Rho-GTPases subfamily: cellular defectors orchestrating viral infection
et al., Cellular & Molecular Biology Letters, doi:10.1186/s11658-025-00722-w, May 2025
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
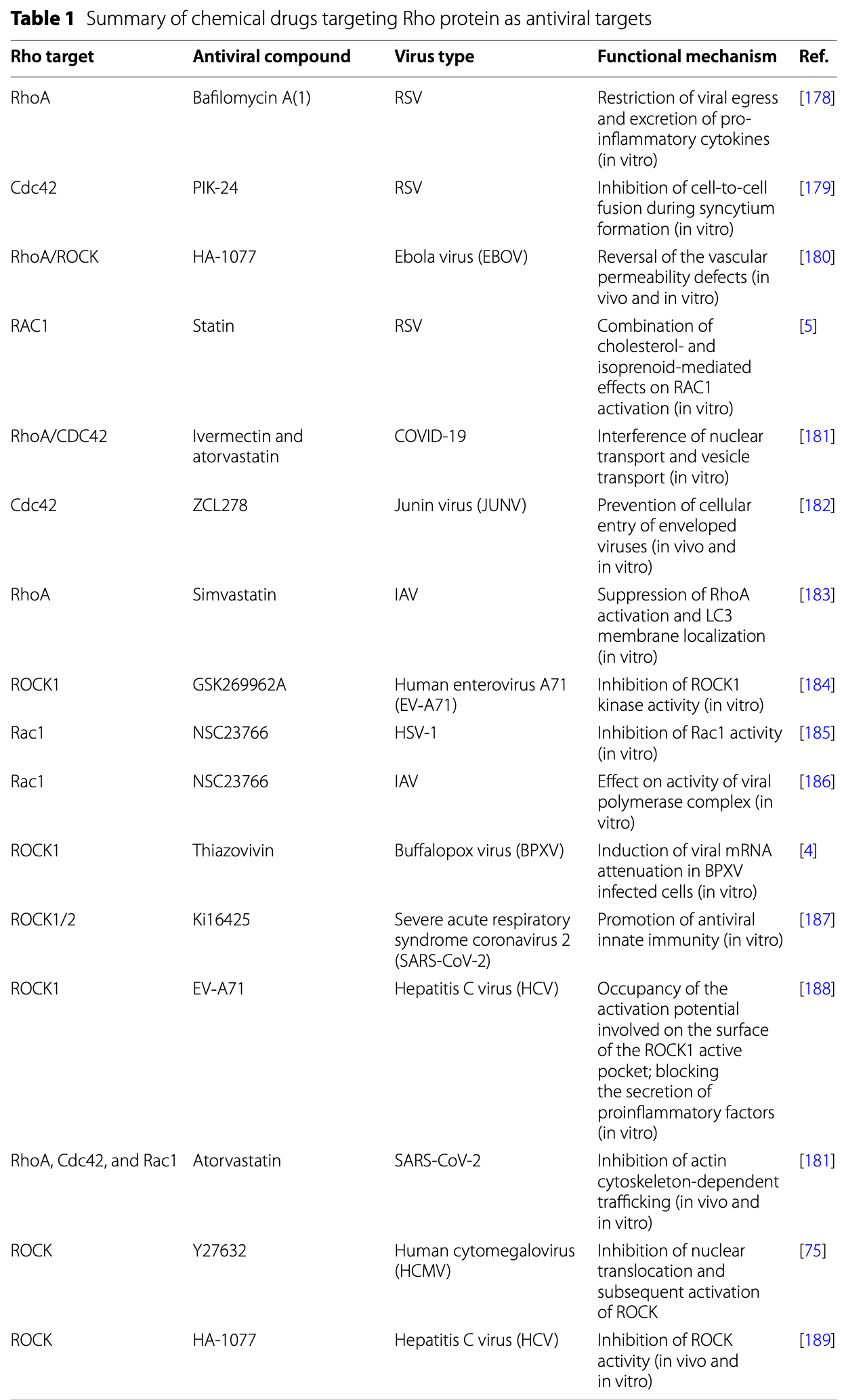
Review of Rho-GTPases as pivotal host factors commandeered by SARS-CoV-2 and other viruses, noting ivermectin as one of many compounds targeting this axis. Authors list ivermectin + atorvastatin as agents that suppress RhoA/CDC42 signaling; in cell-culture models this combination interfered with nuclear import and vesicle trafficking, key steps for coronavirus replication, thereby curbing viral growth. By disrupting cytoskeleton-dependent transport processes rather than viral proteins directly, the review contends that ivermectin exemplifies a host-directed strategy that could complement classical antivirals and curb resistance.
1.
Reich, S., Methodological Analysis of Bias Risks in Adaptive Multi-Arm Platform Trials: A Case-Series from Three COVID-19 Studies, Center for Open Science, doi:10.31222/osf.io/h5kc8_v1.
2.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
3.
Zhang et al., Rho-GTPases subfamily: cellular defectors orchestrating viral infection, Cellular & Molecular Biology Letters, doi:10.1186/s11658-025-00722-w.
4.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
5.
Ulloa-Aguilar et al., The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection, Cells, doi:10.3390/cells13181591.
6.
Enyeji et al., Effective Treatment of COVID-19 Infection with Repurposed Drugs: Case Reports, Viral Immunology, doi:10.1089/vim.2024.0034.
7.
Wimalawansa, S., Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity, Heliyon, doi:10.1016/j.heliyon.2024.e34691.
8.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
9.
Mehraeen et al., Treatments for Olfactory Dysfunction in COVID-19: A Systematic Review, International Archives of Otorhinolaryngology, doi:10.1055/s-0044-1786046.
10.
Scheim et al., Back to the Basics of SARS-CoV-2 Biochemistry: Microvascular Occlusive Glycan Bindings Govern Its Morbidities and Inform Therapeutic Responses, Viruses, doi:10.3390/v16040647.
11.
Yagisawa et al., Global trends in clinical trials of ivermectin for COVID-19—Part 2, The Japanese Journal of Antibiotics, doi:10.11553/antibiotics.77.1_45.
12.
Liu et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
13.
Scheim (B) et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
14.
Yemeke et al., Impact of the COVID-19 pandemic on the quality of medical products in Zimbabwe: a qualitative study based on key informant interviews with health system stakeholders, BMJ Open, doi:10.1136/bmjopen-2022-068923.
15.
Kory, P., The Global War on Ivermectin, International Covid Summit III, European Parliament, Brussels, covid19criticalcare.com/wp-content/uploads/2023/05/GLOBAL-WAR-ON-IVERMECTIN-PARLIAMENT.pdf.
16.
Babalola et al., The Place of Ivermectin in the Management of Covid-19: State of the Evidence, Medical Research Archives, doi:10.18103/mra.v11i4.3778.
17.
Loo et al., Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-023-03520-1.
18.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
19.
Kory (B), P., The Criminal Censorship of Ivermectin's Efficacy By The High-Impact Medical Journals - Part 1, Pierre Kory’s Medical Musings, pierrekory.substack.com/p/the-criminal-censorship-of-ivermectins.
20.
Al-kuraishy et al., Central effects of Ivermectin in alleviation of Covid-19-induced dysautonomia, Current Drug Targets, doi:10.2174/1389450123666220810102406.
21.
Schwartz, E., Does ivermectin have a place in the treatment of mild Covid-19?, New Microbes and New Infections, doi:10.1016/j.nmni.2022.100989.
22.
Marques et al., Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols, Brazilian Journal of Biology, doi:10.1590/1519-6984.258325.
23.
Semiz, S., SIT1 transporter as a potential novel target in treatment of COVID-19, Biomolecular Concepts, doi:10.1515/bmc-2021-0017.
24.
Zaidi et al., The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review, The Journal of Antibiotics, doi:10.1038/s41429-021-00491-6.
25.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
26.
Low et al., Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166294.
27.
Fordham et al., The uses and abuses of systematic reviews, OSF Preprints, doi:10.31219/osf.io/mp4f2.
28.
Kow et al., Pitfalls in Reporting Sample Size Calculation Across Randomized Controlled Trials Involving Ivermectin for the treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001441.
29.
Santin et al., Ivermectin: a multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes and New Infections, doi:10.1016/j.nmni.2021.100924.
30.
Adegboro et al., A review of the anti-viral effects of ivermectin, African Journal of Clinical and Experimental Microbiology, doi:10.4314/ajcem.v22i3.2.
31.
Turkia, M., A Continuation of a Timeline of Ivermectin-Related Events in the COVID-19 Pandemic [June 30, 2021], ResearchGate, doi:10.13140/RG.2.2.16973.36326.
32.
Jagiasi et al., Variation in therapeutic strategies for the management of severe COVID-19 in India- A nationwide cross-sectional survey, The International Journal of Clinical Practice, doi:10.1111/ijcp.14574.
33.
Lind et al., Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis, Journal of General Internal Medicine, doi:10.1007/s11606-021-06948-6.
34.
Wang et al., Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19, medRxiv, doi:10.1101/2021.06.01.21258147.
35.
Kory (C) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001377.
36.
DiNicolantonio et al., Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors, Open Heart, doi:10.1136/openhrt-2021-001655.
37.
Turkia (B), M., A timeline of ivermectin-related events in the COVID-19 pandemic, Research Gate, www.researchgate.net/publication/350610718_A_Timeline_of_Ivermectin-Related_Events_in_the_COVID-19_Pandemic_April_3_2021.
38.
Wehbe et al., Repurposing Ivermectin for COVID-19: Molecular Aspects and Therapeutic Possibilities, Front. Immunol., doi:10.3389/fimmu.2021.663586.
39.
Yagisawa (B) et al., Global trends in clinical studies of ivermectin in COVID-19, The Japanese Journal of Antibiotics, 74-1, Mar 2021, jja-contents.wdc-jp.com/pdf/JJA74/74-1-open/74-1_44-95.pdf.
40.
Jans et al., The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.10.042.
41.
Kory (D) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Frontiers in Pharmacology, doi:10.3389/fphar.2021.643369.
42.
Formiga et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J. Control Release, doi:10.1016/j.jconrel.2020.10.009.
43.
Scheim (D), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
44.
Turkia (C), M., FLCCC Alliance MATH+ ascorbic acid and I-MASK+ ivermectin protocols for COVID-19 — a brief review, ResearchGate, www.researchgate.net/profile/Mika_Turkia/publication/345694745_FLCCC_Alliance_MATH_ascorbic_acid_and_I-MASK_ivermectin_protocols_for_COVID-19_-_A_Brief_Review/links/5fab010f4585150781078260/FLCCC-Alliance-MATH-ascorbic-acid-and-I-MASK-ivermectin-protocols-for-COVID-19-A-Brief-Review.pdf.
45.
Jans (B) et al., Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?, Cells 2020, 9:9, 2100, doi:10.3390/cells9092100.
46.
Elkholy et al., Ivermectin: A Closer Look at a Potential Remedy, Cureus, doi:10.7759/cureus.10378.
47.
DiNicolantonio (B) et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
48.
Vora et al., White paper on Ivermectin as a potential therapy for COVID-19, Indian Journal of Tuberculosis, doi:10.1016/j.ijtb.2020.07.031.
Zhang et al., 2 May 2025, peer-reviewed, 6 authors.
Contact: duanhong0924@126.com.
