Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols
et al., Brazilian Journal of Biology, doi:10.1590/1519-6984.258325, May 2022
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of ivermectin as a possible treatment for COVID-19, focusing on 2022 protocols. Authors discuss the long history of safe ivermectin use in humans for parasitic infections, noting its promising in vitro activity against SARS-CoV-2, including a 5,000-fold reduction in viral RNA within 48 hours. This effect may be attributed to the inhibition of viral protein import into the nucleus. One in vivo study found ivermectin was associated with lower mortality, especially in patients requiring oxygen or ventilatory support. In Brazil, some private and public entities have been using 12 mg ivermectin protocols for COVID-19 prophylaxis and early treatment. Authors note that sub-Saharan Africa, where ivermectin is widely used in mass drug administration programs, has the lowest COVID-19 mortality rates.
1.
Reich, S., Methodological Analysis of Bias Risks in Adaptive Multi-Arm Platform Trials: A Case-Series from Three COVID-19 Studies, Center for Open Science, doi:10.31222/osf.io/h5kc8_v1.
2.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
3.
Zhang et al., Rho-GTPases subfamily: cellular defectors orchestrating viral infection, Cellular & Molecular Biology Letters, doi:10.1186/s11658-025-00722-w.
4.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
5.
Ulloa-Aguilar et al., The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection, Cells, doi:10.3390/cells13181591.
6.
Enyeji et al., Effective Treatment of COVID-19 Infection with Repurposed Drugs: Case Reports, Viral Immunology, doi:10.1089/vim.2024.0034.
7.
Wimalawansa, S., Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity, Heliyon, doi:10.1016/j.heliyon.2024.e34691.
8.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
9.
Mehraeen et al., Treatments for Olfactory Dysfunction in COVID-19: A Systematic Review, International Archives of Otorhinolaryngology, doi:10.1055/s-0044-1786046.
10.
Scheim et al., Back to the Basics of SARS-CoV-2 Biochemistry: Microvascular Occlusive Glycan Bindings Govern Its Morbidities and Inform Therapeutic Responses, Viruses, doi:10.3390/v16040647.
11.
Yagisawa et al., Global trends in clinical trials of ivermectin for COVID-19—Part 2, The Japanese Journal of Antibiotics, doi:10.11553/antibiotics.77.1_45.
12.
Liu et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
13.
Scheim (B) et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
14.
Yemeke et al., Impact of the COVID-19 pandemic on the quality of medical products in Zimbabwe: a qualitative study based on key informant interviews with health system stakeholders, BMJ Open, doi:10.1136/bmjopen-2022-068923.
15.
Kory, P., The Global War on Ivermectin, International Covid Summit III, European Parliament, Brussels, covid19criticalcare.com/wp-content/uploads/2023/05/GLOBAL-WAR-ON-IVERMECTIN-PARLIAMENT.pdf.
16.
Babalola et al., The Place of Ivermectin in the Management of Covid-19: State of the Evidence, Medical Research Archives, doi:10.18103/mra.v11i4.3778.
17.
Loo et al., Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-023-03520-1.
18.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
19.
Kory (B), P., The Criminal Censorship of Ivermectin's Efficacy By The High-Impact Medical Journals - Part 1, Pierre Kory’s Medical Musings, pierrekory.substack.com/p/the-criminal-censorship-of-ivermectins.
20.
Al-kuraishy et al., Central effects of Ivermectin in alleviation of Covid-19-induced dysautonomia, Current Drug Targets, doi:10.2174/1389450123666220810102406.
21.
Schwartz, E., Does ivermectin have a place in the treatment of mild Covid-19?, New Microbes and New Infections, doi:10.1016/j.nmni.2022.100989.
22.
Marques et al., Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols, Brazilian Journal of Biology, doi:10.1590/1519-6984.258325.
23.
Semiz, S., SIT1 transporter as a potential novel target in treatment of COVID-19, Biomolecular Concepts, doi:10.1515/bmc-2021-0017.
24.
Zaidi et al., The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review, The Journal of Antibiotics, doi:10.1038/s41429-021-00491-6.
