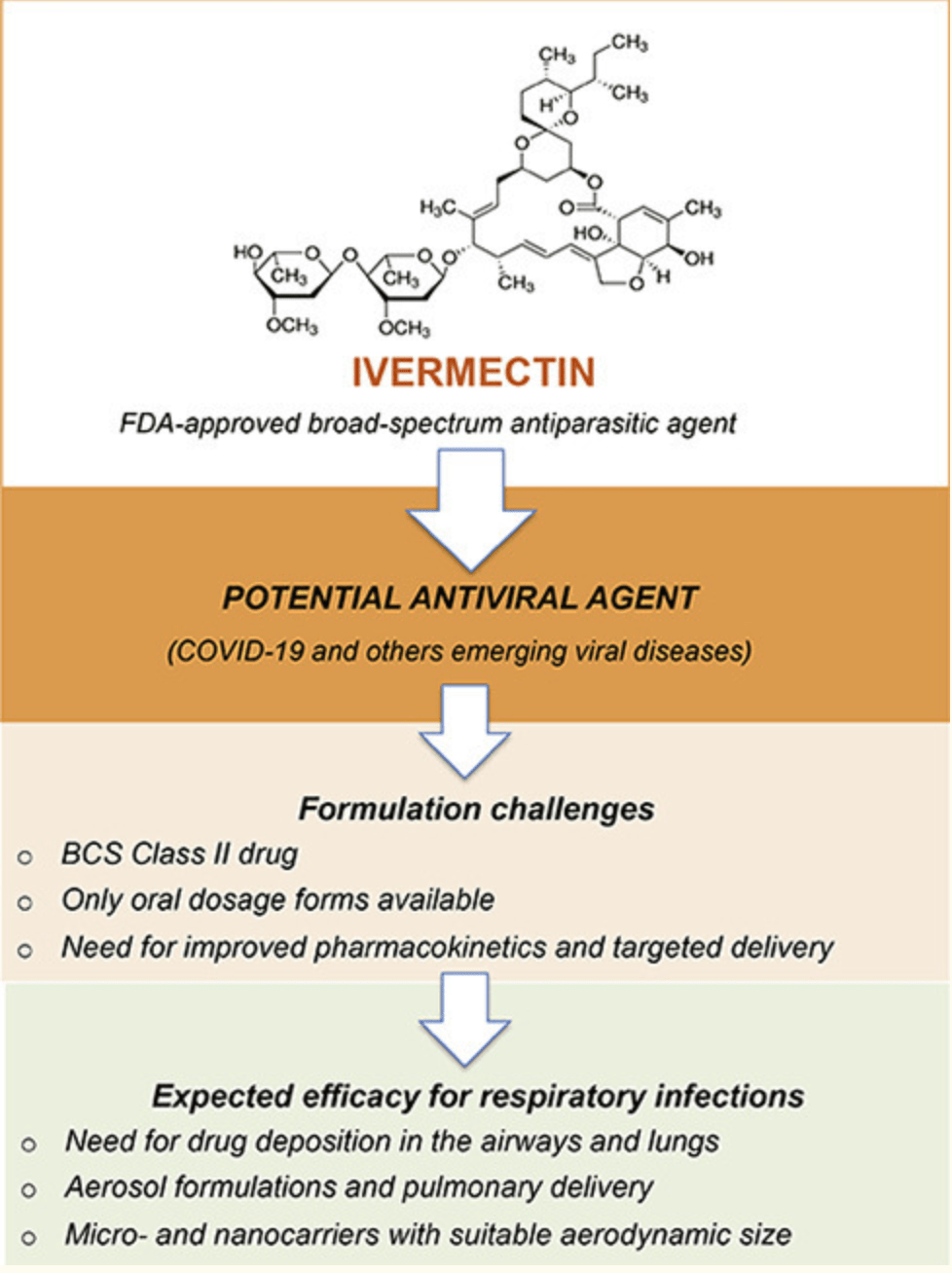
Ivermectin: an award-winning drug with expected antiviral activity against COVID-19
et al., J. Control Release, doi:10.1016/j.jconrel.2020.10.009, Jan 2021
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review hypothesizing that micro- and nanotechnology-based formulations of ivermectin for the pulmonary delivery of ivermectin may be beneficial for use with COVID-19.