Rho-GTPases subfamily: cellular defectors orchestrating viral infection
Cellular & Molecular Biology Letters, doi:10.1186/s11658-025-00722-w
Ras homolog gene family-guanosine triphosphatases (Rho-GTPases), key molecular switches regulating cytoskeletal dynamics and cellular signaling, play a pivotal role in viral infections by modulating critical processes such as viral entry, replication, and release. This review elucidates the intricate mechanisms through which Rho-GTPases, via interactions with guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and other signaling pathways, including the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), rat sarcoma (Ras), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways, facilitate viral pathogenesis. Specific viruses, such as influenza A virus (IAV), herpesviruses, human immunodeficiency virus (HIV), and respiratory syncytial virus (RSV), exploit Rho-GTPase-mediated cytoskeletal reorganization to enhance infectivity. For example, Rho-GTPases promote actin remodeling and membrane fusion, which are essential for viral entry and intracellular transport. Furthermore, Rho-GTPases modulate immune responses, often suppressing antiviral defenses to favor viral replication. Despite these insights, the molecular mechanisms underlying Rho-GTPase regulation during viral infections remain incompletely understood. Future research should focus on delineating the precise roles of Rho-GTPases in distinct viral life cycles, uncovering novel regulatory mechanisms, and developing targeted antiviral therapies that selectively inhibit Rho-GTPase signaling without compromising host cell functions. Such advancements could pave the way for broad-spectrum antiviral strategies, particularly against viruses that heavily rely on cytoskeletal manipulation for infection.
Declarations Competing interests The authors declare no competing interest.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Acharya, Reis, Volcic, Liu, Wang et al., Actin cytoskeleton remodeling primes RIG-I-like receptor activation, Cell
Agrelli, De Moura, Crovella, Brandao, ZIKA virus entry mechanisms in human cells, Infect Genet Evol
Alarifi, Alkahtani, Al-Qahtani, Stournaras, Sourvinos, Induction of interleukin-11 mediated by RhoA GTPase during human cytomegalovirus lytic infection, Cell Signal
Amin, Dubey, Zhang, Gremer, Dvorsky et al., Rho-kinase: regulation, (dys)function, and inhibition, Biol Chem
Anwar, Fazal, Malik, Rahman, RhoA/Rho-associated kinase pathway selectively regulates thrombininduced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65, J Immunol
Arakawa, Cordeiro, Way, F11L-mediated inhibition of RhoA-mDia signaling stimulates microtubule dynamics during vaccinia virus infection, Cell Host Microbe
Arozarena, Matallanas, Crespo, Maintenance of CDC42 GDP-bound state by Rho-GDI inhibits MAP kinase activation by the exchange factor Ras-GRF. Evidence for Ras-GRF function being inhibited by Cdc42-GDP but unaffected by CDC42-GTP, J Biol Chem
Ayasoufi, Pfaller, Seek and hide: the manipulating interplay of measles virus with the innate immune system, Curr Opin Virol
Bagci, Sriskandarajah, Robert, Boulais, Elkholi et al., Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms, Nat Cell Biol
Bandekar, Chen, Ravala, Cash, Avramova et al., Structural/functional studies of Trio provide insights into its configuration and show that conserved linker elements enhance its activity for Rac1, J Biol Chem
Basbous, Azzarelli, Pacary, Moreau, Pathophysiological functions of Rnd proteins, Small GTPases
Becker, Pettee, Sugrue, Reinard, Schroeder et al., The cytoskeleton effectors rho-kinase (ROCK) and mammalian diaphanous-related (mDia) formin have dynamic roles in tumor microtube formation in invasive glioblastoma cells, Cells
Bedi, Ono, Friend or foe: the role of the cytoskeleton in influenza A virus assembly, Viruses
Biro, Munoz, Weninger, Targeting Rho-GTPases in immune cell migration and inflammation, Br J Pharmacol
Boehmer, Nimonkar, Herpes virus replication, IUBMB Life
Bos, Rehmann, Wittinghofer, GEFs and GAPs: critical elements in the control of small G proteins, Cell
Bostanciklioglu, Igci, Sativa, Anthemis hyaline and Citrus sinensis extracts reduce SARS-CoV-2 replication by fluctuating Rho GTPase, PI3K-AKT, and MAPK/ERK pathways in HeLa-CEACAM1a cells, Gene
Brar, Dey, Bhardwaj, Pande, Singh et al., Dendrite regeneration in C. elegans is controlled by the RAC GTPase CED-10 and the RhoGEF TIAM-1, PLoS Genet
Budge, Graham, Inhibition of respiratory syncytial virus by RhoA-derived peptides: implications for the development of improved antiviral agents targeting heparin-binding viruses, J Antimicrob Chemother
Burbage, Keppler, Gasparrini, Martinez-Martin, Gaya et al., Cdc42 is a key regulator of B cell differentiation and is required for antiviral humoral immunity, J Exp Med
Burbage, Keppler, Montaner, Mattila, Batista, The small Rho GTPase TC10 modulates B cell immune responses, J Immunol
Burrel, Topalis, Boutolleau, Herpes simplex virus resistance to antivirals, Virologie
Chen, Li, Diao, Cao, Fu et al., Up-regulation of RhoB by glucocorticoids and its effects on the cell proliferation and NF-kappaB transcriptional activity, J Steroid Biochem Mol Biol
Cherfils, Zeghouf, Regulation of small GTPases by GEFs, GAPs, and GDIs, Physiol Rev
Chichili, Chew, Shankar, Chin, Jobichen, Structural basis for p50RhoGAP BCH domainmediated regulation of Rho inactivation, Proc Natl Acad Sci
Chmielewski, Schmid, Simmons, Chiu, Chikungunya virus assembly and budding visualized in situ using cryogenic electron tomography, Nat Microbiol
Crosas-Molist, Samain, Kohlhammer, Orgaz, George et al., Rho GTPase signaling in cancer progression and dissemination, Physiol Rev
Cuartas-Lopez, Hernandez-Cuellar, Gomez, Disentangling the role of PI3K/Akt, Rho GTPase and the actin cytoskeleton on dengue virus infection, Virus Res
Dam, Kracht, Pleschka, Schmitz, The influenza A virus genotype determines the antiviral function of NF-kappaB, J Virol
Daniels, Holman, Cruz-Orengo, Jujjavarapu, Durrant et al., Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals, MBio
Dewane, Salvi, Demali, Fueling the cytoskeleton-links between cell metabolism and actin remodeling, J Cell Sci
Diamond, Kanneganti, Innate immunity: the first line of defense against SARS-CoV-2, Nat Immunol
Dogterom, Koenderink, Actin-microtubule crosstalk in cell biology, Nat Rev Mol Cell Biol
Du, Hu, Menendez-Arias, Zhan, Liu, Target-based drug design strategies to overcome resistance to antiviral agents: opportunities and challenges, Drug Resist Updat
Ebrahimi, Kharazmi, Ghanaatian, Miraghel, Amiri et al., Role of the Wnt and GTPase pathways in breast cancer tumorigenesis and treatment, Cytokine Growth Factor Rev
Eliyahu, Tirosh, Dobesova, Nachshon, Schwartz et al., Rho-associated coiled-coil kinase 1 translocates to the nucleus and inhibits human cytomegalovirus propagation, J Virol
Fan, Luo, Zhang, Wang, Chen et al., Cell division control protein 42 interacts with hepatitis E virus capsid protein and participates in hepatitis E virus infection, Front Microbiol
Fan, Wu, Zhang, Wang, Wang et al., Effect of the Rho GTPase inhibitor-1 on the entry of dengue serotype 2 virus into EAhy926 cells, Mol Biol Rep
Fares, Fares, Khachfe, Salhab, Fares, Molecular principles of metastasis: a hallmark of cancer revisited, Signal Transduct Target Ther
Favoreel, Enquist, Feierbach, Actin and Rho GTPases in herpesvirus biology, Trends Microbiol
Finke, Hungerland, Solov'yov, Ia, Schuhmann, Different receptor models show differences in ligand binding strength and location: a computational drug screening for the tick-borne encephalitis virus, Mol Divers
Finkielstein, Overduin, Capelluto, Cell migration and signaling specificity is determined by the phosphatidylserine recognition motif of Rac1, J Biol Chem
Frank, Adelstein, Hansen, GIT2 represses Crk-and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover, EMBO J
Fu, Wang, Yang, Chen, Kuerban et al., Septin11 promotes hepatocellular carcinoma cell motility by activating RhoA to regulate cytoskeleton and cell adhesion, Cell Death Dis
Fu, Wang, Zhai, Qi, Jing et al., Changes in apoptosis, proliferation and T lymphocyte subtype on thymic cells of SPF chickens infected with reticuloendotheliosis virus, Mol Immunol
Gao, Raduka, Rezaee, Vitamin D(3) protects against respiratory syncytial virus-induced barrier dysfunction in airway epithelial cells via PKA signaling pathway, Eur J Cell Biol
Gerlt, Mayr, Sarto, Ludwig, Boergeling, Cellular Protein Phosphatase 2A Regulates Cell Survival Mechanisms in Influenza A Virus Infection, Int J Mol Sci
Glotfelty, Tovar, Hsueh, Tweedie, Li et al., The RhoA-ROCK1/ROCK2 pathway exacerbates inflammatory signaling in immortalized and primary microglia, Cells
Grzywa, Klicka, Wlodarski, Regulators at every step-how microRNAs drive tumor cell invasiveness and metastasis, Cancers
Guan, Cannon, Coates, Mei, Effect of the Rho-Kinase/ROCK signaling pathway on cytoskeleton components, Genes
Gupta, Saxena, Chen, HIV/AIDS: current updates on the disease, treatment and prevention, Proc Natl Acad Sci India Sect B Biol Sci
Haga, Garg, Collu, Borda, Agua et al., RhoBTB1 interacts with ROCKs and inhibits invasion, Biochem J
Haidari, Zhang, Ganjehei, Ali, Chen, Inhibition of MLC phosphorylation restricts replication of influenza virus-a mechanism of action for anti-influenza agents, PLoS ONE
Han, Zou, Zhou, Zeng, Zheng et al., The small GTPase NtRHO1 negatively regulates tobacco defense response to tobacco mosaic virus by interacting with NtWRKY50, J Exp Bot
Haspel, Jang, Nussinov, Allosteric activation of RhoA complexed with p115-RhoGEF deciphered by conformational dynamics, J Chem Inf Model
Hemshekhar, Choi, Mookherjee, Host defense peptide LL-37-mediated chemoattractant properties, but not anti-inflammatory cytokine IL-1RA production, is selectively controlled by Cdc42 Rho GTPase via G proteincoupled receptors and JNK mitogen-activated protein kinase, Front Immunol
Hodges, Sharrocks, Edelmann, Baban, Moris et al., Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication, Nat Immunol
Hong, Kim, Yanpallewar, Lin, The Rho/Rac guanine nucleotide exchange factor Vav1 regulates Hif-1alpha and Glut-1 expression and glucose uptake in the brain, Int J Mol Sci
Hornikova, Brustikova, Huerfano, Forstova, Nuclear cytoskeleton in virus infection, Int J Mol Sci
Howden, Michael, Hight-Warburton, Parsons, alpha2beta1 integrins spatially restrict Cdc42 activity to stabilise adherens junctions, BMC Biol
Hu, Chong, Lu, Qin, Ren et al., Loss of the BCR-FGFR1 GEF domain suppresses RHOA activation and enhances B-lymphomagenesis in mice, Cancer Res
Hunter, Campbell, Kerridge, Fraser, Hannaway et al., Up-regulation of the PI3K/AKT and RHO/RAC/PAK signalling pathways in CHK1 inhibitor resistant Emicro-Myc lymphoma cells, Biochem J
Iesato, Tatsumi, Saito, Ogasawara, Sakao et al., Tiotropium bromide attenuates respiratory syncytial virus replication in epithelial cells, Drug News Perspect