25.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
26.
Low et al., Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166294.
27.
Fordham et al., The uses and abuses of systematic reviews, OSF Preprints, doi:10.31219/osf.io/mp4f2.
28.
Kow et al., Pitfalls in Reporting Sample Size Calculation Across Randomized Controlled Trials Involving Ivermectin for the treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001441.
29.
Santin et al., Ivermectin: a multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes and New Infections, doi:10.1016/j.nmni.2021.100924.
30.
Adegboro et al., A review of the anti-viral effects of ivermectin, African Journal of Clinical and Experimental Microbiology, doi:10.4314/ajcem.v22i3.2.
31.
Turkia, M., A Continuation of a Timeline of Ivermectin-Related Events in the COVID-19 Pandemic [June 30, 2021], ResearchGate, doi:10.13140/RG.2.2.16973.36326.
32.
Jagiasi et al., Variation in therapeutic strategies for the management of severe COVID-19 in India- A nationwide cross-sectional survey, The International Journal of Clinical Practice, doi:10.1111/ijcp.14574.
33.
Lind et al., Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis, Journal of General Internal Medicine, doi:10.1007/s11606-021-06948-6.
34.
Wang et al., Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19, medRxiv, doi:10.1101/2021.06.01.21258147.
35.
Kory (C) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001377.
36.
DiNicolantonio et al., Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors, Open Heart, doi:10.1136/openhrt-2021-001655.
37.
Turkia (B), M., A timeline of ivermectin-related events in the COVID-19 pandemic, Research Gate, www.researchgate.net/publication/350610718_A_Timeline_of_Ivermectin-Related_Events_in_the_COVID-19_Pandemic_April_3_2021.
38.
Wehbe et al., Repurposing Ivermectin for COVID-19: Molecular Aspects and Therapeutic Possibilities, Front. Immunol., doi:10.3389/fimmu.2021.663586.
39.
Yagisawa (B) et al., Global trends in clinical studies of ivermectin in COVID-19, The Japanese Journal of Antibiotics, 74-1, Mar 2021, jja-contents.wdc-jp.com/pdf/JJA74/74-1-open/74-1_44-95.pdf.
40.
Jans et al., The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.10.042.
41.
Kory (D) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Frontiers in Pharmacology, doi:10.3389/fphar.2021.643369.
42.
Formiga et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J. Control Release, doi:10.1016/j.jconrel.2020.10.009.
43.
Scheim (D), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
44.
Turkia (C), M., FLCCC Alliance MATH+ ascorbic acid and I-MASK+ ivermectin protocols for COVID-19 — a brief review, ResearchGate, www.researchgate.net/profile/Mika_Turkia/publication/345694745_FLCCC_Alliance_MATH_ascorbic_acid_and_I-MASK_ivermectin_protocols_for_COVID-19_-_A_Brief_Review/links/5fab010f4585150781078260/FLCCC-Alliance-MATH-ascorbic-acid-and-I-MASK-ivermectin-protocols-for-COVID-19-A-Brief-Review.pdf.
45.
Jans (B) et al., Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?, Cells 2020, 9:9, 2100, doi:10.3390/cells9092100.
46.
Elkholy et al., Ivermectin: A Closer Look at a Potential Remedy, Cureus, doi:10.7759/cureus.10378.
47.
DiNicolantonio (B) et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
48.
Vora et al., White paper on Ivermectin as a potential therapy for COVID-19, Indian Journal of Tuberculosis, doi:10.1016/j.ijtb.2020.07.031.
Marques et al., 23 May 2022, peer-reviewed, 4 authors.
Contact: reitz@utfpr.edu.br.
Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols
Brazilian Journal of Biology, doi:10.1590/1519-6984.258325
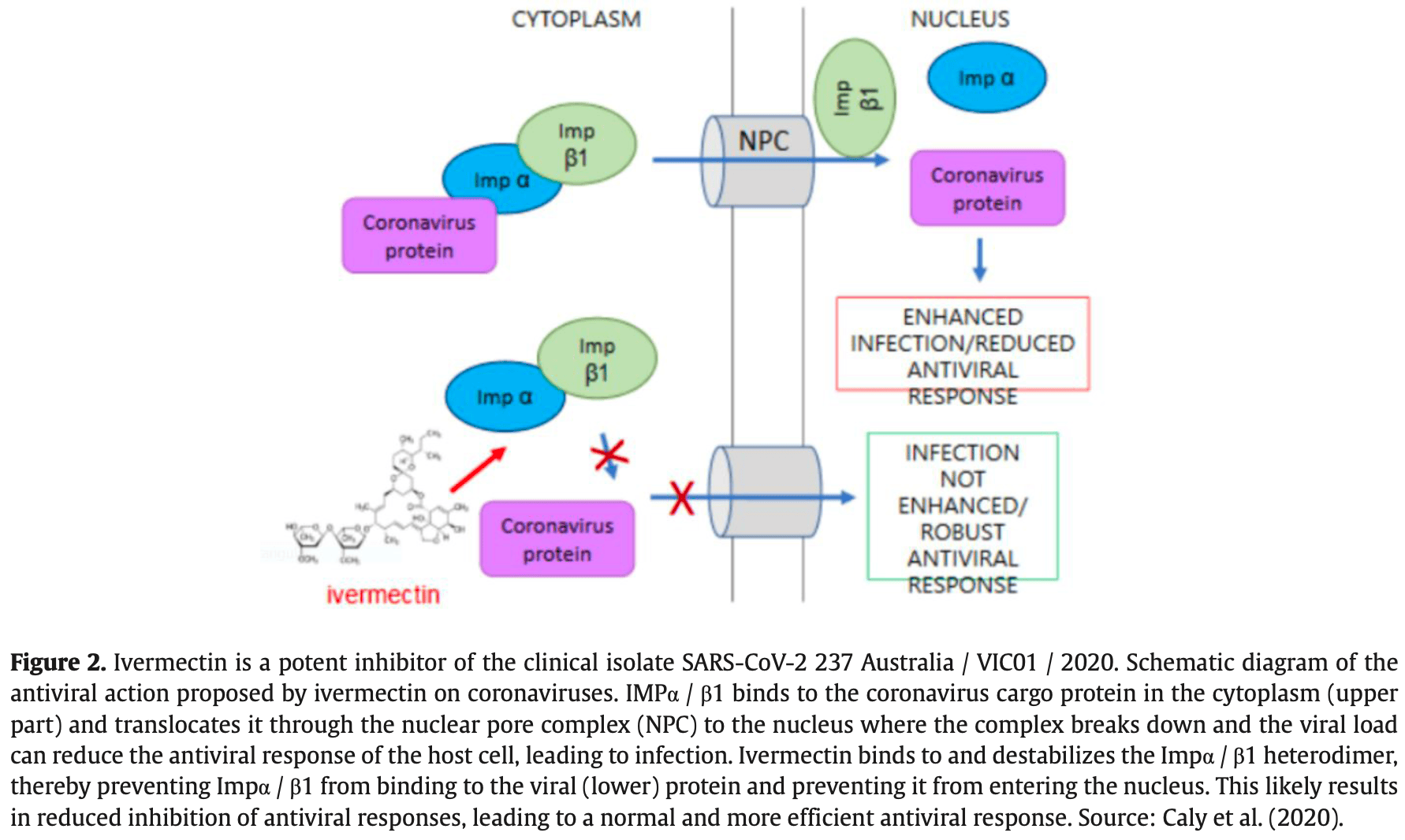
Ivermectin is a safe and effective drug in humans and has been approved for use in numerous parasitic infections for over 50 years. In addition, many studies have already shown its antiviral activity. Ivermectin is generally well tolerated, with no indication of central nervous system-associated toxicity at doses up to 10 times the highest FDAapproved dose of 200 µg/kg. The in vitro results of ivermectin for reducing SARS-CoV-2 viral load are promising and show that Ivermectin kills SARS-CoV-2 within 48 hours. A hypothesized mechanism of action for this drug is a likely inhibition of IMPα/β1-mediated nuclear import of viral proteins as demonstrated for other RNA viruses. However, controlled and randomized studies are needed to prove its effectiveness in COVID-19 in humans. In a single in vivo study with published results, patients confirmed to be infected with SARS-CoV-2 received at least one dose of ivermectin at any time during hospitalization. The use of ivermectin was associated with lower mortality during treatment with COVID-19, especially in patients who required increased inspired oxygen or ventilatory support. Additionally, 81 studies with the clinical use of ivermectin in humans are being carried out worldwide according to ClinicalTrials.gov. However, none of these data has been published so far. However, private and public entities in Brazil have been adopting this drug in their protocols as prophylaxis and in the initial phase of the disease. In addition, ivermectin has been used in mass treatment to prevent onchocerciasis and lymphatic filariasis in sub-Saharan Africa for many years. Surprisingly, this region has the lowest proportional mortality rate among the continents, despite the increasing numbers of infected people released by the World Health Organization.
