, administered on an empty stomach, which significantly reduces bioavailability compared to administration with a high-fat meal. Delayed treatment initiation, with patients often treated several days after symptom onset, was identified as further limiting potential antiviral benefits. Author also notes that positive findings in secondary outcomes were often downplayed or omitted in final publications, and that transparency was lacking due to the failure to share de-identified participant data despite registry promises. Details of all issues reported, and many more, can be found in the individual paper analyses1-3.
1.
Naggie et al., Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.18590.
2.
Reis et al., Effect of Early Treatment with Ivermectin among Patients with Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2115869.
3.
Hayward et al., Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes, Journal of Infection, doi:10.1016/j.jinf.2024.106130.
4.
Reich, S., Methodological Analysis of Bias Risks in Adaptive Multi-Arm Platform Trials: A Case-Series from Three COVID-19 Studies, Center for Open Science, doi:10.31222/osf.io/h5kc8_v1.
5.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
6.
Zhang et al., Rho-GTPases subfamily: cellular defectors orchestrating viral infection, Cellular & Molecular Biology Letters, doi:10.1186/s11658-025-00722-w.
7.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
8.
Ulloa-Aguilar et al., The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection, Cells, doi:10.3390/cells13181591.
9.
Enyeji et al., Effective Treatment of COVID-19 Infection with Repurposed Drugs: Case Reports, Viral Immunology, doi:10.1089/vim.2024.0034.
10.
Wimalawansa, S., Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity, Heliyon, doi:10.1016/j.heliyon.2024.e34691.
11.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
12.
Mehraeen et al., Treatments for Olfactory Dysfunction in COVID-19: A Systematic Review, International Archives of Otorhinolaryngology, doi:10.1055/s-0044-1786046.
13.
Scheim et al., Back to the Basics of SARS-CoV-2 Biochemistry: Microvascular Occlusive Glycan Bindings Govern Its Morbidities and Inform Therapeutic Responses, Viruses, doi:10.3390/v16040647.
14.
Yagisawa et al., Global trends in clinical trials of ivermectin for COVID-19—Part 2, The Japanese Journal of Antibiotics, doi:10.11553/antibiotics.77.1_45.
15.
Liu et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
16.
Scheim (B) et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
17.
Yemeke et al., Impact of the COVID-19 pandemic on the quality of medical products in Zimbabwe: a qualitative study based on key informant interviews with health system stakeholders, BMJ Open, doi:10.1136/bmjopen-2022-068923.
18.
Kory, P., The Global War on Ivermectin, International Covid Summit III, European Parliament, Brussels, covid19criticalcare.com/wp-content/uploads/2023/05/GLOBAL-WAR-ON-IVERMECTIN-PARLIAMENT.pdf.
19.
Babalola et al., The Place of Ivermectin in the Management of Covid-19: State of the Evidence, Medical Research Archives, doi:10.18103/mra.v11i4.3778.
20.
Loo et al., Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-023-03520-1.
21.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
22.
Kory (B), P., The Criminal Censorship of Ivermectin's Efficacy By The High-Impact Medical Journals - Part 1, Pierre Kory’s Medical Musings, pierrekory.substack.com/p/the-criminal-censorship-of-ivermectins.
23.
Al-kuraishy et al., Central effects of Ivermectin in alleviation of Covid-19-induced dysautonomia, Current Drug Targets, doi:10.2174/1389450123666220810102406.
24.
Schwartz, E., Does ivermectin have a place in the treatment of mild Covid-19?, New Microbes and New Infections, doi:10.1016/j.nmni.2022.100989.
25.
Marques et al., Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols, Brazilian Journal of Biology, doi:10.1590/1519-6984.258325.
26.
Semiz, S., SIT1 transporter as a potential novel target in treatment of COVID-19, Biomolecular Concepts, doi:10.1515/bmc-2021-0017.
27.
Zaidi et al., The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review, The Journal of Antibiotics, doi:10.1038/s41429-021-00491-6.
28.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
29.
Low et al., Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166294.
30.
Fordham et al., The uses and abuses of systematic reviews, OSF Preprints, doi:10.31219/osf.io/mp4f2.
31.
Kow et al., Pitfalls in Reporting Sample Size Calculation Across Randomized Controlled Trials Involving Ivermectin for the treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001441.
32.
Santin et al., Ivermectin: a multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes and New Infections, doi:10.1016/j.nmni.2021.100924.
33.
Adegboro et al., A review of the anti-viral effects of ivermectin, African Journal of Clinical and Experimental Microbiology, doi:10.4314/ajcem.v22i3.2.
34.
Turkia, M., A Continuation of a Timeline of Ivermectin-Related Events in the COVID-19 Pandemic [June 30, 2021], ResearchGate, doi:10.13140/RG.2.2.16973.36326.
35.
Jagiasi et al., Variation in therapeutic strategies for the management of severe COVID-19 in India- A nationwide cross-sectional survey, The International Journal of Clinical Practice, doi:10.1111/ijcp.14574.
36.
Lind et al., Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis, Journal of General Internal Medicine, doi:10.1007/s11606-021-06948-6.
37.
Wang et al., Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19, medRxiv, doi:10.1101/2021.06.01.21258147.
38.
Kory (C) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001377.
39.
DiNicolantonio et al., Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors, Open Heart, doi:10.1136/openhrt-2021-001655.
40.