Jasenosky, Cadena, Mire, Borisevich, Haridas et al., The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus, iScience
Jiang, Wang, Chen, Gao, Song et al., Influenza A virus NS1 induces G0/G1 cell cycle arrest by inhibiting the expression and activity of RhoA protein, J Virol
Khosravi-Far, Der, The Ras signal transduction pathway, Cancer Metastasis Rev
Kralj, Jukic, Bren, Bagchi, Commercial SARS-CoV-2 targeted, protease inhibitor focused and protein-protein interaction inhibitor focused molecular libraries for virtual screening and drug design, Adv Protein Chem Struct Biol
Krishnan, Zeichner, Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency, J Virol
Kumar, Barua, Tripathi, Kumar, Role of ROCK signaling in virus replication, Virus Res
Kumar, Chander, Khandelwal, Verma, Rawat et al., ROCK1/MLC2 inhibition induces decay of viral mRNA in BPXV infected cells, Sci Rep
Kumar, Chandran, KSHV entry and trafficking in target cells-hijacking of cell signal pathways, actin and membrane dynamics, Viruses
Kumar, Mishra, Kumar, Raut, Sato et al., Essential role of Rnd1 in innate immunity during viral and bacterial infections, Cell Death Dis
Kumar, Sharma, Kumar, Tripathi, Barua et al., Host-directed antiviral therapy, Clin Microbiol Rev
Kunschmann, Puder, Fischer, Steffen, Rottner et al., The small GTPase Rac1 increases cell surface stiffness and enhances 3D migration into extracellular matrices, Sci Rep
Kuroki, Hatta, Natsume, Sakai, Yagi et al., ARHGAP1 transported with influenza viral genome ensures integrity of viral particle surface through efficient budozone formation, MBio
Lamote, Glorieux, Nauwynck, Favoreel, The US3 protein of pseudorabies virus drives viral passage across the basement membrane in porcine respiratory mucosa explants, J Virol
Langedijk, Bont, Respiratory syncytial virus infection and novel interventions, Nat Rev Microbiol
Lee, Choi, Lee, Yoon, Kim et al., Hepatocyte growth factor-dependent antiviral activity of activated cdc42-associated kinase 1 against hepatitis B virus, Front Microbiol
Lee, Moon, Pak, Kim, Lee et al., HA1077 displays synergistic activity with daclatasvir against hepatitis C virus and suppresses the emergence of NS5A resistance-associated substitutions in mice, Sci Rep
Li, Stupack, Bokoch, Nemerow, Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases, J Virol
Li, Wang, Guo, Zhao, CDC42 regulates cell proliferation and apoptosis in bladder cancer via the IQGAP3mediated Ras/ERK pathway, Biochem Genet
Li, Wang, Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis, Int J Biol Sci
Li, Wang, Wang, Tang, Wan et al., RhoA suppresses pseudorabies virus replication in vitro, Virol J
Li, Yang, Liu, Meng, Li et al., Kaposi's sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus, J Virol
Li, Zhou, Li, Pan, Guo et al., Comprehensive characterization of human-virus protein-protein interactions reveals disease comorbidities and potential antiviral drugs, Comput Struct Biotechnol J
Lin, Chang, Chen, Shih, RhoA/ROCK1 regulates Avian Reovirus S1133-induced switch from autophagy to apoptosis, BMC Vet Res
Linden, Jones, Potential multi-modal effects of provirus integration on HIV-1 persistence: lessons from other viruses, Trends Immunol
Linfield, Gao, Raduka, Harford, Piedimonte et al., RSV attenuates epithelial cell restitution by inhibiting actin cytoskeleton-dependent cell migration, Am J Physiol Lung Cell Mol Physiol
Liu, Cohen, The role of PI3K/Akt in human herpesvirus infection: from the bench to the bedside, Virology
Liu, Jiang, Zhou, Zhao, Li et al., Dasabuvir suppresses esophageal squamous cell carcinoma growth in vitro and in vivo through targeting ROCK1, Cell Death Dis
Liu, Lin, Wang, Hsu, Liao et al., Activation of small GTPases RhoA and Rac1 is required for avian reovirus p10-induced syncytium formation, Mol Cells
Liu, Xu, Zhang, Wang, Guo et al., RABV induces biphasic actin cytoskeletal rearrangement through Rac1 activity modulation, J Virol
Lou, Liu, Bai, Cheng, Zhang et al., Kinesin-1 regulates endocytic trafficking of classical swine fever virus along acetylated microtubules, J Virol
Lu, Wu, Plemenitas, Yu, Sawai et al., CDC42 and Rac1 are implicated in the activation of the Nefassociated kinase and replication of HIV-1, Curr Biol
Lucera, Fleissner, Tabler, Schlatzer, Troyer et al., HIV signaling through CD4 and CCR5 activates Rho family GTPases that are required for optimal infection of primary CD4+ T cells, Retrovirology
Lv, Li, Guan, Hu, Zhang et al., Porcine hemagglutinating encephalomyelitis virus activation of the integrin alpha5beta1-FAK-Cofilin pathway causes cytoskeletal rearrangement to promote its invasion of N2a cells, J Virol
Ma, Cavallin, Leung, Chiozzini, Goldschmidt-Clermont et al., A role for virally induced reactive oxygen species in Kaposi's sarcoma herpesvirus tumorigenesis, Antioxid Redox Signal
Ma, Yasunaga, Shimura, Takemoto, Watanabe et al., Human retroviral antisense mRNAs are retained in the nuclei of infected cells for viral persistence, Proc Natl Acad Sci
Maldonado, Medina, Velazquez, Dharmawardhane, Targeting Rac and Cdc42 GEFs in metastatic cancer, Front Cell Dev Biol
Malhi, Norris, Duan, Moraes, Maynes, Statin-mediated disruption of Rho GTPase prenylation and activity inhibits respiratory syncytial virus infection, Commun Biol
Manukyan, Nalbant, Luxen, Hahn, Knaus, RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B, J Immunol
Martin, Reimann, Fritz, Ryu, Jeon et al., Spatio-temporal co-ordination of RhoA, Rac1 and Cdc42 activation during prototypical edge protrusion and retraction dynamics, Sci Rep
Matoba, Kawanami, Tsukamoto, Kinoshita, Ito et al., Rho-kinase regulation of TNF-alphainduced nuclear translocation of NF-kappaB RelA/p65 and M-CSF expression via p38 MAPK in mesangial cells, Am J Physiol Renal Physiol
Matos, Rehfeld, Guedes, Lobato, Bovine vaccinia: insights into the disease in cattle, Viruses
Messias, Loss-Morais, Carvalho, Gonzalez, Cunha et al., Zika virus targets the human thymic epithelium, Sci Rep
Miranda-Saksena, Denes, Diefenbach, Cunningham, Infection and transport of herpes simplex virus type 1 in neurons: role of the cytoskeleton, Viruses
Mitchell, Hobbs, Aghajanian, Campbell, Redox regulation of Ras and Rho GTPases: mechanism and function, Antioxid Redox Signal
Montazersaheb, Khatibi, Hejazi, Tarhriz, Farjami et al., COVID-19 infection: an overview on cytokine storm and related interventions, Virol J
Mosaddeghzadeh, Ahmadian, The RHO family GTPases: mechanisms of regulation and signaling, Cells
Mosaddeghzadeh, Jasemi, Majolee, Zhang, Hordijk et al., Electrostatic forces mediate the specificity of RHO GTPase-GDI interactions, Int J Mol Sci
Mufti, Sufyan, Shahid, Alzahrani, Shahzad et al., Computer-aided identification of dengue virus NS2B/NS3 protease inhibitors: an integrated molecular modelling approach for screening of phytochemicals, J Biomol Struct Dyn
Myster, Palmeira, Sorel, Bouillenne, Depauw et al., Viral semaphorin inhibits dendritic cell phagocytosis and migration but is not essential for gammaherpesvirus-induced lymphoproliferation in malignant catarrhal fever, J Virol
Nakano, Nishikawa, Asaoka, Ishikawa, Ohwaki et al., DBS is activated by EPHB2/SRC signalingmediated tyrosine phosphorylation in HEK293 cells, Mol Cell Biochem
Narumiya, Thumkeo, Rho signaling research: history, current status and future directions, FEBS Lett
Nguyen, Ralbovska, Kugler, RhoBTB proteins regulate the hippo pathway by antagonizing ubiquitination of LKB1, GBethesda
Nie, Hui, Guo, Yang, Huang et al., Rearrangement of actin cytoskeleton by Zika virus infection facilitates blood-testis barrier hyperpermeability, Virol Sin
Nishi, Saigo, Cellular internalization of green fluorescent protein fused with herpes simplex virus protein VP22 via a lipid raft-mediated endocytic pathway independent of caveolae and Rho family GTPases but dependent on dynamin and Arf6, J Biol Chem
Ocaranza, Moya, Jalil, Lavandero, Kalergis et al., Rho-kinase pathway activation and apoptosis in circulating leucocytes in patients with heart failure with reduced ejection fraction, J Cell Mol Med
Pancione, Cerulo, Remo, Giordano, Gutierrez-Uzquiza et al., Centrosome dynamics and its role in inflammatory response and metastatic process, Biomolecules
Patel, Kukol, Integrating molecular modelling methods to advance influenza A virus drug discovery, Drug Discov Today
Peng, Yang, Li, Shi, Luan et al., Exosome and virus infection, Front Immunol
Pochynyuk, Stockand, Staruschenko, Ion channel regulation by Ras, Rho, and Rab small GTPases, Exp Biol Med
Qu, Li, Zhang, He, Liu et al., AKR1B10 promotes breast cancer cell proliferation and migration via the PI3K/AKT/NF-kappaB signaling pathway, Cell Biosci
Quetglas, Hernaez, Galindo, Munoz-Moreno, Cuesta-Geijo, Small rho GTPases and cholesterol biosynthetic pathway intermediates in African swine fever virus infection, J Virol
Raghu, Sharma-Walia, Veettil, Sadagopan, Caballero et al., Lipid rafts of primary endothelial cells are essential for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry, J Virol
Rahman, Saha, Islam, Suman, Biswas et al., Virtual screening, molecular dynamics and structure-activity relationship studies to identify potent approved drugs for Covid-19 treatment, J Biomol Struct Dyn
Rajan, Kudryashov, Reisler, Actin bundles dynamics and architecture, Biomolecules
Richerioux, Blondeau, Wiedemann, Remy, Vautherot et al., Rho-ROCK and Rac-PAK signaling pathways have opposing effects on the cell-to-cell spread of Marek's Disease Virus, PLoS ONE
Rodenburg, Van Buul, Rho GTPase signalling networks in cancer cell transendothelial migration, Vasc Biol
Rojas, Fuentes, Rausell, Valencia, The Ras protein superfamily: evolutionary tree and role of conserved amino acids, J Cell Biol
Sala, Oakes, Stress fiber strain recognition by the LIM protein testin is cryptic and mediated by RhoA, Mol Biol Cell
Salloum, Jaafar, El-Sibai, Rho A and Rac1: antagonists moving forward, Tissue Cell
Sastre, Montoro, Galvez-Martin, Lacerda, Lucia et al., Small GTPases of the Ras and Rho families switch on/off signaling pathways in neurodegenerative diseases, Int J Mol Sci
Semblat, Doerig, PAK in pathogen-host interactions, Cell Logist
Senoo, Kamimura, Kimura, Nakajima, Sawai et al., Phosphorylated Rho-GDP directly activates mTORC2 kinase towards AKT through dimerization with Ras-GTP to regulate cell migration, Nat Cell Biol
Sharif, Baek, Naveed, Stalin, Kang et al., Porcine sapovirus-induced tight junction dissociation via activation of RhoA/ROCK/MLC signaling pathway, J Virol
Sharma, Marin, Wu, Prikryl, Melikyan, Human immunodeficiency virus 1 preferentially fuses with pHneutral endocytic vesicles in cell lines and human primary CD4+ T-cells, ACS Nano
Shinn, Chen, Ferrer, Itkin, Klumpp-Thomas et al., High-throughput screening for drug combinations, Methods Mol Biol
Simpson, Yamauchi, Microtubules in influenza virus entry and egress, Viruses