References
Awadzi, Opoku, Addy, Quartey, The chemotherapy of onchocerciasis XIX: the clinical and laboratory tolerance of-high dose ivermectin
Baraka, Mahmoud, Marschke, Geary, Homeida et al., Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus, European Journal of Clinical Pharmacology, doi:10.1007/s002280050131
Bezerra, Medeiros, Mota, Costa, Protocolo de manejo para síndromes gripais frente à pandemia de coronavírus (COVID-19)
Brasil, Dispõe sobre o protocolo para o uso dos medicamentos Ivermectina e Cloroquina/Hidroxicloroquina nos pacientes com suspeita ou confirmação de COVID-19
Brasil, None
Bryant, Lawrie, Dowswell, Fordham, Mitchell et al., Ivermectin for prevention and treatment of COVID-19 Infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001402
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787
Cascella, Rajnik, Cuomo, Dulebohn, Napoli, Features, evaluation and treatment coronavirus (COVID-19)
Chaccour, Casellas, Blanco-Di, Matteo, Pineda et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2020.100720
Chaccour, Hammann, Rabinovich, Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety, Malaria Journal, doi:10.1186/s12936-017-1801-4
Chaccour, Ruiz-Castillo, Richardson, Moncunill, Casellas et al., The SARS-CoV-2 ivermectin Navarra-ISGlobal Trial (SAINT) to evaluate the potential of ivermectin to reduce COVID-19 transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol, Trials, doi:10.1186/s13063-020-04421-z
Crump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, The Journal of Antibiotics, doi:10.1038/ja.2017.11
Daley, %E2%80%93%20shared,centuries%20 %E2%80%93%20elephantiasis%20and%20river%20blindness
Edwards, Dingsdale, Helsby, Orme, Breckenridge, The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution, European Journal of Clinical Pharmacology, doi:10.1007/BF00637608
Fink, Porras, Pharmacokinetics of ivermectin in animals and humans, doi:10.1007/978-1-4612-3626-9_7
González Canga, Prieto, Diez Liébana, Fernández Martínez, Sierra Vega et al., The pharmacokinetics and interactions of ivermectin in humans: a mini-review, The AAPS Journal, doi:10.1208/s12248-007-9000-9
Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, Journal of Clinical Pharmacology, doi:10.1177/009127002401382731
Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, The Journal of Antibiotics, doi:10.1038/s41429-020-0336-z
King, Is ivermectin safe in pregnancy? The Lancet, Global Health, doi:10.1016/S2214-109X(19)30490-5
Krolewiecki, Lifschitz, Moragas, Travacio, Alonso et al., Antiviral effect of highdose ivermectin in adults with COVID-19: A proof-of-concept randomized trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100959
Merck, Tablets Stromectol (Ivermectina)
Ménez, Sutra, Prichard, Lespine, Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (-/-) mice and effects on mammalian GABA(A) channel activity, PLoS Neglected Tropical Diseases, doi:10.1371/journal.pntd.0001883
National, Coronavírus: Unimed Belém tem cerca de 450 pacientes recuperados
Navarro, Camprubí, Requena-Méndez, Buonfrate, Giorli et al., Safety of high-dose ivermectin: a systematic review and meta-analysis, The Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkz524
Nicolas, Maia, Bassat, Kobylinski, Monteiro et al., Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis, The Lancet. Global Health, doi:10.1016/S2214-109X(19)30453-X
Nunes, Lima, harmacotherapy for COVID-19 treatment in patients with renal impairment: a updated review, Scielo Preprints
Oosting, Njoo, Kijlstra, Van Wilgenburg, Keukens et al., Ivermectin detection in serum of onchocerciasis patients: relationship to adverse reactions, The American Journal of Tropical Medicine and Hygiene, doi:10.4269/ajtmh.1995.52.94
Patrì, Fabbrocini, Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment, Journal of the American Academy of Dermatology, doi:10.1016/j.jaad.2020.04.017