1.
Reich, S., Methodological Analysis of Bias Risks in Adaptive Multi-Arm Platform Trials: A Case-Series from Three COVID-19 Studies, Center for Open Science, doi:10.31222/osf.io/h5kc8_v1.
2.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
3.
Zhang et al., Rho-GTPases subfamily: cellular defectors orchestrating viral infection, Cellular & Molecular Biology Letters, doi:10.1186/s11658-025-00722-w.
4.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
5.
Ulloa-Aguilar et al., The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection, Cells, doi:10.3390/cells13181591.
6.
Enyeji et al., Effective Treatment of COVID-19 Infection with Repurposed Drugs: Case Reports, Viral Immunology, doi:10.1089/vim.2024.0034.
7.
Wimalawansa, S., Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity, Heliyon, doi:10.1016/j.heliyon.2024.e34691.
8.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
9.
Mehraeen et al., Treatments for Olfactory Dysfunction in COVID-19: A Systematic Review, International Archives of Otorhinolaryngology, doi:10.1055/s-0044-1786046.
10.
Scheim et al., Back to the Basics of SARS-CoV-2 Biochemistry: Microvascular Occlusive Glycan Bindings Govern Its Morbidities and Inform Therapeutic Responses, Viruses, doi:10.3390/v16040647.
11.
Yagisawa et al., Global trends in clinical trials of ivermectin for COVID-19—Part 2, The Japanese Journal of Antibiotics, doi:10.11553/antibiotics.77.1_45.
12.
Liu et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
13.
Scheim (B) et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
14.
Yemeke et al., Impact of the COVID-19 pandemic on the quality of medical products in Zimbabwe: a qualitative study based on key informant interviews with health system stakeholders, BMJ Open, doi:10.1136/bmjopen-2022-068923.
15.
Kory, P., The Global War on Ivermectin, International Covid Summit III, European Parliament, Brussels, covid19criticalcare.com/wp-content/uploads/2023/05/GLOBAL-WAR-ON-IVERMECTIN-PARLIAMENT.pdf.
16.
Babalola et al., The Place of Ivermectin in the Management of Covid-19: State of the Evidence, Medical Research Archives, doi:10.18103/mra.v11i4.3778.
17.
Loo et al., Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-023-03520-1.
18.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
19.
Kory (B), P., The Criminal Censorship of Ivermectin's Efficacy By The High-Impact Medical Journals - Part 1, Pierre Kory’s Medical Musings, pierrekory.substack.com/p/the-criminal-censorship-of-ivermectins.
20.
Al-kuraishy et al., Central effects of Ivermectin in alleviation of Covid-19-induced dysautonomia, Current Drug Targets, doi:10.2174/1389450123666220810102406.
21.
Schwartz, E., Does ivermectin have a place in the treatment of mild Covid-19?, New Microbes and New Infections, doi:10.1016/j.nmni.2022.100989.
22.
Marques et al., Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols, Brazilian Journal of Biology, doi:10.1590/1519-6984.258325.
23.
Semiz, S., SIT1 transporter as a potential novel target in treatment of COVID-19, Biomolecular Concepts, doi:10.1515/bmc-2021-0017.
24.
Zaidi et al., The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review, The Journal of Antibiotics, doi:10.1038/s41429-021-00491-6.
25.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
26.
Low et al., Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166294.
27.
Fordham et al., The uses and abuses of systematic reviews, OSF Preprints, doi:10.31219/osf.io/mp4f2.
28.
Kow et al., Pitfalls in Reporting Sample Size Calculation Across Randomized Controlled Trials Involving Ivermectin for the treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001441.
29.