Soriano, Alcon-Perez, Manzanares, Castellano, The crossroads between RAS and RHO signaling pathways in cellular transformation, motility and contraction, Genes
Stella, Turville, All-round manipulation of the actin cytoskeleton by HIV, Viruses
Stoletov, Gong, Terman, Nck and Crk mediate distinct VEGF-induced signaling pathways that serve overlapping functions in focal adhesion turnover and integrin activation, Exp Cell Res
Su, Chen, Qi, Shi, Feng et al., A mini-review on cell cycle regulation of coronavirus infection, Front Vet Sci
Sugrue, Tan, Defining the assembleome of the respiratory syncytial virus, Subcell Biochem
Suttitheptumrong, Mahutchariyakul, Rawarak, Reamtong, Boonnak et al., Altered moesin and actin cytoskeleton protein rearrangements affect transendothelial permeability in human endothelial cells upon dengue virus infection and TNF-alpha treatment, Viruses
Tang, Guo, Lin, Shi, Qian et al., hCLOCK causes rho-kinase-mediated endothelial dysfunction and NF-kappaB-mediated inflammatory responses, Oxid Med Cell Longev
Thomas, Mariani-Floderer, Lopez-Huertas, Gros, Hamard-Peron et al., Involvement of the Rac1-IRSp53-Wave2-Arp2/3 signaling pathway in HIV-1 gag particle release in CD4 T cells, J Virol
Tomas, Yermen, Regazzi, Pessin, Halban, Regulation of insulin secretion by phosphatidylinositol-4,5-bisphosphate, Traffic
Trejo-Cerro, Aguilar-Hernandez, Silva-Ayala, Lopez, Arias, The actin cytoskeleton is important for rotavirus internalization and RNA genome replication, Virus Res
Tripathi, Grant, Qian, Zhou, Mertins et al., Receptor tyrosine kinase activation of RhoA is mediated by AKT phosphorylation of DLC1, J Cell Biol
Tseliou, Al-Qahtani, Alarifi, Alkahtani, Stournaras et al., The role of RhoA, RhoB and RhoC GTPases in cell morphology, proliferation and migration in human cytomegalovirus (HCMV) infected glioblastoma cells, Cell Physiol Biochem
Upasani, Vo, Ung, Heng, Laurent et al., Impaired antibody-independent immune response of B cells in patients with acute dengue infection, Front Immunol
Valderrama, Cordeiro, Schleich, Frischknecht, Way, Vaccinia virus-induced cell motility requires F11Lmediated inhibition of RhoA signaling, Science
Van Den Broeke, Favoreel, Actin' up: herpesvirus interactions with Rho GTPase signaling, Viruses
Van Den Broeke, Jacob, Favoreel, Rho'ing in and out of cells: viral interactions with Rho GTPase signaling, Small GTPases
Wang, Burckhardt, Yakimovich, Greber, Imaging, tracking and computational analyses of virus entry and egress with the cytoskeleton, Viruses
Wang, Chen, Xiao, Han, Wang et al., Cylindrospermopsin induces abnormal vascular development through impairing cytoskeleton and promoting vascular endothelial cell apoptosis by the Rho/ROCK signaling pathway, Environ Res
Wang, Jiang, Li, Yao, Wang, Identification of key proteins of cytopathic biotype bovine viral diarrhoea virus involved in activating NF-kappaB pathway in BVDV-induced inflammatory response, Virulence
Wang, Sun, Liang, Liu, Zhao et al., GPER stabilizes F-actin cytoskeleton and activates TAZ via PLCbeta-PKC and Rho/ROCK-LIMK-Cofilin pathway, Biochem Biophys Res Commun
Wang, Zhang, Chen, Xu, Gao et al., Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection, PLoS Negl Trop Dis
Wennerberg, Forget, Ellerbroek, Arthur, Burridge et al., Rnd proteins function as RhoA antagonists by activating p190 RhoGAP, Curr Biol
Whitehead, Zohn, Der, Rho GTPase-dependent transformation by G protein-coupled receptors, Oncogene
Wissing, Bruggemann, Steinmann, Todt, Virus-host cell interplay during hepatitis E virus infection, Trends Microbiol
Wu, Gu, Shen, Jia, Yin et al., The role of host cell Rab GTPases in influenza A virus infections, Future Microbiol
Wu, Yin, Jiang, Xu, Structure genomics of SARS-CoV-2 and its Omicron variant: drug design templates for COVID-19, Acta Pharmacol Sin
Xiang, Li, Ma, Yue, Lu et al., HCF-1 promotes cell cycle progression by regulating the expression of CDC42, Cell Death Dis
Xu, He, Chen, Hou, Fan et al., Different types of effective fractions from Radix Isatidis revealed a multiple-target synergy effect against respiratory syncytial virus through RIG-I and MDA5 signaling pathways, a pilot study to testify the theory of superposition of traditional Chinese Medicine efficacy, J Ethnopharmacol
Xu, Qi, Luo, Yang, Xie et al., Hepatitis B virus X protein stimulates proliferation, wound closure and inhibits apoptosis of HuH-7 cells via CDC42, Int J Mol Sci
Xu, Ren, Xie, Zhang, Hu et al., Rac2 mediate foam cell formation and associated immune responses in THP-1 to promote the process of atherosclerotic plaques, Mol Immunol
Xu, Zhou, Chen, Wu, Wang et al., Immediate early response protein 2 promotes the migration and invasion of hepatocellular carcinoma cells via regulating the activity of Rho GTPases, Neoplasma
Yang, Harding, Sweeney, Miao, Swan et al., Control of antiviral innate immune response by protein geranylgeranylation, Sci Adv
Yang, Kotomura, Ho, Zhi, Bixler et al., Complex cell cycle abnormalities caused by human T-lymphotropic virus type 1 Tax, J Virol
Yau, Yang, Chen, Umstead, Stanley et al., SP-R210 isoforms of Myosin18A modulate endosomal sorting and recognition of influenza A virus infection in macrophages, Microbes Infect
Ye, Han, Yu, Zhang, Wang et al., Infectious bursal disease virus activates c-Src to promote alpha4beta1 integrin-dependent viral entry by modulating the downstream Akt-RhoA GTPase-actin rearrangement cascade, J Virol
Ye, Wei, Wei, Zhang, Sun et al., RKIP suppresses the influenza A virus-induced airway inflammatory response via the ERK/MAPK pathway, Int J Mol Med
Yi, Kim, Jang, Lee, Ahn et al., Cellular signals involved in cyclooxygenase-2 expression induced by human cytomegalovirus, Virus Res
Yin, Chen, Hakim, Wang, Xu et al., 6-Thioguanine inhibits rotavirus replication through suppression of Rac1 GDP/GTP cycling, Antiviral Res
Yin, Huang, Petela, Jiang, Zhang et al., Targeting small GTPases: emerging grasps on previously untamable targets, pioneered by KRAS, Signal Transduct Target Ther
Yoneda, Hyun, Jakubski, Saito, Nakajima et al., Hepatitis B virus and DNA stimulation trigger a rapid innate immune response through NF-kappaB, J Immunol
Yu, Sun, Goie, Zhang, Regulation of host immune responses against influenza A virus infection by mitogen-activated protein kinases (MAPKs), Microorganisms
Yu, Wang, Wang, Recent advances in application of computer-aided drug design in anti-influenza A virus drug discovery, Int J Mol Sci
Zhang, Fan, Luo, Wang, Wang, Avian hepatitis E virus ORF2 protein interacts with rap1b to induce cytoskeleton rearrangement that facilitates virus internalization, Microbiol Spectr
Zhong, Hennig, Toborek, Intact lipid rafts regulate HIV-1 Tat protein-induced activation of the Rho signaling and upregulation of P-glycoprotein in brain endothelial cells, J Cereb Blood Flow Metab
Zhou, Jiang, Liu, Liu, Liang, Virus infection and death receptor-mediated apoptosis, Viruses
Zhu, Li, Du, Wang, Cao et al., Foot-and-mouth disease virus infection inhibits LGP2 protein expression to exaggerate inflammatory response and promote viral replication, Cell Death Dis
DOI record:
{
"DOI": "10.1186/s11658-025-00722-w",
"ISSN": [
"1689-1392"
],
"URL": "http://dx.doi.org/10.1186/s11658-025-00722-w",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Ras homolog gene family-guanosine triphosphatases (Rho-GTPases), key molecular switches regulating cytoskeletal dynamics and cellular signaling, play a pivotal role in viral infections by modulating critical processes such as viral entry, replication, and release. This review elucidates the intricate mechanisms through which Rho-GTPases, via interactions with guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and other signaling pathways, including the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), rat sarcoma (Ras), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways, facilitate viral pathogenesis. Specific viruses, such as influenza A virus (IAV), herpesviruses, human immunodeficiency virus (HIV), and respiratory syncytial virus (RSV), exploit Rho-GTPase-mediated cytoskeletal reorganization to enhance infectivity. For example, Rho-GTPases promote actin remodeling and membrane fusion, which are essential for viral entry and intracellular transport. Furthermore, Rho-GTPases modulate immune responses, often suppressing antiviral defenses to favor viral replication. Despite these insights, the molecular mechanisms underlying Rho-GTPase regulation during viral infections remain incompletely understood. Future research should focus on delineating the precise roles of Rho-GTPases in distinct viral life cycles, uncovering novel regulatory mechanisms, and developing targeted antiviral therapies that selectively inhibit Rho-GTPase signaling without compromising host cell functions. Such advancements could pave the way for broad-spectrum antiviral strategies, particularly against viruses that heavily rely on cytoskeletal manipulation for infection.</jats:p>",
"alternative-id": [
"722"
],
"article-number": "55",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "31 October 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "27 March 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "2 May 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no competing interest."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-7022-5403",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Beibei",
"sequence": "first"
},
{
"affiliation": [],
"family": "Li",
"given": "Shuli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ding",
"given": "Juntao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Jingxia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Zhenghai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duan",
"given": "Hong",
"sequence": "additional"
}
],
"container-title": "Cellular & Molecular Biology Letters",
"container-title-short": "Cell Mol Biol Lett",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T15:24:43Z",
"timestamp": 1746199483000
},
"deposited": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T16:03:48Z",
"timestamp": 1746201828000
},
"funder": [
{
"name": "Tianchi Talent Youth of Doctoral Talent Program in Xinjiang Uygur Autonomous Region"
},
{
"award": [
"2022D01C697"
],
"name": "Youth Fund of the Natural Science Foundation of Xinjiang Uygur Autonomous Region"
}
],
"indexed": {
"date-parts": [
[
2025,
5,
3
]
],
"date-time": "2025-05-03T04:09:08Z",
"timestamp": 1746245348812,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
5,
2
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T00:00:00Z",
"timestamp": 1746144000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T00:00:00Z",
"timestamp": 1746144000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s11658-025-00722-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s11658-025-00722-w/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s11658-025-00722-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
5,
2
]
]
},
"published-online": {
"date-parts": [
