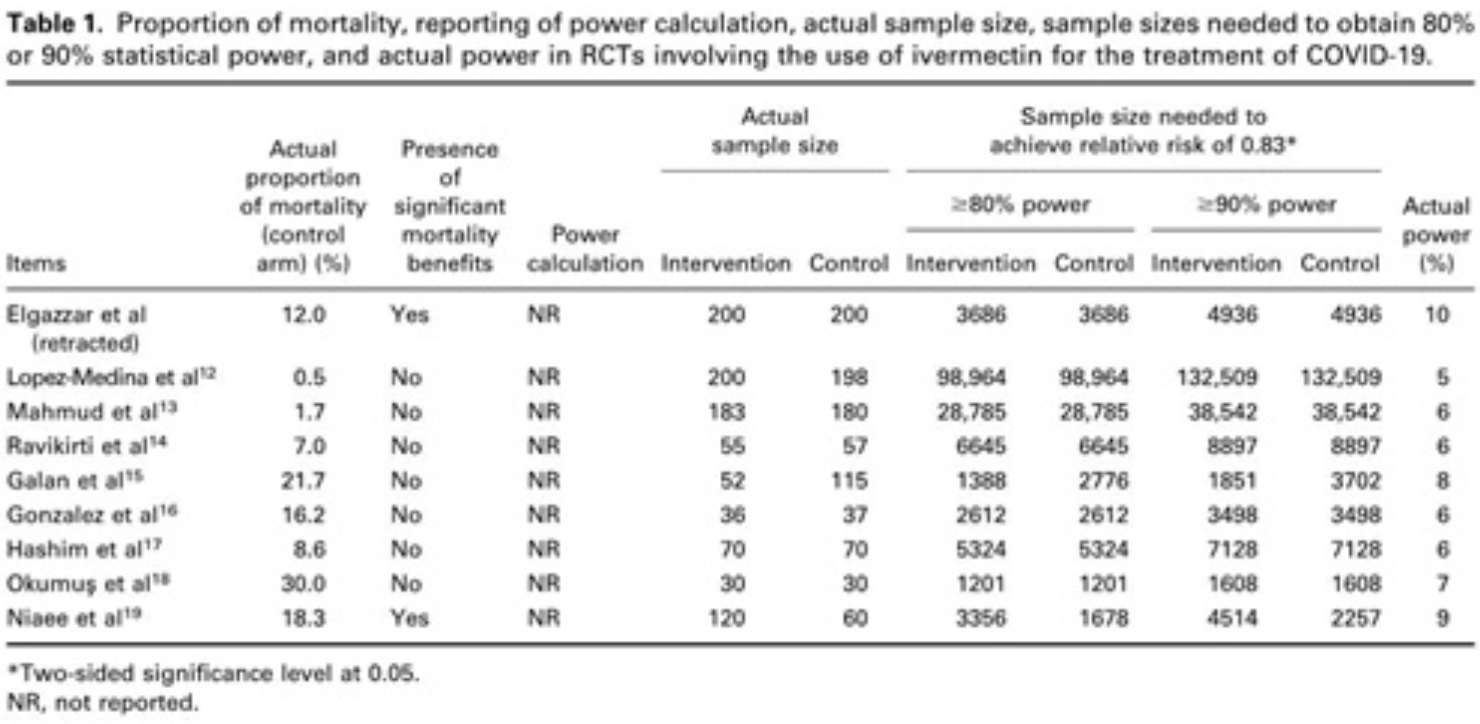
Pitfalls in Reporting Sample Size Calculation Across Randomized Controlled Trials Involving Ivermectin for the treatment of COVID-19
et al., American Journal of Therapeutics, doi:10.1097/MJT.0000000000001441, Aug 2021
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of sample size calculations in ivermectin RCTs, showing that existing RCTs are very underpowered.
1.
Reich, S., Methodological Analysis of Bias Risks in Adaptive Multi-Arm Platform Trials: A Case-Series from Three COVID-19 Studies, Center for Open Science, doi:10.31222/osf.io/h5kc8_v1.
2.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
3.
Zhang et al., Rho-GTPases subfamily: cellular defectors orchestrating viral infection, Cellular & Molecular Biology Letters, doi:10.1186/s11658-025-00722-w.
4.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
5.