Santin et al., Ivermectin: a multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes and New Infections, doi:10.1016/j.nmni.2021.100924.
30.
Adegboro et al., A review of the anti-viral effects of ivermectin, African Journal of Clinical and Experimental Microbiology, doi:10.4314/ajcem.v22i3.2.
31.
Turkia, M., A Continuation of a Timeline of Ivermectin-Related Events in the COVID-19 Pandemic [June 30, 2021], ResearchGate, doi:10.13140/RG.2.2.16973.36326.
32.
Jagiasi et al., Variation in therapeutic strategies for the management of severe COVID-19 in India- A nationwide cross-sectional survey, The International Journal of Clinical Practice, doi:10.1111/ijcp.14574.
33.
Lind et al., Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis, Journal of General Internal Medicine, doi:10.1007/s11606-021-06948-6.
34.
Wang et al., Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19, medRxiv, doi:10.1101/2021.06.01.21258147.
35.
Kory (C) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001377.
36.
DiNicolantonio et al., Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors, Open Heart, doi:10.1136/openhrt-2021-001655.
37.
Turkia (B), M., A timeline of ivermectin-related events in the COVID-19 pandemic, Research Gate, www.researchgate.net/publication/350610718_A_Timeline_of_Ivermectin-Related_Events_in_the_COVID-19_Pandemic_April_3_2021.
38.
Wehbe et al., Repurposing Ivermectin for COVID-19: Molecular Aspects and Therapeutic Possibilities, Front. Immunol., doi:10.3389/fimmu.2021.663586.
39.
Yagisawa (B) et al., Global trends in clinical studies of ivermectin in COVID-19, The Japanese Journal of Antibiotics, 74-1, Mar 2021, jja-contents.wdc-jp.com/pdf/JJA74/74-1-open/74-1_44-95.pdf.
40.
Jans et al., The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.10.042.
41.
Kory (D) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Frontiers in Pharmacology, doi:10.3389/fphar.2021.643369.
42.
Formiga et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J. Control Release, doi:10.1016/j.jconrel.2020.10.009.
43.
Scheim (D), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
44.
Turkia (C), M., FLCCC Alliance MATH+ ascorbic acid and I-MASK+ ivermectin protocols for COVID-19 — a brief review, ResearchGate, www.researchgate.net/profile/Mika_Turkia/publication/345694745_FLCCC_Alliance_MATH_ascorbic_acid_and_I-MASK_ivermectin_protocols_for_COVID-19_-_A_Brief_Review/links/5fab010f4585150781078260/FLCCC-Alliance-MATH-ascorbic-acid-and-I-MASK-ivermectin-protocols-for-COVID-19-A-Brief-Review.pdf.
45.
Jans (B) et al., Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?, Cells 2020, 9:9, 2100, doi:10.3390/cells9092100.
46.
Elkholy et al., Ivermectin: A Closer Look at a Potential Remedy, Cureus, doi:10.7759/cureus.10378.
47.
DiNicolantonio (B) et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
48.
Vora et al., White paper on Ivermectin as a potential therapy for COVID-19, Indian Journal of Tuberculosis, doi:10.1016/j.ijtb.2020.07.031.
Formiga et al., 10 Jan 2021, peer-reviewed, 6 authors.
Ivermectin: an award-winning drug with expected antiviral activity against COVID-19
Journal of Controlled Release, doi:10.1016/j.jconrel.2020.10.009
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre -including this research content -immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
Declaration of Competing Interest The authors deny the existence of any conflicts of interest.