[
2025,
5,
2
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.3390/cells10071831",
"author": "N Mosaddeghzadeh",
"doi-asserted-by": "publisher",
"first-page": "1831",
"issue": "7",
"journal-title": "Cells",
"key": "722_CR1",
"unstructured": "Mosaddeghzadeh N, Ahmadian MR. The RHO family GTPases: mechanisms of regulation and signaling. Cells. 2021;10(7):1831.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.4161/sgtp.28318",
"author": "C Van den Broeke",
"doi-asserted-by": "publisher",
"journal-title": "Small GTPases",
"key": "722_CR2",
"unstructured": "Van den Broeke C, Jacob T, Favoreel HW. Rho’ing in and out of cells: viral interactions with Rho GTPase signaling. Small GTPases. 2014;5: e28318.",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.1515/hsz-2013-0181",
"author": "E Amin",
"doi-asserted-by": "publisher",
"first-page": "1399",
"issue": "11",
"journal-title": "Biol Chem",
"key": "722_CR3",
"unstructured": "Amin E, Dubey BN, Zhang SC, Gremer L, Dvorsky R, Moll JM, et al. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394(11):1399–410.",
"volume": "394",
"year": "2013"
},
{
"DOI": "10.1016/j.virusres.2023.199105",
"author": "R Kumar",
"doi-asserted-by": "publisher",
"journal-title": "Virus Res",
"key": "722_CR4",
"unstructured": "Kumar R, Barua S, Tripathi BN, Kumar N. Role of ROCK signaling in virus replication. Virus Res. 2023;329: 199105.",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1038/s42003-021-02754-2",
"author": "M Malhi",
"doi-asserted-by": "publisher",
"first-page": "1239",
"issue": "1",
"journal-title": "Commun Biol",
"key": "722_CR5",
"unstructured": "Malhi M, Norris MJ, Duan W, Moraes TJ, Maynes JT. Statin-mediated disruption of Rho GTPase prenylation and activity inhibits respiratory syncytial virus infection. Commun Biol. 2021;4(1):1239.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1083/jcb.201103008",
"author": "AM Rojas",
"doi-asserted-by": "publisher",
"first-page": "189",
"issue": "2",
"journal-title": "J Cell Biol",
"key": "722_CR6",
"unstructured": "Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol. 2012;196(2):189–201.",
"volume": "196",
"year": "2012"
},
{
"DOI": "10.1159/000438612",
"author": "M Tseliou",
"doi-asserted-by": "publisher",
"first-page": "94",
"issue": "1",
"journal-title": "Cell Physiol Biochem",
"key": "722_CR7",
"unstructured": "Tseliou M, Al-Qahtani A, Alarifi S, Alkahtani SH, Stournaras C, Sourvinos G. The role of RhoA, RhoB and RhoC GTPases in cell morphology, proliferation and migration in human cytomegalovirus (HCMV) infected glioblastoma cells. Cell Physiol Biochem. 2016;38(1):94–109.",
"volume": "38",
"year": "2016"
},
{
"DOI": "10.1016/j.molimm.2023.10.004",
"author": "L Xu",
"doi-asserted-by": "publisher",
"first-page": "196",
"journal-title": "Mol Immunol",
"key": "722_CR8",
"unstructured": "Xu L, Ren H, Xie D, Zhang F, Hu X, Fang S, et al. Rac2 mediate foam cell formation and associated immune responses in THP-1 to promote the process of atherosclerotic plaques. Mol Immunol. 2023;163:196–206.",
"volume": "163",
"year": "2023"
},
{
"DOI": "10.1038/s41598-019-43975-0",
"author": "T Kunschmann",
"doi-asserted-by": "publisher",
"first-page": "7675",
"issue": "1",
"journal-title": "Sci Rep",
"key": "722_CR9",
"unstructured": "Kunschmann T, Puder S, Fischer T, Steffen A, Rottner K, Mierke CT. The small GTPase Rac1 increases cell surface stiffness and enhances 3D migration into extracellular matrices. Sci Rep. 2019;9(1):7675.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1074/jbc.M605560200",
"author": "CV Finkielstein",
"doi-asserted-by": "publisher",
"first-page": "27317",
"issue": "37",
"journal-title": "J Biol Chem",
"key": "722_CR10",
"unstructured": "Finkielstein CV, Overduin M, Capelluto DG. Cell migration and signaling specificity is determined by the phosphatidylserine recognition motif of Rac1. J Biol Chem. 2006;281(37):27317–26.",
"volume": "281",
"year": "2006"
},
{
"DOI": "10.1002/1873-3468.13087",
"author": "S Narumiya",
"doi-asserted-by": "publisher",
"first-page": "1763",
"issue": "11",
"journal-title": "FEBS Lett",
"key": "722_CR11",
"unstructured": "Narumiya S, Thumkeo D. Rho signaling research: history, current status and future directions. FEBS Lett. 2018;592(11):1763–76.",
"volume": "592",
"year": "2018"
},
{
"DOI": "10.3389/fmicb.2021.775083",
"author": "M Fan",
"doi-asserted-by": "publisher",
"journal-title": "Front Microbiol",
"key": "722_CR12",
"unstructured": "Fan M, Luo Y, Zhang B, Wang J, Chen T, Liu B, et al. Cell division control protein 42 interacts with hepatitis E virus capsid protein and participates in hepatitis E virus infection. Front Microbiol. 2021;12: 775083.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1128/spectrum.02265-21",
"author": "B Zhang",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Microbiol Spectr",
"key": "722_CR13",
"unstructured": "Zhang B, Fan M, Fan J, Luo Y, Wang J, Wang Y, et al. Avian hepatitis E virus ORF2 protein interacts with rap1b to induce cytoskeleton rearrangement that facilitates virus internalization. Microbiol Spectr. 2022;10(1): e0226521.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1007/s10528-022-10223-6",
"author": "G Li",
"doi-asserted-by": "publisher",
"first-page": "2383",
"issue": "6",
"journal-title": "Biochem Genet",
"key": "722_CR14",
"unstructured": "Li G, Wang Y, Guo XB, Zhao B. CDC42 regulates cell proliferation and apoptosis in bladder cancer via the IQGAP3-mediated Ras/ERK pathway. Biochem Genet. 2022;60(6):2383–98.",
"volume": "60",
"year": "2022"
},
{
"DOI": "10.1038/s41419-020-03094-5",
"author": "P Xiang",
"doi-asserted-by": "publisher",
"first-page": "907",
"issue": "10",
"journal-title": "Cell Death Dis",
"key": "722_CR15",
"unstructured": "Xiang P, Li F, Ma Z, Yue J, Lu C, You Y, et al. HCF-1 promotes cell cycle progression by regulating the expression of CDC42. Cell Death Dis. 2020;11(10):907.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1080/21541248.2020.1829914",
"author": "S Basbous",
"doi-asserted-by": "publisher",
"first-page": "336",
"issue": "5–6",
"journal-title": "Small GTPases",
"key": "722_CR16",
"unstructured": "Basbous S, Azzarelli R, Pacary E, Moreau V. Pathophysiological functions of Rnd proteins. Small GTPases. 2021;12(5–6):336–57.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/S0960-9822(03)00418-4",
"author": "K Wennerberg",
"doi-asserted-by": "publisher",
"first-page": "1106",
"issue": "13",
"journal-title": "Curr Biol",
"key": "722_CR17",
"unstructured": "Wennerberg K, Forget MA, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, et al. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr Biol. 2003;13(13):1106–15.",
"volume": "13",
"year": "2003"
},
{
"author": "TH Nguyen",
"first-page": "1319",
"issue": "4",
"journal-title": "GBethesda.",
"key": "722_CR18",
"unstructured": "Nguyen TH, Ralbovska A, Kugler JM. RhoBTB proteins regulate the hippo pathway by antagonizing ubiquitination of LKB1. GBethesda. 2020;10(4):1319–25.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1042/BCJ20190203",
"author": "RB Haga",
"doi-asserted-by": "publisher",
"first-page": "2499",
"issue": "17",
"journal-title": "Biochem J",
"key": "722_CR19",
"unstructured": "Haga RB, Garg R, Collu F, Borda D’Agua B, Menendez ST, Colomba A, et al. RhoBTB1 interacts with ROCKs and inhibits invasion. Biochem J. 2019;476(17):2499–514.",
"volume": "476",
"year": "2019"
},
{
"DOI": "10.3390/v3040278",
"author": "C Van den Broeke",
"doi-asserted-by": "publisher",
"first-page": "278",
"issue": "4",
"journal-title": "Viruses",
"key": "722_CR20",
"unstructured": "Van den Broeke C, Favoreel HW. Actin’ up: herpesvirus interactions with Rho GTPase signaling. Viruses. 2011;3(4):278–92.",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1016/j.tim.2020.07.002",
"author": "MH Wissing",
"doi-asserted-by": "publisher",
"first-page": "309",
"issue": "4",
"journal-title": "Trends Microbiol",
"key": "722_CR21",
"unstructured": "Wissing MH, Bruggemann Y, Steinmann E, Todt D. Virus-host cell interplay during hepatitis E virus infection. Trends Microbiol. 2021;29(4):309–19.",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1016/j.meegid.2019.01.018",
"author": "A Agrelli",
"doi-asserted-by": "publisher",
"first-page": "22",
"journal-title": "Infect Genet Evol",
"key": "722_CR22",
"unstructured": "Agrelli A, de Moura RR, Crovella S, Brandao LAC. ZIKA virus entry mechanisms in human cells. Infect Genet Evol. 2019;69:22–9.",
"volume": "69",
"year": "2019"
},
{
"DOI": "10.3389/fimmu.2023.1154217",
"author": "Y Peng",
"doi-asserted-by": "publisher",
"first-page": "1154217",
"journal-title": "Front Immunol",
"key": "722_CR23",
"unstructured": "Peng Y, Yang Y, Li Y, Shi T, Luan Y, Yin C. Exosome and virus infection. Front Immunol. 2023;14:1154217.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41564-022-01164-2",
"author": "D Chmielewski",
"doi-asserted-by": "publisher",
"first-page": "1270",
"issue": "8",
"journal-title": "Nat Microbiol",
"key": "722_CR24",
"unstructured": "Chmielewski D, Schmid MF, Simmons G, Jin J, Chiu W. Chikungunya virus assembly and budding visualized in situ using cryogenic electron tomography. Nat Microbiol. 2022;7(8):1270–9.",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.3390/v9110316",
"author": "X Zhou",
"doi-asserted-by": "publisher",
"first-page": "316",
"issue": "11",
"journal-title": "Viruses",
"key": "722_CR25",
"unstructured": "Zhou X, Jiang W, Liu Z, Liu S, Liang X. Virus infection and death receptor-mediated apoptosis. Viruses. 2017;9(11):316.",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1128/mbio.00721-22",
"author": "T Kuroki",
"doi-asserted-by": "publisher",
"first-page": "e0072122",
"issue": "3",
"journal-title": "MBio",
"key": "722_CR26",
"unstructured": "Kuroki T, Hatta T, Natsume T, Sakai N, Yagi A, Kato K, et al. ARHGAP1 transported with influenza viral genome ensures integrity of viral particle surface through efficient budozone formation. MBio. 2022;13(3):e0072122.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1093/jxb/erab408",
"author": "H Han",
"doi-asserted-by": "publisher",
"first-page": "366",
"issue": "1",
"journal-title": "J Exp Bot",
"key": "722_CR27",
"unstructured": "Han H, Zou J, Zhou J, Zeng M, Zheng D, Yuan X, et al. The small GTPase NtRHO1 negatively regulates tobacco defense response to tobacco mosaic virus by interacting with NtWRKY50. J Exp Bot. 2022;73(1):366–81.",
"volume": "73",
"year": "2022"
},
{
"DOI": "10.1016/j.virusres.2018.08.013",
"author": "AM Cuartas-Lopez",
"doi-asserted-by": "publisher",
"first-page": "153",
"journal-title": "Virus Res",
"key": "722_CR28",
"unstructured": "Cuartas-Lopez AM, Hernandez-Cuellar CE, Gallego-Gomez JC. Disentangling the role of PI3K/Akt, Rho GTPase and the actin cytoskeleton on dengue virus infection. Virus Res. 2018;256:153–65.",
"volume": "256",
"year": "2018"
},
{
"DOI": "10.1128/JVI.01577-16",
"author": "JAS Lamote",
"doi-asserted-by": "publisher",
"first-page": "10945",