Ulloa-Aguilar et al., The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection, Cells, doi:10.3390/cells13181591.
6.
Enyeji et al., Effective Treatment of COVID-19 Infection with Repurposed Drugs: Case Reports, Viral Immunology, doi:10.1089/vim.2024.0034.
7.
Wimalawansa, S., Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity, Heliyon, doi:10.1016/j.heliyon.2024.e34691.
8.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
9.
Mehraeen et al., Treatments for Olfactory Dysfunction in COVID-19: A Systematic Review, International Archives of Otorhinolaryngology, doi:10.1055/s-0044-1786046.
10.
Scheim et al., Back to the Basics of SARS-CoV-2 Biochemistry: Microvascular Occlusive Glycan Bindings Govern Its Morbidities and Inform Therapeutic Responses, Viruses, doi:10.3390/v16040647.
11.
Yagisawa et al., Global trends in clinical trials of ivermectin for COVID-19—Part 2, The Japanese Journal of Antibiotics, doi:10.11553/antibiotics.77.1_45.
12.
Liu et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
13.
Scheim (B) et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
14.
Yemeke et al., Impact of the COVID-19 pandemic on the quality of medical products in Zimbabwe: a qualitative study based on key informant interviews with health system stakeholders, BMJ Open, doi:10.1136/bmjopen-2022-068923.
15.
Kory, P., The Global War on Ivermectin, International Covid Summit III, European Parliament, Brussels, covid19criticalcare.com/wp-content/uploads/2023/05/GLOBAL-WAR-ON-IVERMECTIN-PARLIAMENT.pdf.
16.
Babalola et al., The Place of Ivermectin in the Management of Covid-19: State of the Evidence, Medical Research Archives, doi:10.18103/mra.v11i4.3778.
17.
Loo et al., Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-023-03520-1.
18.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
19.
Kory (B), P., The Criminal Censorship of Ivermectin's Efficacy By The High-Impact Medical Journals - Part 1, Pierre Kory’s Medical Musings, pierrekory.substack.com/p/the-criminal-censorship-of-ivermectins.
20.
Al-kuraishy et al., Central effects of Ivermectin in alleviation of Covid-19-induced dysautonomia, Current Drug Targets, doi:10.2174/1389450123666220810102406.
21.
Schwartz, E., Does ivermectin have a place in the treatment of mild Covid-19?, New Microbes and New Infections, doi:10.1016/j.nmni.2022.100989.
22.
Marques et al., Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols, Brazilian Journal of Biology, doi:10.1590/1519-6984.258325.
23.
Semiz, S., SIT1 transporter as a potential novel target in treatment of COVID-19, Biomolecular Concepts, doi:10.1515/bmc-2021-0017.
24.
Zaidi et al., The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review, The Journal of Antibiotics, doi:10.1038/s41429-021-00491-6.
25.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
26.
Low et al., Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166294.
27.
Fordham et al., The uses and abuses of systematic reviews, OSF Preprints, doi:10.31219/osf.io/mp4f2.
28.
Kow et al., Pitfalls in Reporting Sample Size Calculation Across Randomized Controlled Trials Involving Ivermectin for the treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001441.
29.
Santin et al., Ivermectin: a multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes and New Infections, doi:10.1016/j.nmni.2021.100924.
30.
Adegboro et al., A review of the anti-viral effects of ivermectin, African Journal of Clinical and Experimental Microbiology, doi:10.4314/ajcem.v22i3.2.
31.
Turkia, M., A Continuation of a Timeline of Ivermectin-Related Events in the COVID-19 Pandemic [June 30, 2021], ResearchGate, doi:10.13140/RG.2.2.16973.36326.
32.
Jagiasi et al., Variation in therapeutic strategies for the management of severe COVID-19 in India- A nationwide cross-sectional survey, The International Journal of Clinical Practice, doi:10.1111/ijcp.14574.
33.
Lind et al., Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis, Journal of General Internal Medicine, doi:10.1007/s11606-021-06948-6.
34.
Wang et al., Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19, medRxiv, doi:10.1101/2021.06.01.21258147.
35.
Kory (C) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001377.
36.
DiNicolantonio et al., Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors, Open Heart, doi:10.1136/openhrt-2021-001655.
37.