References
Ali, Afzal, Verma, Bhattacharya, Ahmad et al., Therapeutic efficacy of poly (lactic-co-glycolic acid) nanoparticles encapsulated ivermectin (nano-ivermectin) against brugian filariasis in experimental rodent model, Parasitol. Res, doi:10.1007/s00436-013-3696-5
Ali, Afzal, Verma, Misra-Bhattacharya, Ahmad et al., Improved antifilarial activity of ivermectin in chitosan-alginate nanoparticles against human lymphatic filarial parasite, Brugia malayi, Parasitol. Res, doi:10.1007/s00436-013-3466-4
Barrows, Campos, Powell, Prasanth, Schott-Lerner et al., A screen of FDA-approved drugs for inhibitors of Zika virus infection, Cell Host Microbe, doi:10.1016/j.chom.2016.07.004
Callaway, Cyranoski, Anti-parasite drugs sweep Nobel prize in medicine 2015, Nature, doi:10.1038/nature.2015.18507
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antivir. Res, doi:10.1016/j.antiviral.2020.104787
Camargo, Sapin, Daloz, Maincent, Ivermectin-loaded microparticles for parenteral sustained release: in vitro characterization and effect of some formulation variables, J. Microencapsul, doi:10.3109/02652048.2010.501397
Chaccour, Ruiz-Castillo, Richardson, Moncunill, Casellas et al., The SARS-CoV-2 Ivermectin Navarra-ISGlobal trial (SAINT) to evaluate the potential of ivermectin to reduce COVID-19 transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol for a randomized control pilot trial, Trials, doi:10.1186/s13063-020-04421-z
Chan, Nano research for COVID-19, ACS Nano, doi:10.1021/acsnano.0c02540
Cojocaru, Botezat, Gardikiotis, Uritu, Dodi et al., Nanomaterials designed for antiviral drug delivery transport across biological barriers, Pharmaceutics, doi:10.3390/pharmaceutics12020171
Croci, Bottaro, Chan, Watanabe, Pezzullo et al., Liposomal systems as nanocarriers for the antiviral agent Ivermectin, Int. J. Biomater, doi:10.1155/2016/8043983
Ebbelaar, Venema, Van Dijk, Topical ivermectin in the treatment of Papulopustular rosacea: a systematic review of evidence and clinical guideline recommendations, Dermatol. Ther. (Heidelb), doi:10.1007/s13555-018-0249-y
Ghadiri, Young, Traini, Strategies to enhance drug absorption via nasal and pulmonary routes, Pharmaceutics, doi:10.3390/pharmaceutics11030113
Ho, Nichols, Edgar, Murgia, Loretz et al., Challenges and strategies in drug delivery systems for treatment of pulmonary infections, Eur. J. Pharm. Biopharm, doi:10.1016/j.ejpb.2019.09.002
Ketkar, Yang, Wormser, Wang, Lack of efficacy of ivermectin for prevention of a lethal Zika virus infection in a murine system, Diagn. Microbiol. Infect. Dis, doi:10.1016/j.diagmicrobio.2019.03.012
Liu, Guan, Qin, Zhang, Mao, Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery, Drug Discov. Today, doi:10.1016/j.drudis.2019.09.023
Lv, Liu, Wang, Dang, Qiu et al., Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo, Antivir. Res, doi:10.1016/j.antiviral.2018.09.010
Newman, Drug delivery to the lungs: challenges and opportunities, Ther. Deliv, doi:10.4155/tde-2017-0037
Ono, Tatsuo, Hidaka, Aoki, Minagawa et al., Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor, J. Virol, doi:10.1128/JVI.75.9.4399-4401.2001
Onoue, Yamada, Chan, Nanodrugs: pharmacokinetics and safety, Int. J. Nanomedicine, doi:10.2147/IJN.S38378
Paranjpe, Müller-Goymann, Nanoparticle-mediated pulmonary drug delivery: a review, Int. J. Mol. Sci, doi:10.3390/ijms15045852
Pedersen, Ho, SARS-CoV-2: a storm is raging, J. Clin. Invest, doi:10.1172/JCI137647
Rizzo, Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action, Naunyn Schmiedeberg's, Arch. Pharmacol, doi:10.1007/s00210-020-01902-5
Sandler, Firpo, Omoba, Vu, Menachery et al., Novel ionophores active against La Crosse virus identified through rapid antiviral screening, Antimicrob. Agents Chemother, doi:10.1128/AAC.00086-20
Schmith, Zhou, Lohmer, The approved dose of ivermectin alone is not the Ideal dose for the treatment of COVID-19, Clin. Pharmacol. Ther, doi:10.1002/cpt.1889
Surnar, Kamran, Shah, Basu, Kolishetti et al., Orally administrable therapeutic synthetic nanoparticle for Zika virus, ACS Nano, doi:10.1021/acsnano.9b02807