"issue": "23",
"journal-title": "J Virol",
"key": "722_CR29",
"unstructured": "Lamote JAS, Glorieux S, Nauwynck HJ, Favoreel HW. The US3 protein of pseudorabies virus drives viral passage across the basement membrane in porcine respiratory mucosa explants. J Virol. 2016;90(23):10945–50.",
"volume": "90",
"year": "2016"
},
{
"DOI": "10.1128/jvi.00606-24",
"author": "X Liu",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "722_CR30",
"unstructured": "Liu X, Xu J, Zhang M, Wang H, Guo X, Zhao M, et al. RABV induces biphasic actin cytoskeletal rearrangement through Rac1 activity modulation. J Virol. 2024;2024: e0060624.",
"volume": "2024",
"year": "2024"
},
{
"DOI": "10.1089/ars.2012.4584",
"author": "Q Ma",
"doi-asserted-by": "publisher",
"first-page": "80",
"issue": "1",
"journal-title": "Antioxid Redox Signal",
"key": "722_CR31",
"unstructured": "Ma Q, Cavallin LE, Leung HJ, Chiozzini C, Goldschmidt-Clermont PJ, Mesri EA. A role for virally induced reactive oxygen species in Kaposi’s sarcoma herpesvirus tumorigenesis. Antioxid Redox Signal. 2013;18(1):80–90.",
"volume": "18",
"year": "2013"
},
{
"DOI": "10.3389/fvets.2020.586826",
"author": "M Su",
"doi-asserted-by": "publisher",
"journal-title": "Front Vet Sci",
"key": "722_CR32",
"unstructured": "Su M, Chen Y, Qi S, Shi D, Feng L, Sun D. A mini-review on cell cycle regulation of coronavirus infection. Front Vet Sci. 2020;7: 586826.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00086-10",
"author": "L Yang",
"doi-asserted-by": "publisher",
"first-page": "3001",
"issue": "6",
"journal-title": "J Virol",
"key": "722_CR33",
"unstructured": "Yang L, Kotomura N, Ho YK, Zhi H, Bixler S, Schell MJ, et al. Complex cell cycle abnormalities caused by human T-lymphotropic virus type 1 Tax. J Virol. 2011;85(6):3001–9.",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.1126/sciadv.aav7999",
"author": "S Yang",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "Sci Adv",
"key": "722_CR34",
"unstructured": "Yang S, Harding AT, Sweeney C, Miao D, Swan G, Zhou C, et al. Control of antiviral innate immune response by protein geranylgeranylation. Sci Adv. 2019;5(5): eaav7999.",
"volume": "5",
"year": "2019"
},
{
"DOI": "10.3390/genes12060819",
"author": "O Soriano",
"doi-asserted-by": "publisher",
"first-page": "819",
"issue": "6",
"journal-title": "Genes (Basel).",
"key": "722_CR35",
"unstructured": "Soriano O, Alcon-Perez M, Vicente-Manzanares M, Castellano E. The crossroads between RAS and RHO signaling pathways in cellular transformation, motility and contraction. Genes (Basel). 2021;12(6):819.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2007.05.018",
"author": "JL Bos",
"doi-asserted-by": "publisher",
"first-page": "865",
"issue": "5",
"journal-title": "Cell",
"key": "722_CR36",
"unstructured": "Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129(5):865–77.",
"volume": "129",
"year": "2007"
},
{
"DOI": "10.1038/s41392-023-01441-4",
"author": "G Yin",
"doi-asserted-by": "publisher",
"first-page": "212",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "722_CR37",
"unstructured": "Yin G, Huang J, Petela J, Jiang H, Zhang Y, Gong S, et al. Targeting small GTPases: emerging grasps on previously untamable targets, pioneered by KRAS. Signal Transduct Target Ther. 2023;8(1):212.",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1152/physrev.00045.2020",
"author": "E Crosas-Molist",
"doi-asserted-by": "publisher",
"first-page": "455",
"issue": "1",
"journal-title": "Physiol Rev",
"key": "722_CR38",
"unstructured": "Crosas-Molist E, Samain R, Kohlhammer L, Orgaz JL, George SL, Maiques O, et al. Rho GTPase signaling in cancer progression and dissemination. Physiol Rev. 2022;102(1):455–510.",
"volume": "102",
"year": "2022"
},
{
"DOI": "10.1530/VB-21-0008",
"author": "WS Rodenburg",
"doi-asserted-by": "publisher",
"first-page": "R77",
"issue": "1",
"journal-title": "Vasc Biol",
"key": "722_CR39",
"unstructured": "Rodenburg WS, van Buul JD. Rho GTPase signalling networks in cancer cell transendothelial migration. Vasc Biol. 2021;3(1):R77–95.",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.7150/ijbs.44943",
"author": "X Li",
"doi-asserted-by": "publisher",
"first-page": "2014",
"issue": "12",
"journal-title": "Int J Biol Sci",
"key": "722_CR40",
"unstructured": "Li X, Wang J. Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis. Int J Biol Sci. 2020;16(12):2014–28.",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.3390/cancers12123709",
"author": "TM Grzywa",
"doi-asserted-by": "publisher",
"first-page": "3709",
"issue": "12",
"journal-title": "Cancers (Basel).",
"key": "722_CR41",
"unstructured": "Grzywa TM, Klicka K, Wlodarski PK. Regulators at every step-how microRNAs drive tumor cell invasiveness and metastasis. Cancers (Basel). 2020;12(12):3709.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/biom11050629",
"author": "M Pancione",
"doi-asserted-by": "publisher",
"first-page": "629",
"issue": "5",
"journal-title": "Biomolecules",
"key": "722_CR42",
"unstructured": "Pancione M, Cerulo L, Remo A, Giordano G, Gutierrez-Uzquiza A, Bragado P, et al. Centrosome dynamics and its role in inflammatory response and metastatic process. Biomolecules. 2021;11(5):629.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41392-020-0134-x",
"author": "J Fares",
"doi-asserted-by": "publisher",
"first-page": "28",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "722_CR43",
"unstructured": "Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5(1):28.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.cytogfr.2022.05.001",
"author": "N Ebrahimi",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "Cytokine Growth Factor Rev",
"key": "722_CR44",
"unstructured": "Ebrahimi N, Kharazmi K, Ghanaatian M, Miraghel SA, Amiri Y, Seyedebrahimi SS, et al. Role of the Wnt and GTPase pathways in breast cancer tumorigenesis and treatment. Cytokine Growth Factor Rev. 2022;67:11–24.",
"volume": "67",
"year": "2022"
},
{
"DOI": "10.1111/jcmm.14819",
"author": "MP Ocaranza",
"doi-asserted-by": "publisher",
"first-page": "1413",
"issue": "2",
"journal-title": "J Cell Mol Med",
"key": "722_CR45",
"unstructured": "Ocaranza MP, Moya J, Jalil JE, Lavandero S, Kalergis AM, Molina C, et al. Rho-kinase pathway activation and apoptosis in circulating leucocytes in patients with heart failure with reduced ejection fraction. J Cell Mol Med. 2020;24(2):1413–27.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1038/s41419-023-05726-y",
"author": "L Fu",
"doi-asserted-by": "publisher",
"first-page": "280",
"issue": "4",
"journal-title": "Cell Death Dis",
"key": "722_CR46",
"unstructured": "Fu L, Wang X, Yang Y, Chen M, Kuerban A, Liu H, et al. Septin11 promotes hepatocellular carcinoma cell motility by activating RhoA to regulate cytoskeleton and cell adhesion. Cell Death Dis. 2023;14(4):280.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3390/biom13030450",
"author": "S Rajan",
"doi-asserted-by": "publisher",
"first-page": "450",
"issue": "3",
"journal-title": "Biomolecules",
"key": "722_CR47",
"unstructured": "Rajan S, Kudryashov DS, Reisler E. Actin bundles dynamics and architecture. Biomolecules. 2023;13(3):450.",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1371/journal.pntd.0000809",
"author": "JL Wang",
"doi-asserted-by": "publisher",
"first-page": "e809",
"issue": "8",
"journal-title": "PLoS Negl Trop Dis",
"key": "722_CR48",
"unstructured": "Wang JL, Zhang JL, Chen W, Xu XF, Gao N, Fan DY, et al. Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection. PLoS Negl Trop Dis. 2010;4(8):e809.",
"volume": "4",
"year": "2010"
},
{
"DOI": "10.3390/v10040166",
"doi-asserted-by": "crossref",
"key": "722_CR49",
"unstructured": "Wang IH, Burckhardt CJ, Yakimovich A, Greber UF. Imaging, tracking and computational analyses of virus entry and egress with the cytoskeleton. Viruses. 2018;10(4)."
},
{
"DOI": "10.3390/genes14020272",
"author": "G Guan",
"doi-asserted-by": "publisher",
"first-page": "272",
"issue": "2",
"journal-title": "Genes (Basel).",
"key": "722_CR50",
"unstructured": "Guan G, Cannon RD, Coates DE, Mei L. Effect of the Rho-Kinase/ROCK signaling pathway on cytoskeleton components. Genes (Basel). 2023;14(2):272.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3390/cells11091559",
"author": "KN Becker",
"doi-asserted-by": "publisher",
"first-page": "1559",
"issue": "9",
"journal-title": "Cells",
"key": "722_CR51",
"unstructured": "Becker KN, Pettee KM, Sugrue A, Reinard KA, Schroeder JL, Eisenmann KM. The cytoskeleton effectors rho-kinase (ROCK) and mammalian diaphanous-related (mDia) formin have dynamic roles in tumor microtube formation in invasive glioblastoma cells. Cells. 2022;11(9):1559.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1091/mbc.E21-03-0156",
"author": "S Sala",
"doi-asserted-by": "publisher",
"first-page": "1758",
"issue": "18",
"journal-title": "Mol Biol Cell",
"key": "722_CR52",
"unstructured": "Sala S, Oakes PW. Stress fiber strain recognition by the LIM protein testin is cryptic and mediated by RhoA. Mol Biol Cell. 2021;32(18):1758–71.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1038/srep21901",
"author": "K Martin",
"doi-asserted-by": "publisher",
"first-page": "21901",
"journal-title": "Sci Rep",
"key": "722_CR53",
"unstructured": "Martin K, Reimann A, Fritz RD, Ryu H, Jeon NL, Pertz O. Spatio-temporal co-ordination of RhoA, Rac1 and Cdc42 activation during prototypical edge protrusion and retraction dynamics. Sci Rep. 2016;6:21901.",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1242/jcs.248385",
"doi-asserted-by": "crossref",
"key": "722_CR54",
"unstructured": "DeWane G, Salvi AM, DeMali KA. Fueling the cytoskeleton—links between cell metabolism and actin remodeling. J Cell Sci. 2021;134(3)."
},
{
"DOI": "10.1016/j.cell.2022.08.011",
"author": "D Acharya",
"doi-asserted-by": "publisher",
"first-page": "3588",
"issue": "19",
"journal-title": "Cell",
"key": "722_CR55",
"unstructured": "Acharya D, Reis R, Volcic M, Liu G, Wang MK, Chia BS, et al. Actin cytoskeleton remodeling primes RIG-I-like receptor activation. Cell. 2022;185(19):3588-602 e21.",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1128/JVI.01891-16",
"doi-asserted-by": "crossref",
"key": "722_CR56",
"unstructured": "Ye C, Han X, Yu Z, Zhang E, Wang L, Liu H. Infectious bursal disease virus activates c-Src to promote alpha4beta1 integrin-dependent viral entry by modulating the downstream Akt-RhoA GTPase-actin rearrangement cascade. J Virol. 2017;91(3)."
},
{
"DOI": "10.3390/v8110305",
"doi-asserted-by": "crossref",
"key": "722_CR57",
"unstructured": "Kumar B, Chandran B. KSHV entry and trafficking in target cells-hijacking of cell signal pathways, actin and membrane dynamics. Viruses. 2016;8(11)."
},
{
"DOI": "10.3390/ijms23010578",
"doi-asserted-by": "crossref",
"key": "722_CR58",
"unstructured": "Hornikova L, Brustikova K, Huerfano S, Forstova J. Nuclear cytoskeleton in virus infection. Int J Mol Sci. 2022;23(1)."