Rajter, Sherman, Fatteh, Vogel, Sacks et al., ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19, Chest Journal, doi:10.1016/j.chest.2020.10.009
Richards, Eigege, Umaru, Kahansim, Adelamo et al., The interruption of transmission of human onchocerciasis by an annual mass drug administration program in Plateau and Nasarawa States, Nigeria, The American Journal of Tropical Medicine and Hygiene, doi:10.4269/ajtmh.19-0577
Romani, Marks, Sokana, Nasi, Kamoriki et al., Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single-arm community intervention trial, The Lancet. Infectious Diseases, doi:10.1016/S1473-3099(18)30790-4
Rupp, Balneário Camboriú vai oferecer tratamento em fase inicial a pacientes com Covid-19
Sharun, Dhama, Patel, Pathak, Tiwari et al., Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19, Ann Clin Microbiol Antimicrob, doi:10.1186/s12941-020-00368-w
Vilanova, Baía, Abelardo Santos' registra mais de 900 recuperados da Covid-19
Walker, Whittaker, Watson, Baguelin, Winskill et al., The global impact of COVID-19 and strategies for mitigation and suppression, Science, Brazilian Journal of Biology, doi:10.1126/science.abc0035
Zeng, Andrew, Arison, Luffer-Atlas, Wang, Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes, Xenobiotica, doi:10.1080/004982598239597
DOI record:
{
"DOI": "10.1590/1519-6984.258325",
"ISSN": [
"1678-4375",
"1519-6984"
],
"URL": "http://dx.doi.org/10.1590/1519-6984.258325",
"abstract": "<jats:p>Abstract Ivermectin is a safe and effective drug in humans and has been approved for use in numerous parasitic infections for over 50 years. In addition, many studies have already shown its antiviral activity. Ivermectin is generally well tolerated, with no indication of central nervous system-associated toxicity at doses up to 10 times the highest FDA-approved dose of 200 µg/kg. The in vitro results of ivermectin for reducing SARS-CoV-2 viral load are promising and show that Ivermectin kills SARS-CoV-2 within 48 hours. A hypothesized mechanism of action for this drug is a likely inhibition of IMPα/β1-mediated nuclear import of viral proteins as demonstrated for other RNA viruses. However, controlled and randomized studies are needed to prove its effectiveness in COVID-19 in humans. In a single in vivo study with published results, patients confirmed to be infected with SARS-CoV-2 received at least one dose of ivermectin at any time during hospitalization. The use of ivermectin was associated with lower mortality during treatment with COVID-19, especially in patients who required increased inspired oxygen or ventilatory support. Additionally, 81 studies with the clinical use of ivermectin in humans are being carried out worldwide according to ClinicalTrials.gov. However, none of these data has been published so far. However, private and public entities in Brazil have been adopting this drug in their protocols as prophylaxis and in the initial phase of the disease. In addition, ivermectin has been used in mass treatment to prevent onchocerciasis and lymphatic filariasis in sub-Saharan Africa for many years. Surprisingly, this region has the lowest proportional mortality rate among the continents, despite the increasing numbers of infected people released by the World Health Organization.</jats:p>",
"alternative-id": [
"S1519-69842024000100303"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5024-0542",
"affiliation": [
{
"name": "Universidade Tecnológica Federal do Paraná, Brasil"
}
],
"authenticated-orcid": false,
"family": "Marques",
"given": "L. L. M.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7888-5820",
"affiliation": [
{
"name": "Universidade Tecnológica Federal do Paraná, Brasil"
}
],
"authenticated-orcid": false,
"family": "Beneti",
"given": "S. C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5719-5633",
"affiliation": [
{
"name": "Universidade Tecnológica Federal do Paraná, Brasil"