Takano, Sugano, Higashida, Hayashi, Machida et al., Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test, Pharm. Res, doi:10.1007/s11095-006-0162-4
Ullio-Gamboa, Palma, Benoit, Allemandi, Picollo et al., Ivermectin lipid-based nanocarriers as novel formulations against head lice, Parasitol. Res, doi:10.1007/s00436-017-5510-2
Varghese, Kaukinen, Glasker, Bespalov, Hanski et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antivir. Res, doi:10.1016/j.antiviral.2015.12.012
Wang, Lv, Ji, Wang, Qiu et al., Ivermectin treatment inhibits the replication of porcine circovirus 2 (PCV2) in vitro and mitigates the impact of viral infection in piglets, Virus Res, doi:10.1016/j.virusres.2019.01.010
Wölfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Xu, Han, Liu, Pang, Zheng et al., Antivirus effectiveness of ivermectin on dengue virus type 2 in Aedes albopictus, PLoS Negl. Trop. Dis, doi:10.1371/journal.pntd.0006934
Yan, Ci, Chen, Chen, Li et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflamm. Res, doi:10.1007/s00011-011-0307-8
Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin alpha/beta1 heterodimer, Antivir. Res, doi:10.1016/j.antiviral.2020.104760
Zhang, Song, Ci, An, Ju et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm. Res, doi:10.1007/s00011-008-8007-8
Zhang, Zhou, Wei, Yue, Wang et al., Histopathologic changes and SARS-CoV-2 imunostaining in the lung of a patient with COVID-19, Ann. Intern. Med, doi:10.7326/M20-0533
Zheng, Fan, Yu, Feng, Lou et al., Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang Province, China, BMJ, doi:10.1136/bmj.m1443
Zhou, Leung, Tang, Parumasivam, Loh et al., Inhaled formulations and pulmonary drug delivery systems for respiratory infections, Adv. Drug Deliv. Rev, doi:10.1016/j.addr.2014.10.022
Zhou, Yang, Wang, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N. Engl. J. Med, doi:10.1056/NEJMc2001737
DOI record:
{
"DOI": "10.1016/j.jconrel.2020.10.009",
"ISSN": [
"0168-3659"
],
"URL": "http://dx.doi.org/10.1016/j.jconrel.2020.10.009",
"alternative-id": [
"S0168365920305800"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Ivermectin: an award-winning drug with expected antiviral activity against COVID-19"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Controlled Release"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jconrel.2020.10.009"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier B.V. All rights reserved."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1553-0533",
"affiliation": [],
"authenticated-orcid": false,
"family": "Formiga",
"given": "Fabio Rocha",
"sequence": "first"
},
{
"affiliation": [],
"family": "Leblanc",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Souza Rebouças",
"given": "Juliana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farias",
"given": "Leonardo Paiva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Oliveira",
"given": "Ronaldo Nascimento",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pena",
"given": "Lindomar",
"sequence": "additional"
}
],
"container-title": "Journal of Controlled Release",
"container-title-short": "Journal of Controlled Release",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
10,
7
]
],
"date-time": "2020-10-07T15:10:29Z",
"timestamp": 1602083429000
},
"deposited": {
"date-parts": [
[
2022,
6,
26
]
],
"date-time": "2022-06-26T11:46:04Z",
"timestamp": 1656243964000
},
"funder": [
{
"DOI": "10.13039/501100003593",
"doi-asserted-by": "publisher",
"name": "Conselho Nacional de Desenvolvimento Científico e Tecnológico"
},
{
"DOI": "10.13039/501100000193",
"doi-asserted-by": "publisher",
"name": "International Development Research Centre"
},
{
"DOI": "10.13039/501100002322",
"doi-asserted-by": "publisher",
"name": "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior"
},
{
"DOI": "10.13039/501100006162",
"doi-asserted-by": "publisher",
"name": "Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco"
},
{
"DOI": "10.13039/501100006507",
"doi-asserted-by": "publisher",
"name": "Fundação Oswaldo Cruz"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
14
]
],
"date-time": "2024-05-14T02:35:27Z",
"timestamp": 1715654127631