},
{
"DOI": "10.1016/j.virusres.2019.01.003",
"author": "O Trejo-Cerro",
"doi-asserted-by": "publisher",
"first-page": "27",
"journal-title": "Virus Res",
"key": "722_CR59",
"unstructured": "Trejo-Cerro O, Aguilar-Hernandez N, Silva-Ayala D, Lopez S, Arias CF. The actin cytoskeleton is important for rotavirus internalization and RNA genome replication. Virus Res. 2019;263:27–33.",
"volume": "263",
"year": "2019"
},
{
"DOI": "10.1128/JVI.00051-21",
"doi-asserted-by": "crossref",
"key": "722_CR60",
"unstructured": "Sharif M, Baek YB, Naveed A, Stalin N, Kang MI, Park SI, et al. Porcine sapovirus-induced tight junction dissociation via activation of RhoA/ROCK/MLC signaling pathway. J Virol. 2021;95(11)."
},
{
"DOI": "10.1128/JVI.00469-15",
"author": "A Thomas",
"doi-asserted-by": "publisher",
"first-page": "8162",
"issue": "16",
"journal-title": "J Virol",
"key": "722_CR61",
"unstructured": "Thomas A, Mariani-Floderer C, Lopez-Huertas MR, Gros N, Hamard-Peron E, Favard C, et al. Involvement of the Rac1-IRSp53-Wave2-Arp2/3 signaling pathway in HIV-1 gag particle release in CD4 T cells. J Virol. 2015;89(16):8162–81.",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.3389/fcell.2020.00201",
"author": "MDM Maldonado",
"doi-asserted-by": "publisher",
"first-page": "201",
"journal-title": "Front Cell Dev Biol",
"key": "722_CR62",
"unstructured": "Maldonado MDM, Medina JI, Velazquez L, Dharmawardhane S. Targeting Rac and Cdc42 GEFs in metastatic cancer. Front Cell Dev Biol. 2020;8:201.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1371/journal.pgen.1010127",
"author": "HK Brar",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "PLoS Genet",
"key": "722_CR63",
"unstructured": "Brar HK, Dey S, Bhardwaj S, Pande D, Singh P, Dey S, et al. Dendrite regeneration in C. elegans is controlled by the RAC GTPase CED-10 and the RhoGEF TIAM-1. PLoS Genet. 2022;18(3): e1010127.",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.3390/ijms21041341",
"author": "J Hong",
"doi-asserted-by": "publisher",
"first-page": "1341",
"issue": "4",
"journal-title": "Int J Mol Sci",
"key": "722_CR64",
"unstructured": "Hong J, Kim Y, Yanpallewar S, Lin PC. The Rho/Rac guanine nucleotide exchange factor Vav1 regulates Hif-1alpha and Glut-1 expression and glucose uptake in the brain. Int J Mol Sci. 2020;21(4):1341.",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.jbc.2022.102209",
"author": "SJ Bandekar",
"doi-asserted-by": "publisher",
"issue": "8",
"journal-title": "J Biol Chem",
"key": "722_CR65",
"unstructured": "Bandekar SJ, Chen CL, Ravala SK, Cash JN, Avramova LV, Zhalnina MV, et al. Structural/functional studies of Trio provide insights into its configuration and show that conserved linker elements enhance its activity for Rac1. J Biol Chem. 2022;298(8): 102209.",
"volume": "298",
"year": "2022"
},
{
"DOI": "10.1007/s11010-019-03552-5",
"author": "S Nakano",
"doi-asserted-by": "publisher",
"first-page": "83",
"issue": "1–2",
"journal-title": "Mol Cell Biochem",
"key": "722_CR66",
"unstructured": "Nakano S, Nishikawa M, Asaoka R, Ishikawa N, Ohwaki C, Sato K, et al. DBS is activated by EPHB2/SRC signaling-mediated tyrosine phosphorylation in HEK293 cells. Mol Cell Biochem. 2019;459(1–2):83–93.",
"volume": "459",
"year": "2019"
},
{
"DOI": "10.1186/s12915-021-01054-9",
"author": "JD Howden",
"doi-asserted-by": "publisher",
"first-page": "130",
"issue": "1",
"journal-title": "BMC Biol",
"key": "722_CR67",
"unstructured": "Howden JD, Michael M, Hight-Warburton W, Parsons M. alpha2beta1 integrins spatially restrict Cdc42 activity to stabilise adherens junctions. BMC Biol. 2021;19(1):130.",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.3390/ijms222212493",
"author": "N Mosaddeghzadeh",
"doi-asserted-by": "publisher",
"first-page": "12493",
"issue": "22",
"journal-title": "Int J Mol Sci",
"key": "722_CR68",
"unstructured": "Mosaddeghzadeh N, Kazemein Jasemi NS, Majolee J, Zhang SC, Hordijk PL, Dvorsky R, et al. Electrostatic forces mediate the specificity of RHO GTPase-GDI interactions. Int J Mol Sci. 2021;22(22):12493.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1152/physrev.00003.2012",
"author": "J Cherfils",
"doi-asserted-by": "publisher",
"first-page": "269",
"issue": "1",
"journal-title": "Physiol Rev",
"key": "722_CR69",
"unstructured": "Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93(1):269–309.",
"volume": "93",
"year": "2013"
},
{
"DOI": "10.1038/sj.onc.1204188",
"author": "IP Whitehead",
"doi-asserted-by": "publisher",
"first-page": "1547",
"issue": "13",
"journal-title": "Oncogene",
"key": "722_CR70",
"unstructured": "Whitehead IP, Zohn IE, Der CJ. Rho GTPase-dependent transformation by G protein-coupled receptors. Oncogene. 2001;20(13):1547–55.",
"volume": "20",
"year": "2001"
},
{
"DOI": "10.1016/j.tice.2020.101364",
"author": "G Salloum",
"doi-asserted-by": "publisher",
"journal-title": "Tissue Cell",
"key": "722_CR71",
"unstructured": "Salloum G, Jaafar L, El-Sibai M. Rho A and Rac1: antagonists moving forward. Tissue Cell. 2020;65: 101364.",
"volume": "65",
"year": "2020"
},
{
"DOI": "10.1016/j.envres.2020.109236",
"author": "L Wang",
"doi-asserted-by": "publisher",
"journal-title": "Environ Res",
"key": "722_CR72",
"unstructured": "Wang L, Chen G, Xiao G, Han L, Wang Q, Hu T. Cylindrospermopsin induces abnormal vascular development through impairing cytoskeleton and promoting vascular endothelial cell apoptosis by the Rho/ROCK signaling pathway. Environ Res. 2020;183: 109236.",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2019.06.132",
"author": "Z Wang",
"doi-asserted-by": "publisher",
"first-page": "976",
"issue": "3",
"journal-title": "Biochem Biophys Res Commun",
"key": "722_CR73",
"unstructured": "Wang Z, Sun L, Liang S, Liu ZC, Zhao ZY, Yang J, et al. GPER stabilizes F-actin cytoskeleton and activates TAZ via PLCbeta-PKC and Rho/ROCK-LIMK-Cofilin pathway. Biochem Biophys Res Commun. 2019;516(3):976–82.",
"volume": "516",
"year": "2019"
},
{
"DOI": "10.1128/JVI.01736-18",
"doi-asserted-by": "crossref",
"key": "722_CR74",
"unstructured": "Lv X, Li Z, Guan J, Hu S, Zhang J, Lan Y, et al. Porcine hemagglutinating encephalomyelitis virus activation of the integrin alpha5beta1-FAK-Cofilin pathway causes cytoskeletal rearrangement to promote its invasion of N2a cells. J Virol. 2019;93(5)."
},
{
"DOI": "10.1128/JVI.00453-19",
"doi-asserted-by": "crossref",
"key": "722_CR75",
"unstructured": "Eliyahu E, Tirosh O, Dobesova M, Nachshon A, Schwartz M, Stern-Ginossar N. Rho-associated coiled-coil kinase 1 translocates to the nucleus and inhibits human cytomegalovirus propagation. J Virol. 2019;93(19)."
},
{
"DOI": "10.1111/j.1600-0854.2009.00996.x",
"author": "A Tomas",
"doi-asserted-by": "publisher",
"first-page": "123",
"issue": "1",
"journal-title": "Traffic",
"key": "722_CR76",
"unstructured": "Tomas A, Yermen B, Regazzi R, Pessin JE, Halban PA. Regulation of insulin secretion by phosphatidylinositol-4,5-bisphosphate. Traffic. 2010;11(1):123–37.",
"volume": "11",
"year": "2010"
},
{
"DOI": "10.1021/acsnano.3c05508",
"author": "M Sharma",
"doi-asserted-by": "publisher",
"first-page": "17436",
"issue": "17",
"journal-title": "ACS Nano",
"key": "722_CR77",
"unstructured": "Sharma M, Marin M, Wu H, Prikryl D, Melikyan GB. Human immunodeficiency virus 1 preferentially fuses with pH-neutral endocytic vesicles in cell lines and human primary CD4+ T-cells. ACS Nano. 2023;17(17):17436–50.",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1016/j.chom.2007.04.007",
"author": "Y Arakawa",
"doi-asserted-by": "publisher",
"first-page": "213",
"issue": "3",
"journal-title": "Cell Host Microbe",
"key": "722_CR78",
"unstructured": "Arakawa Y, Cordeiro JV, Way M. F11L-mediated inhibition of RhoA-mDia signaling stimulates microtubule dynamics during vaccinia virus infection. Cell Host Microbe. 2007;1(3):213–26.",
"volume": "1",
"year": "2007"
},
{
"DOI": "10.1038/sj.emboj.7601092",
"author": "SR Frank",
"doi-asserted-by": "publisher",
"first-page": "1848",
"issue": "9",
"journal-title": "EMBO J",
"key": "722_CR79",
"unstructured": "Frank SR, Adelstein MR, Hansen SH. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. EMBO J. 2006;25(9):1848–59.",
"volume": "25",
"year": "2006"
},
{
"DOI": "10.1016/j.yexcr.2004.01.008",
"author": "KV Stoletov",
"doi-asserted-by": "publisher",
"first-page": "258",
"issue": "1",
"journal-title": "Exp Cell Res",
"key": "722_CR80",
"unstructured": "Stoletov KV, Gong C, Terman BI. Nck and Crk mediate distinct VEGF-induced signaling pathways that serve overlapping functions in focal adhesion turnover and integrin activation. Exp Cell Res. 2004;295(1):258–68.",
"volume": "295",
"year": "2004"
},
{
"DOI": "10.1038/s41598-020-58135-y",
"author": "CV Messias",
"doi-asserted-by": "publisher",
"first-page": "1378",
"issue": "1",
"journal-title": "Sci Rep",
"key": "722_CR81",
"unstructured": "Messias CV, Loss-Morais G, Carvalho JB, Gonzalez MN, Cunha DP, Vasconcelos Z, et al. Zika virus targets the human thymic epithelium. Sci Rep. 2020;10(1):1378.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.4161/cl.20222",
"author": "JP Semblat",
"doi-asserted-by": "publisher",
"first-page": "126",
"issue": "2",
"journal-title": "Cell Logist",
"key": "722_CR82",
"unstructured": "Semblat JP, Doerig C. PAK in pathogen–host interactions. Cell Logist. 2012;2(2):126–31.",
"volume": "2",
"year": "2012"
},
{
"DOI": "10.3892/ijmm.2022.5204",
"doi-asserted-by": "crossref",
"key": "722_CR83",
"unstructured": "Ye JJ, Wei SL, Wei YY, Zhang DW, Sun L, Wu HM, et al. RKIP suppresses the influenza A virus‑induced airway inflammatory response via the ERK/MAPK pathway. Int J Mol Med. 2023;51(1)."