}
],
"authenticated-orcid": false,
"family": "Pinzon",
"given": "C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0432-9191",
"affiliation": [
{
"name": "Universidade Tecnológica Federal do Paraná, Brasil"
}
],
"authenticated-orcid": false,
"family": "Cardoso",
"given": "F. A. R.",
"sequence": "additional"
}
],
"container-title": "Brazilian Journal of Biology",
"container-title-short": "Braz. J. Biol.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
13
]
],
"date-time": "2022-05-13T12:33:35Z",
"timestamp": 1652445215000
},
"deposited": {
"date-parts": [
[
2022,
5,
13
]
],
"date-time": "2022-05-13T12:34:12Z",
"timestamp": 1652445252000
},
"indexed": {
"date-parts": [
[
2024,
7,
31
]
],
"date-time": "2024-07-31T18:35:45Z",
"timestamp": 1722450945579
},
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2024
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
}
],
"link": [
{
"URL": "http://www.scielo.br/scielo.php?script=sci_pdf&pid=S1519-69842024000100303&tlng=en",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "530",
"original-title": [
"Ivermectina como um possível tratamento para COVID-19: uma revisão dos protocolos de 2022"
],
"prefix": "10.1590",
"published": {
"date-parts": [
[
2024
]
]
},
"published-online": {
"date-parts": [
[
2024
]
]
},
"publisher": "FapUNIFESP (SciELO)",
"reference": [
{
"key": "ref1",
"series-title": "Bulário eletrônico",
"year": "2021"
},
{
"key": "ref2",
"series-title": "Secretaria de Saúde de Natal recomenda usar ivermectina para prevenir e tratar coronavírus",
"year": "2020"
},
{
"article-title": "The chemotherapy of onchocerciasis XIX: the clinical and laboratory tolerance of-high dose ivermectin",
"author": "AWADZI K.",
"first-page": "131",
"issue": "2",
"journal-title": "Tropical Medicine and Parasitology",
"key": "ref3",
"volume": "46",
"year": "1995"
},
{
"DOI": "10.1007/s002280050131",
"article-title": "Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus",
"author": "BARAKA O.Z.",
"doi-asserted-by": "crossref",
"first-page": "407",
"issue": "5",
"journal-title": "European Journal of Clinical Pharmacology",
"key": "ref4",
"volume": "50",
"year": "1996"
},
{
"author": "BEZERRA H.M.",
"key": "ref5",
"series-title": "Protocolo de manejo para síndromes gripais frente à pandemia de coronavírus (COVID-19).",
"year": "2020"
},
{
"key": "ref6",
"series-title": "Decreto no 11.940, de 07 de Julho de 2020, que regulamenta a distribuição do medicamento ivermectina no Município de Itajaí",
"year": "2020"
},
{
"key": "ref7",
"series-title": "Portaria no 022/2020. Dispõe sobre o protocolo para o uso dos medicamentos Ivermectina e Cloroquina/Hidroxicloroquina nos pacientes com suspeita ou confirmação de COVID-19.",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"article-title": "Ivermectin for prevention and treatment of COVID-19 Infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines",
"author": "BRYANT A.",
"doi-asserted-by": "crossref",
"first-page": "e434",
"issue": "4",
"journal-title": "American Journal of Therapeutics",
"key": "ref8",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "CALY L.",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Research",
"key": "ref9",
"volume": "178",
"year": "2020"
},
{
"author": "CASCELLA M.",
"key": "ref10",
"series-title": "Features, evaluation and treatment coronavirus (COVID-19).",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"article-title": "The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial",
"author": "CHACCOUR C.",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "ref11",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1186/s12936-017-1801-4",
"article-title": "Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety",
"author": "CHACCOUR C.",
"doi-asserted-by": "crossref",
"first-page": "161",
"issue": "1",
"journal-title": "Malaria Journal",
"key": "ref12",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1186/s13063-020-04421-z",