Turkia (B), M., A timeline of ivermectin-related events in the COVID-19 pandemic, Research Gate, www.researchgate.net/publication/350610718_A_Timeline_of_Ivermectin-Related_Events_in_the_COVID-19_Pandemic_April_3_2021.
38.
Wehbe et al., Repurposing Ivermectin for COVID-19: Molecular Aspects and Therapeutic Possibilities, Front. Immunol., doi:10.3389/fimmu.2021.663586.
39.
Yagisawa (B) et al., Global trends in clinical studies of ivermectin in COVID-19, The Japanese Journal of Antibiotics, 74-1, Mar 2021, jja-contents.wdc-jp.com/pdf/JJA74/74-1-open/74-1_44-95.pdf.
40.
Jans et al., The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.10.042.
41.
Kory (D) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Frontiers in Pharmacology, doi:10.3389/fphar.2021.643369.
42.
Formiga et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J. Control Release, doi:10.1016/j.jconrel.2020.10.009.
43.
Scheim (D), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
44.
Turkia (C), M., FLCCC Alliance MATH+ ascorbic acid and I-MASK+ ivermectin protocols for COVID-19 — a brief review, ResearchGate, www.researchgate.net/profile/Mika_Turkia/publication/345694745_FLCCC_Alliance_MATH_ascorbic_acid_and_I-MASK_ivermectin_protocols_for_COVID-19_-_A_Brief_Review/links/5fab010f4585150781078260/FLCCC-Alliance-MATH-ascorbic-acid-and-I-MASK-ivermectin-protocols-for-COVID-19-A-Brief-Review.pdf.
45.
Jans (B) et al., Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?, Cells 2020, 9:9, 2100, doi:10.3390/cells9092100.
46.
Elkholy et al., Ivermectin: A Closer Look at a Potential Remedy, Cureus, doi:10.7759/cureus.10378.
47.
DiNicolantonio (B) et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
48.
Vora et al., White paper on Ivermectin as a potential therapy for COVID-19, Indian Journal of Tuberculosis, doi:10.1016/j.ijtb.2020.07.031.
Kow et al., 6 Aug 2021, peer-reviewed, 2 authors.
DOI record:
{
"DOI": "10.1097/mjt.0000000000001441",
"ISSN": [
"1075-2765"
],
"URL": "http://dx.doi.org/10.1097/MJT.0000000000001441",
"author": [
{
"affiliation": [
{
"name": "School of Pharmacy, Monash University Malaysia Bandar Sunway, Selangor, Malaysia"
},
{
"name": "School of Postgraduate Studies, International Medical University, Kuala Lumpur, Malaysia"
}
],
"family": "Kow",
"given": "Chia Siang",
"sequence": "first"
},
{
"affiliation": [
{
"name": "School of Applied Sciences, University of Huddersfield, Huddersfield, United Kingdom"
},
{
"name": "School of Biomedical Sciences and Pharmacy University of Newcastle, Callaghan, Australia"
}
],
"family": "Hasan",
"given": "Syed S.",
"sequence": "additional"
}
],
"container-title": "American Journal of Therapeutics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
8,
13
]
],
"date-time": "2021-08-13T13:55:18Z",
"timestamp": 1628862918000
},
"deposited": {
"date-parts": [
[
2023,
9,
17
]
],
"date-time": "2023-09-17T04:19:01Z",
"timestamp": 1694924341000
},
"indexed": {
"date-parts": [
[
2023,
9,
18
]
],
"date-time": "2023-09-18T04:35:25Z",
"timestamp": 1695011725354
},
"is-referenced-by-count": 4,
"issue": "5",
"issued": {
"date-parts": [
[
2021,
8,
6
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2021
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.1097/MJT.0000000000001441",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "e616-e619",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2021,
8,
6
]
]
},
"published-online": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"article-title": "An interactive web-based dashboard to track COVID-19 in real time",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "533",
"journal-title": "Lancet Infect Dis.",
"key": "R1-20230917",
"volume": "20",
"year": "2020"
},
{
"article-title": "The use of remdesivir for the management of patients with moderate-to-severe COVID-19: a systematic review",
"author": "Thiruchelvam",
"journal-title": "Expert Rev Anti Infect Ther.",
"key": "R2-20230917",
"year": "2021"
},
{
"DOI": "10.1080/17476348.2021.1925546",
"article-title": "Does methylprednisolone reduce the mortality risk in hospitalized COVID-19 patients? A meta-analysis of randomized control trials",
"author": "Hasan",
"doi-asserted-by": "crossref",
"journal-title": "Expert Rev Respir Med.",
"key": "R3-20230917",
"year": "2021"
},
{
"DOI": "10.1007/s43440-021-00245-z",
"article-title": "The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis",
"author": "Kow",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol Rep.",
"key": "R4-20230917",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antivir Res",
"key": "R5-20230917",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1371/journal.pbio.3001162",