},
"is-referenced-by-count": 50,
"issued": {
"date-parts": [
[
2021,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0168365920305800?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0168365920305800?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "758-761",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/nature.2015.18507",
"article-title": "Anti-parasite drugs sweep Nobel prize in medicine 2015",
"author": "Callaway",
"doi-asserted-by": "crossref",
"first-page": "174",
"journal-title": "Nature.",
"key": "10.1016/j.jconrel.2020.10.009_bb0005",
"volume": "526",
"year": "2015"
},
{
"DOI": "10.1016/j.antiviral.2018.09.010",
"article-title": "Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo",
"author": "Lv",
"doi-asserted-by": "crossref",
"first-page": "55",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0010",
"volume": "159",
"year": "2018"
},
{
"DOI": "10.1016/j.virusres.2019.01.010",
"article-title": "Ivermectin treatment inhibits the replication of porcine circovirus 2 (PCV2) in vitro and mitigates the impact of viral infection in piglets",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "80",
"journal-title": "Virus Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0015",
"volume": "263",
"year": "2019"
},
{
"DOI": "10.1371/journal.pntd.0006934",
"article-title": "Antivirus effectiveness of ivermectin on dengue virus type 2 in Aedes albopictus",
"author": "Xu",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Negl. Trop. Dis.",
"key": "10.1016/j.jconrel.2020.10.009_bb0020",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.1016/j.antiviral.2015.12.012",
"article-title": "Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses",
"author": "Varghese",
"doi-asserted-by": "crossref",
"first-page": "117",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0025",
"volume": "126",
"year": "2016"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"article-title": "The broad spectrum antiviral ivermectin targets the host nuclear transport importin alpha/beta1 heterodimer",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "104760",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0030",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2016.07.004",
"article-title": "A screen of FDA-approved drugs for inhibitors of Zika virus infection",
"author": "Barrows",
"doi-asserted-by": "crossref",
"first-page": "259",
"journal-title": "Cell Host Microbe",
"key": "10.1016/j.jconrel.2020.10.009_bb0035",
"volume": "20",
"year": "2016"
},
{
"DOI": "10.1016/j.diagmicrobio.2019.03.012",
"article-title": "Lack of efficacy of ivermectin for prevention of a lethal Zika virus infection in a murine system",
"author": "Ketkar",
"doi-asserted-by": "crossref",
"first-page": "38",
"journal-title": "Diagn. Microbiol. Infect. Dis.",
"key": "10.1016/j.jconrel.2020.10.009_bb0040",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0045",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1128/JVI.75.9.4399-4401.2001",
"article-title": "Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor",
"author": "Ono",
"doi-asserted-by": "crossref",
"first-page": "4399",
"journal-title": "J. Virol.",
"key": "10.1016/j.jconrel.2020.10.009_bb0050",
"volume": "75",
"year": "2001"
},
{
"article-title": "Histopathologic changes and SARS-CoV-2 imunostaining in the lung of a patient with COVID-19",
"author": "Zhang",
"first-page": "M20",
"journal-title": "Ann. Intern. Med.",
"key": "10.1016/j.jconrel.2020.10.009_bb0055",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1889",
"article-title": "The approved dose of ivermectin alone is not the Ideal dose for the treatment of COVID-19",
"author": "Schmith",
"doi-asserted-by": "crossref",
"first-page": "762",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "10.1016/j.jconrel.2020.10.009_bb0060",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1007/s00210-020-01902-5",
"article-title": "Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action",
"author": "Rizzo",
"doi-asserted-by": "crossref",
"first-page": "1153",
"journal-title": "Naunyn Schmiedeberg’s Arch. Pharmacol.",