"article-title": "The SARS-CoV-2 ivermectin Navarra-ISGlobal Trial (SAINT) to evaluate the potential of ivermectin to reduce COVID-19 transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol",
"author": "CHACCOUR C.",
"doi-asserted-by": "crossref",
"first-page": "498",
"issue": "1",
"journal-title": "Trials",
"key": "ref13",
"volume": "21",
"year": "2020"
},
{
"key": "ref14",
"series-title": "Boletim ética na pesquisa",
"year": "2020"
},
{
"DOI": "10.1038/ja.2017.11",
"article-title": "Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations",
"author": "CRUMP A.",
"doi-asserted-by": "crossref",
"first-page": "495",
"issue": "5",
"journal-title": "The Journal of Antibiotics",
"key": "ref15",
"volume": "70",
"year": "2017"
},
{
"author": "DALEY B.",
"key": "ref16",
"series-title": "How 2015 Nobel Prize drug might rid Africa of ancient scourges",
"year": "2015"
},
{
"DOI": "10.1007/BF00637608",
"article-title": "The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution",
"author": "EDWARDS G.",
"doi-asserted-by": "crossref",
"first-page": "681",
"issue": "6",
"journal-title": "European Journal of Clinical Pharmacology",
"key": "ref17",
"volume": "35",
"year": "1988"
},
{
"author": "FINK D.W.",
"first-page": "113",
"key": "ref18",
"series-title": "Ivermectin and abamectin",
"volume-title": "Pharmacokinetics of ivermectin in animals and humans.",
"year": "1989"
},
{
"DOI": "10.1208/s12248-007-9000-9",
"article-title": "The pharmacokinetics and interactions of ivermectin in humans: a mini-review",
"author": "GONZÁLEZ CANGA A.",
"doi-asserted-by": "crossref",
"first-page": "42",
"issue": "1",
"journal-title": "The AAPS Journal",
"key": "ref19",
"volume": "10",
"year": "2008"
},
{
"DOI": "10.1177/009127002401382731",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "GUZZO C.A.",
"doi-asserted-by": "crossref",
"first-page": "1122",
"issue": "10",
"journal-title": "Journal of Clinical Pharmacology",
"key": "ref20",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"article-title": "Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen",
"author": "HEIDARY F.",
"doi-asserted-by": "crossref",
"first-page": "593",
"issue": "9",
"journal-title": "The Journal of Antibiotics",
"key": "ref21",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(19)30490-5",
"article-title": "Is ivermectin safe in pregnancy?",
"author": "KING C.L.",
"doi-asserted-by": "crossref",
"first-page": "e12",
"issue": "1",
"journal-title": "The Lancet. Global Health",
"key": "ref22",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"article-title": "Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial",
"author": "KROLEWIECKI A.",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "ref23",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1371/journal.pntd.0001883",
"article-title": "Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (−/−) mice and effects on mammalian GABA(A) channel activity",
"author": "MÉNEZ C.",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "PLoS Neglected Tropical Diseases",
"key": "ref24",
"volume": "6",
"year": "2012"
},
{
"key": "ref25",
"series-title": "Tablets Stromectol (Ivermectina) FDA approved Package insert 2009",
"year": "2009"
},
{
"DOI": "10.1093/jac/dkz524",
"article-title": "Safety of high-dose ivermectin: a systematic review and meta-analysis",
"author": "NAVARRO M.",
"doi-asserted-by": "crossref",
"first-page": "827",
"issue": "4",
"journal-title": "The Journal of Antimicrobial Chemotherapy",
"key": "ref26",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(19)30453-X",
"article-title": "Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis",
"author": "NICOLAS P.",
"doi-asserted-by": "crossref",
"first-page": "e92",
"issue": "1",
"journal-title": "The Lancet. Global Health",
"key": "ref27",
"volume": "8",
"year": "2020"
},
{