"article-title": "The methodological quality of 176,620 RCTs published between 1966 and 2018 reveals a positive trend but also an urgent need for improvement",
"author": "Vinkers",
"doi-asserted-by": "crossref",
"first-page": "e3001162",
"journal-title": "PLoS Biol.",
"key": "R6-20230917",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.09.1470",
"article-title": "Use of hydroxychloroquine and chloroquine in COVID-19: how good is the quality of RCTs?",
"author": "Mazhar",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Int J Infect Dis.",
"key": "R7-20230917",
"volume": "101",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.03.010",
"article-title": "Sample size estimation in clinical research: from RCTs to observational studies",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "S12",
"journal-title": "Chest",
"key": "R8-20230917",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"article-title": "Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines",
"author": "Bryant",
"doi-asserted-by": "crossref",
"first-page": "e434",
"journal-title": "Am J Ther.",
"key": "R9-20230917",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with covid-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med.",
"key": "R10-20230917",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1186/s12874-020-01101-z",
"article-title": "Statistical design of Phase II/III clinical trials for testing therapeutic interventions in COVID-19 patients",
"author": "Rai",
"doi-asserted-by": "crossref",
"first-page": "220",
"journal-title": "BMC Med Res Methodol",
"key": "R11-20230917",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial",
"author": "López-Medina",
"doi-asserted-by": "crossref",
"first-page": "1426",
"journal-title": "JAMA",
"key": "R12-20230917",
"volume": "325",
"year": "2021"
},
{
"article-title": "Ivermectin as a potential treatment for mild to moderate COVID-19—a double blind randomized placebo-controlled trial",
"author": "Ravikirti",
"first-page": "10",
"journal-title": "medRxiv",
"key": "R14-20230917",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1080/20477724.2021.1890887",
"article-title": "Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection",
"author": "Galan",
"doi-asserted-by": "crossref",
"first-page": "235",
"journal-title": "Pathog Glob Health",
"key": "R15-20230917",
"volume": "115",
"year": "2021"
},
{
"article-title": "Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial",
"author": "Gonzalez",
"journal-title": "Preprint Medrxiv.",
"key": "R16-20230917",
"year": "2021"
},
{
"article-title": "Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq",
"author": "Hashim",
"journal-title": "Preprint Medrxiv.",
"key": "R17-20230917",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06104-9",
"article-title": "Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients",
"author": "Okumus",
"doi-asserted-by": "crossref",
"first-page": "411",
"journal-title": "BMC Infect Dis",
"key": "R18-20230917",
"volume": "21",
"year": "2021"
},
{
"article-title": "Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial",
"author": "Niaee",
"journal-title": "Preprint Res Square",
"key": "R19-20230917",
"year": "2020"
},
{
"article-title": "Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Horby",
"journal-title": "Preprint Medrxiv.",
"key": "R20-20230917",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00149-5",
"article-title": "Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "605",
"journal-title": "Lancet",
"key": "R21-20230917",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2022926",
"article-title": "Effect of hydroxychloroquine in hospitalized patients with covid-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "2030",
"journal-title": "N Engl J Med.",
"key": "R22-20230917",
"volume": "383",
"year": "2020"
},
{
"article-title": "Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Horby",
"first-page": "55",
"journal-title": "Preprint Medrxiv.",
"key": "R23-20230917",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "2049",
"journal-title": "Lancet",
"key": "R24-20230917",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)32013-4",
"article-title": "Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "1345",
"journal-title": "Lancet",
"key": "R25-20230917",
"volume": "396",
"year": "2020"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/MJT.0000000000001441"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Pitfalls in Reporting Sample Size Calculation Across Randomized Controlled Trials Involving Ivermectin for the treatment of COVID-19",
"type": "journal-article",
"volume": "28"
}