"key": "10.1016/j.jconrel.2020.10.009_bb0065",
"volume": "393",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00086-20",
"article-title": "Novel ionophores active against La Crosse virus identified through rapid antiviral screening",
"author": "Sandler",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "10.1016/j.jconrel.2020.10.009_bb0070",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1172/JCI137647",
"article-title": "SARS-CoV-2: a storm is raging",
"author": "Pedersen",
"doi-asserted-by": "crossref",
"first-page": "2202",
"journal-title": "J. Clin. Invest.",
"key": "10.1016/j.jconrel.2020.10.009_bb0075",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"article-title": "A pneumonia outbreak associated with a new coronavirus of probable bat origin",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Nature.",
"key": "10.1016/j.jconrel.2020.10.009_bb0080",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"article-title": "Virological assessment of hospitalized patients with COVID-2019",
"author": "Wölfel",
"doi-asserted-by": "crossref",
"first-page": "465",
"journal-title": "Nature.",
"key": "10.1016/j.jconrel.2020.10.009_bb0085",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2001737",
"article-title": "SARS-CoV-2 viral load in upper respiratory specimens of infected patients",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "1177",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jconrel.2020.10.009_bb0090",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1443",
"article-title": "Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang Province, China, January-march 2020: retrospective cohort study",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "m1443",
"journal-title": "BMJ.",
"key": "10.1016/j.jconrel.2020.10.009_bb0095",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1007/s13555-018-0249-y",
"article-title": "Topical ivermectin in the treatment of Papulopustular rosacea: a systematic review of evidence and clinical guideline recommendations",
"author": "Ebbelaar",
"doi-asserted-by": "crossref",
"first-page": "379",
"journal-title": "Dermatol. Ther. (Heidelb).",
"key": "10.1016/j.jconrel.2020.10.009_bb0100",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1007/s00011-011-0307-8",
"article-title": "Anti-inflammatory effects of ivermectin in mouse model of allergic asthma",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "589",
"journal-title": "Inflamm. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0105",
"volume": "60",
"year": "2011"
},
{
"DOI": "10.1007/s00011-008-8007-8",
"article-title": "Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "524",
"journal-title": "Inflamm. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0110",
"volume": "57",
"year": "2008"
},
{
"DOI": "10.1186/s13063-020-04421-z",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "498",
"journal-title": "Trials.",
"key": "10.1016/j.jconrel.2020.10.009_bb0115",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.ejpb.2019.09.002",
"article-title": "Challenges and strategies in drug delivery systems for treatment of pulmonary infections",
"author": "Ho",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "Eur. J. Pharm. Biopharm.",
"key": "10.1016/j.jconrel.2020.10.009_bb0120",
"volume": "144",
"year": "2019"
},
{
"DOI": "10.1016/j.addr.2014.10.022",
"article-title": "Inhaled formulations and pulmonary drug delivery systems for respiratory infections",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Adv. Drug Deliv. Rev.",
"key": "10.1016/j.jconrel.2020.10.009_bb0125",
"volume": "85",
"year": "2015"
},
{
"DOI": "10.3390/pharmaceutics11030113",
"article-title": "Strategies to enhance drug absorption via nasal and pulmonary routes",
"author": "Ghadiri",
"doi-asserted-by": "crossref",
"first-page": "113",
"journal-title": "Pharmaceutics.",
"key": "10.1016/j.jconrel.2020.10.009_bb0130",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1007/s11095-006-0162-4",
"article-title": "Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test",
"author": "Takano",
"doi-asserted-by": "crossref",
"first-page": "1144",