"article-title": "harmacotherapy for COVID-19 treatment in patients with renal impairment: a updated review",
"author": "NUNES L.",
"journal-title": "Scielo Preprints.",
"key": "ref28",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.1995.52.94",
"article-title": "Ivermectin detection in serum of onchocerciasis patients: relationship to adverse reactions",
"author": "OOSTING J.",
"doi-asserted-by": "crossref",
"first-page": "94",
"issue": "1",
"journal-title": "The American Journal of Tropical Medicine and Hygiene",
"key": "ref29",
"volume": "52",
"year": "1995"
},
{
"DOI": "10.1016/j.jaad.2020.04.017",
"article-title": "Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment?",
"author": "PATRÌ A.",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Journal of the American Academy of Dermatology",
"key": "ref30",
"volume": "82",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"article-title": "ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19",
"author": "RAJTER J.C.",
"doi-asserted-by": "crossref",
"first-page": "85",
"issue": "1",
"journal-title": "Chest Journal",
"key": "ref31",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.19-0577",
"article-title": "The interruption of transmission of human onchocerciasis by an annual mass drug administration program in Plateau and Nasarawa States, Nigeria",
"author": "RICHARDS F.O.",
"doi-asserted-by": "crossref",
"first-page": "582",
"issue": "3",
"journal-title": "The American Journal of Tropical Medicine and Hygiene",
"key": "ref32",
"volume": "102",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(18)30790-4",
"article-title": "Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single-arm community intervention trial",
"author": "ROMANI L.",
"doi-asserted-by": "crossref",
"first-page": "510",
"issue": "5",
"journal-title": "The Lancet. Infectious Diseases",
"key": "ref33",
"volume": "19",
"year": "2019"
},
{
"author": "RUPP I.",
"key": "ref34",
"series-title": "Balneário Camboriú vai oferecer tratamento em fase inicial a pacientes com Covid-19",
"year": "2020"
},
{
"article-title": "Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19",
"author": "SHARUN K.",
"issue": "23",
"journal-title": "Ann Clin Microbiol Antimicrob",
"key": "ref35",
"volume": "19",
"year": "2020"
},
{
"key": "ref36",
"series-title": "ClinicalTrials.gov",
"year": "2022"
},
{
"key": "ref37",
"series-title": "Coronavírus: Unimed Belém tem cerca de 450 pacientes recuperados",
"year": "2020"
},
{
"key": "ref38",
"series-title": "Distribuição de medicamentos para tratamento da Covid-19",
"year": "2020"
},
{
"edition": "5.",
"key": "ref39",
"series-title": "Proposta de tratamento da COVID-19 dependendo da fase, no momento do diagnóstico",
"year": "2020"
},
{
"author": "VILANOVA R.",
"key": "ref40",
"series-title": "Em dois meses, ‘Abelardo Santos’ registra mais de 900 recuperados da Covid-19.",
"year": "2020"
},
{
"DOI": "10.1126/science.abc0035",
"article-title": "The global impact of COVID-19 and strategies for mitigation and suppression",
"author": "WALKER P.G.T.",
"doi-asserted-by": "crossref",
"first-page": "413",
"issue": "6502",
"journal-title": "Science",
"key": "ref41",
"volume": "369",
"year": "2020"
},
{
"key": "ref42",
"series-title": "Novel Coronavirus (COVID-19) health sector preparedness & response.",
"year": "2020"
},
{
"key": "ref43",
"series-title": "WHO Coronavirus disease (COVID-19) dashboard-situation by WHO Region.",
"year": "2020"
},
{
"DOI": "10.1080/004982598239597",
"article-title": "Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes",
"author": "ZENG Z.",
"doi-asserted-by": "crossref",
"first-page": "313",
"issue": "3",
"journal-title": "Xenobiotica",
"key": "ref44",
"volume": "28",
"year": "1998"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1519-69842024000100303&tlng=en"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols",
"type": "journal-article",
"volume": "84"
}