Turkia (B), M., A timeline of ivermectin-related events in the COVID-19 pandemic, Research Gate, www.researchgate.net/publication/350610718_A_Timeline_of_Ivermectin-Related_Events_in_the_COVID-19_Pandemic_April_3_2021.
41.
Wehbe et al., Repurposing Ivermectin for COVID-19: Molecular Aspects and Therapeutic Possibilities, Front. Immunol., doi:10.3389/fimmu.2021.663586.
42.
Yagisawa (B) et al., Global trends in clinical studies of ivermectin in COVID-19, The Japanese Journal of Antibiotics, 74-1, Mar 2021, jja-contents.wdc-jp.com/pdf/JJA74/74-1-open/74-1_44-95.pdf.
43.
Jans et al., The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.10.042.
44.
Kory (D) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Frontiers in Pharmacology, doi:10.3389/fphar.2021.643369.
45.
Formiga et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J. Control Release, doi:10.1016/j.jconrel.2020.10.009.
46.
Scheim (D), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
47.
Turkia (C), M., FLCCC Alliance MATH+ ascorbic acid and I-MASK+ ivermectin protocols for COVID-19 — a brief review, ResearchGate, www.researchgate.net/profile/Mika_Turkia/publication/345694745_FLCCC_Alliance_MATH_ascorbic_acid_and_I-MASK_ivermectin_protocols_for_COVID-19_-_A_Brief_Review/links/5fab010f4585150781078260/FLCCC-Alliance-MATH-ascorbic-acid-and-I-MASK-ivermectin-protocols-for-COVID-19-A-Brief-Review.pdf.
48.
Jans (B) et al., Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?, Cells 2020, 9:9, 2100, doi:10.3390/cells9092100.
49.
Elkholy et al., Ivermectin: A Closer Look at a Potential Remedy, Cureus, doi:10.7759/cureus.10378.
50.
DiNicolantonio (B) et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
51.
Vora et al., White paper on Ivermectin as a potential therapy for COVID-19, Indian Journal of Tuberculosis, doi:10.1016/j.ijtb.2020.07.031.
Reich et al., 6 Jan 2026, preprint, 1 author.
DOI record:
{
"DOI": "10.31222/osf.io/h5kc8_v1",
"URL": "http://dx.doi.org/10.31222/osf.io/h5kc8_v1",
"abstract": "<p>Background: Adaptive platform trials (APTs) were widely promoted during the COVID-19 pandemic as an efficient and flexible approach for evaluating multiple interventions. However, their complexity and frequent protocol amendments may introduce risks of bias. We selected ivermectin as our case study both because of the marked discrepancy between the generally positive findings in the broader literature and the negative results from a few large trials, and because these influential studies were all designed as adaptive platform trials with multiple arms.Methods: We performed a methodological case-series analysis of three major adaptive platform trials: ACTIV-6 (USA), PRINCIPLE (UK), and TOGETHER (Brazil), each of which included an ivermectin arm for outpatient COVID-19. For each trial, we systematically reviewed all publicly available protocols, registry entries, analysis plans, preprints, and publications. We identified key protocol changes, design limitations, and reporting issues, and summarized recurring patterns related to endpoints, dosing, treatment timing, randomization, reporting, and data transparency. Each issue was assessed for its likely direction of bias on the observed efficacy of ivermectin. Results: All three trials exhibited extensive protocol changes, outcome modifications, and reporting inconsistencies, many of which occurred after participant enrollment had begun. Common issues included delayed treatment initiation, suboptimal dosing or administration, selective reporting of results, and inconsistent application of early stopping rules. Positive findings in secondary outcomes or subgroups were often omitted or downplayed in final publications. Data inconsistencies and lack of transparency further limited independent verification. The cumulative effect of these issues was a systematic bias toward underestimating any potential benefit of ivermectin.Conclusion: Our analysis shows that even large adaptive platform trials can be compromised by methodological flaws, losing their intended advantages and potentially distorting the evidence base. In all cases, these flaws systematically biased the assessment of ivermectin, meaning the results of these trials cannot be regarded as definitive evidence against its potential benefit. We propose that small but well-designed randomized trials, when combined in meta-analyses, may provide a more reliable foundation for evidence, even if individual studies are underpowered. Above all, critical appraisal and transparency are essential for trustworthy clinical research and sound policy decisions.</p>",
"author": [
{
"affiliation": [],
"family": "Reich",
"given": "Simon",
"sequence": "first",
"suffix": "MD"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
6
]
],
"date-time": "2026-01-06T18:59:43Z",
"timestamp": 1767725983000
},
"deposited": {
"date-parts": [
[
2026,
1,
7
]
],
"date-time": "2026-01-07T05:01:49Z",
"timestamp": 1767762109000
},
"group-title": "MetaArXiv",
"indexed": {
"date-parts": [
[
2026,
1,
7
]
],
"date-time": "2026-01-07T05:24:18Z",
"timestamp": 1767763458130,
"version": "3.48.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2026,
1,
6
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nd/4.0/legalcode",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
6
]
],
"date-time": "2026-01-06T00:00:00Z",
"timestamp": 1767657600000
}
}
],
"member": "15934",
"original-title": [],
"posted": {
"date-parts": [
[
2026,
1,
6
]
]
},
"prefix": "10.31222",
"published": {
"date-parts": [
[
2026,
1,
6
]
]
},
"publisher": "Center for Open Science",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://osf.io/h5kc8_v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Methodological Analysis of Bias Risks in Adaptive Multi-Arm Platform Trials: A Case-Series from Three COVID-19 Studies",
"type": "posted-content"
}