"journal-title": "Pharm. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0140",
"volume": "23",
"year": "2006"
},
{
"DOI": "10.1021/acsnano.9b02807",
"article-title": "Orally administrable therapeutic synthetic nanoparticle for Zika virus",
"author": "Surnar",
"doi-asserted-by": "crossref",
"first-page": "11034",
"journal-title": "ACS Nano",
"key": "10.1016/j.jconrel.2020.10.009_bb0145",
"volume": "13",
"year": "2019"
},
{
"DOI": "10.2147/IJN.S38378",
"article-title": "Nanodrugs: pharmacokinetics and safety",
"author": "Onoue",
"doi-asserted-by": "crossref",
"first-page": "1025",
"journal-title": "Int. J. Nanomedicine",
"key": "10.1016/j.jconrel.2020.10.009_bb0150",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1007/s00436-017-5510-2",
"article-title": "Ivermectin lipid-based nanocarriers as novel formulations against head lice",
"author": "Ullio-Gamboa",
"doi-asserted-by": "crossref",
"first-page": "2111",
"journal-title": "Parasitol. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0155",
"volume": "116",
"year": "2017"
},
{
"DOI": "10.1007/s00436-013-3466-4",
"article-title": "Improved antifilarial activity of ivermectin in chitosan-alginate nanoparticles against human lymphatic filarial parasite, Brugia malayi",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "2933",
"journal-title": "Parasitol. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0160",
"volume": "112",
"year": "2013"
},
{
"DOI": "10.1007/s00436-013-3696-5",
"article-title": "Therapeutic efficacy of poly (lactic-co-glycolic acid) nanoparticles encapsulated ivermectin (nano-ivermectin) against brugian filariasis in experimental rodent model",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "Parasitol. Res.",
"key": "10.1016/j.jconrel.2020.10.009_bb0165",
"volume": "113",
"year": "2014"
},
{
"DOI": "10.3109/02652048.2010.501397",
"article-title": "Ivermectin-loaded microparticles for parenteral sustained release: in vitro characterization and effect of some formulation variables",
"author": "Camargo",
"doi-asserted-by": "crossref",
"first-page": "609",
"journal-title": "J. Microencapsul.",
"key": "10.1016/j.jconrel.2020.10.009_bb0170",
"volume": "27",
"year": "2010"
},
{
"article-title": "Liposomal systems as nanocarriers for the antiviral agent Ivermectin",
"author": "Croci",
"journal-title": "Int. J. Biomater.",
"key": "10.1016/j.jconrel.2020.10.009_bb0175",
"volume": "8043983",
"year": "2016"
},
{
"DOI": "10.1016/j.drudis.2019.09.023",
"article-title": "Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "150",
"journal-title": "Drug Discov. Today",
"key": "10.1016/j.jconrel.2020.10.009_bb0180",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.4155/tde-2017-0037",
"article-title": "Drug delivery to the lungs: challenges and opportunities",
"author": "Newman",
"doi-asserted-by": "crossref",
"first-page": "647",
"journal-title": "Ther. Deliv.",
"key": "10.1016/j.jconrel.2020.10.009_bb0185",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.3390/ijms15045852",
"article-title": "Nanoparticle-mediated pulmonary drug delivery: a review",
"author": "Paranjpe",
"doi-asserted-by": "crossref",
"first-page": "5852",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.jconrel.2020.10.009_bb0190",
"volume": "15",
"year": "2014"
},
{
"DOI": "10.3390/pharmaceutics12020171",
"article-title": "Nanomaterials designed for antiviral drug delivery transport across biological barriers",
"author": "Cojocaru",
"doi-asserted-by": "crossref",
"first-page": "171",
"journal-title": "Pharmaceutics.",
"key": "10.1016/j.jconrel.2020.10.009_bb0195",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1021/acsnano.0c02540",
"article-title": "Nano research for COVID-19",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "3719",
"journal-title": "ACS Nano",
"key": "10.1016/j.jconrel.2020.10.009_bb0200",
"volume": "14",
"year": "2020"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0168365920305800"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ivermectin: an award-winning drug with expected antiviral activity against COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "329"
}