},
{
"DOI": "10.1074/jbc.M703810200",
"author": "K Nishi",
"doi-asserted-by": "publisher",
"first-page": "27503",
"issue": "37",
"journal-title": "J Biol Chem",
"key": "722_CR84",
"unstructured": "Nishi K, Saigo K. Cellular internalization of green fluorescent protein fused with herpes simplex virus protein VP22 via a lipid raft-mediated endocytic pathway independent of caveolae and Rho family GTPases but dependent on dynamin and Arf6. J Biol Chem. 2007;282(37):27503–17.",
"volume": "282",
"year": "2007"
},
{
"DOI": "10.1007/s12250-020-00343-x",
"author": "Y Nie",
"doi-asserted-by": "publisher",
"first-page": "692",
"issue": "4",
"journal-title": "Virol Sin",
"key": "722_CR85",
"unstructured": "Nie Y, Hui L, Guo M, Yang W, Huang R, Chen J, et al. Rearrangement of actin cytoskeleton by Zika virus infection facilitates blood–testis barrier hyperpermeability. Virol Sin. 2021;36(4):692–705.",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2018.01871",
"author": "M Hemshekhar",
"doi-asserted-by": "publisher",
"first-page": "1871",
"journal-title": "Front Immunol",
"key": "722_CR86",
"unstructured": "Hemshekhar M, Choi KG, Mookherjee N. Host defense peptide LL-37-mediated chemoattractant properties, but not anti-inflammatory cytokine IL-1RA production, is selectively controlled by Cdc42 Rho GTPase via G protein-coupled receptors and JNK mitogen-activated protein kinase. Front Immunol. 2018;9:1871.",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.3389/fmicb.2021.800935",
"author": "HW Lee",
"doi-asserted-by": "publisher",
"journal-title": "Front Microbiol",
"key": "722_CR87",
"unstructured": "Lee HW, Choi Y, Lee AR, Yoon CH, Kim KH, Choi BS, et al. Hepatocyte growth factor-dependent antiviral activity of activated cdc42-associated kinase 1 against hepatitis B virus. Front Microbiol. 2021;12: 800935.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1186/s12985-022-01814-1",
"author": "S Montazersaheb",
"doi-asserted-by": "publisher",
"first-page": "92",
"issue": "1",
"journal-title": "Virol J",
"key": "722_CR88",
"unstructured": "Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J. 2022;19(1):92.",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1186/s13578-021-00677-3",
"author": "J Qu",
"doi-asserted-by": "publisher",
"first-page": "163",
"issue": "1",
"journal-title": "Cell Biosci",
"key": "722_CR89",
"unstructured": "Qu J, Li J, Zhang Y, He R, Liu X, Gong K, et al. AKR1B10 promotes breast cancer cell proliferation and migration via the PI3K/AKT/NF-kappaB signaling pathway. Cell Biosci. 2021;11(1):163.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41556-019-0348-8",
"author": "H Senoo",
"doi-asserted-by": "publisher",
"first-page": "867",
"issue": "7",
"journal-title": "Nat Cell Biol",
"key": "722_CR90",
"unstructured": "Senoo H, Kamimura Y, Kimura R, Nakajima A, Sawai S, Sesaki H, et al. Phosphorylated Rho-GDP directly activates mTORC2 kinase towards AKT through dimerization with Ras-GTP to regulate cell migration. Nat Cell Biol. 2019;21(7):867–78.",
"volume": "21",
"year": "2019"
},
{
"DOI": "10.1042/BCJ20220103",
"author": "JE Hunter",
"doi-asserted-by": "publisher",
"first-page": "2131",
"issue": "19",
"journal-title": "Biochem J",
"key": "722_CR91",
"unstructured": "Hunter JE, Campbell AE, Kerridge S, Fraser C, Hannaway NL, Luli S, et al. Up-regulation of the PI3K/AKT and RHO/RAC/PAK signalling pathways in CHK1 inhibitor resistant Emicro-Myc lymphoma cells. Biochem J. 2022;479(19):2131–51.",
"volume": "479",
"year": "2022"
},
{
"DOI": "10.1083/jcb.201703105",
"author": "BK Tripathi",
"doi-asserted-by": "publisher",
"first-page": "4255",
"issue": "12",
"journal-title": "J Cell Biol",
"key": "722_CR92",
"unstructured": "Tripathi BK, Grant T, Qian X, Zhou M, Mertins P, Wang D, et al. Receptor tyrosine kinase activation of RhoA is mediated by AKT phosphorylation of DLC1. J Cell Biol. 2017;216(12):4255–70.",
"volume": "216",
"year": "2017"
},
{
"DOI": "10.4149/neo_2020_190818N781",
"author": "X Xu",
"doi-asserted-by": "publisher",
"first-page": "614",
"issue": "3",
"journal-title": "Neoplasma",
"key": "722_CR93",
"unstructured": "Xu X, Zhou W, Chen Y, Wu K, Wang H, Zhang J, et al. Immediate early response protein 2 promotes the migration and invasion of hepatocellular carcinoma cells via regulating the activity of Rho GTPases. Neoplasma. 2020;67(3):614–22.",
"volume": "67",
"year": "2020"
},
{
"DOI": "10.1007/BF00690419",
"author": "R Khosravi-Far",
"doi-asserted-by": "publisher",
"first-page": "67",
"issue": "1",
"journal-title": "Cancer Metastasis Rev",
"key": "722_CR94",
"unstructured": "Khosravi-Far R, Der CJ. The Ras signal transduction pathway. Cancer Metastasis Rev. 1994;13(1):67–89.",
"volume": "13",
"year": "1994"
},
{
"DOI": "10.3390/ijms21176312",
"author": "A Arrazola Sastre",
"doi-asserted-by": "publisher",
"first-page": "6312",
"issue": "17",
"journal-title": "Int J Mol Sci",
"key": "722_CR95",
"unstructured": "Arrazola Sastre A, Luque Montoro M, Galvez-Martin P, Lacerda HM, Lucia AM, Llavero F, et al. Small GTPases of the Ras and Rho families switch on/off signaling pathways in neurodegenerative diseases. Int J Mol Sci. 2020;21(17):6312.",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.gene.2024.148366",
"author": "M Bostanciklioglu",
"doi-asserted-by": "publisher",
"journal-title": "Gene",
"key": "722_CR96",
"unstructured": "Bostanciklioglu M, Igci M, Nigella Sativa UM. Anthemis hyaline and Citrus sinensis extracts reduce SARS-CoV-2 replication by fluctuating Rho GTPase, PI3K-AKT, and MAPK/ERK pathways in HeLa-CEACAM1a cells. Gene. 2024;911: 148366.",
"volume": "911",
"year": "2024"
},
{
"DOI": "10.1038/s41556-019-0438-7",
"author": "H Bagci",
"doi-asserted-by": "publisher",
"first-page": "120",
"issue": "1",
"journal-title": "Nat Cell Biol",
"key": "722_CR97",
"unstructured": "Bagci H, Sriskandarajah N, Robert A, Boulais J, Elkholi IE, Tran V, et al. Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms. Nat Cell Biol. 2020;22(1):120–34.",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1158/0008-5472.CAN-18-1889",
"author": "T Hu",
"doi-asserted-by": "publisher",
"first-page": "114",
"issue": "1",
"journal-title": "Cancer Res",
"key": "722_CR98",
"unstructured": "Hu T, Chong Y, Lu S, Qin H, Ren M, Savage NM, et al. Loss of the BCR-FGFR1 GEF domain suppresses RHOA activation and enhances B-lymphomagenesis in mice. Cancer Res. 2019;79(1):114–24.",
"volume": "79",
"year": "2019"
},
{
"DOI": "10.1021/acs.jcim.3c01412",
"author": "N Haspel",
"doi-asserted-by": "publisher",
"first-page": "862",
"issue": "3",
"journal-title": "J Chem Inf Model",
"key": "722_CR99",
"unstructured": "Haspel N, Jang H, Nussinov R. Allosteric activation of RhoA complexed with p115-RhoGEF deciphered by conformational dynamics. J Chem Inf Model. 2024;64(3):862–73.",
"volume": "64",
"year": "2024"
},
{
"DOI": "10.1128/JVI.72.11.8806-8812.1998",
"author": "E Li",
"doi-asserted-by": "publisher",
"first-page": "8806",
"issue": "11",
"journal-title": "J Virol",
"key": "722_CR100",
"unstructured": "Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998;72(11):8806–12.",
"volume": "72",
"year": "1998"
},
{
"DOI": "10.1074/jbc.M011383200",
"author": "I Arozarena",
"doi-asserted-by": "publisher",
"first-page": "21878",
"issue": "24",
"journal-title": "J Biol Chem",
"key": "722_CR101",
"unstructured": "Arozarena I, Matallanas D, Crespo P. Maintenance of CDC42 GDP-bound state by Rho-GDI inhibits MAP kinase activation by the exchange factor Ras-GRF. Evidence for Ras-GRF function being inhibited by Cdc42-GDP but unaffected by CDC42-GTP. J Biol Chem. 2001;276(24):21878–84.",
"volume": "276",
"year": "2001"
},
{
"DOI": "10.1089/ars.2012.4687",
"author": "L Mitchell",
"doi-asserted-by": "publisher",
"first-page": "250",
"issue": "3",
"journal-title": "Antioxid Redox Signal",
"key": "722_CR102",
"unstructured": "Mitchell L, Hobbs GA, Aghajanian A, Campbell SL. Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxid Redox Signal. 2013;18(3):250–8.",
"volume": "18",
"year": "2013"
},
{
"DOI": "10.3181/0703-MR-76",
"author": "O Pochynyuk",
"doi-asserted-by": "publisher",
"first-page": "1258",
"issue": "10",
"journal-title": "Exp Biol Med (Maywood)",
"key": "722_CR103",
"unstructured": "Pochynyuk O, Stockand JD, Staruschenko A. Ion channel regulation by Ras, Rho, and Rab small GTPases. Exp Biol Med (Maywood). 2007;232(10):1258–65.",
"volume": "232",
"year": "2007"
},
{
"DOI": "10.1371/journal.pone.0021444",
"author": "M Haidari",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "PLoS ONE",
"key": "722_CR104",
"unstructured": "Haidari M, Zhang W, Ganjehei L, Ali M, Chen Z. Inhibition of MLC phosphorylation restricts replication of influenza virus—a mechanism of action for anti-influenza agents. PLoS ONE. 2011;6(6): e21444.",
"volume": "6",
"year": "2011"
},
{
"DOI": "10.1080/21505594.2022.2135724",
"author": "W Fan",
"doi-asserted-by": "publisher",
"first-page": "1884",
"issue": "1",
"journal-title": "Virulence",
"key": "722_CR105",
"unstructured": "Fan W, Wang Y, Jiang S, Li Y, Yao X, Wang M, et al. Identification of key proteins of cytopathic biotype bovine viral diarrhoea virus involved in activating NF-kappaB pathway in BVDV-induced inflammatory response. Virulence. 2022;13(1):1884–99.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/cells12101367",
"author": "EJ Glotfelty",
"doi-asserted-by": "publisher",
"first-page": "1367",
"issue": "10",
"journal-title": "Cells",
"key": "722_CR106",
"unstructured": "Glotfelty EJ, Tovar YRLB, Hsueh SC, Tweedie D, Li Y, Harvey BK, et al. The RhoA-ROCK1/ROCK2 pathway exacerbates inflammatory signaling in immortalized and primary microglia. Cells. 2023;12(10):1367.",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.4049/jimmunol.0802280",
"author": "M Manukyan",
"doi-asserted-by": "publisher",
"first-page": "3522",
"issue": "6",
"journal-title": "J Immunol",
"key": "722_CR107",
"unstructured": "Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J Immunol. 2009;182(6):3522–9.",
"volume": "182",
"year": "2009"
},
{
"DOI": "10.1152/ajprenal.00113.2014",
"author": "K Matoba",
"doi-asserted-by": "publisher",
"first-page": "F571",
"issue": "5",
"journal-title": "Am J Physiol Renal Physiol",
"key": "722_CR108",
"unstructured": "Matoba K, Kawanami D, Tsukamoto M, Kinoshita J, Ito T, Ishizawa S, et al. Rho-kinase regulation of TNF-alpha-induced nuclear translocation of NF-kappaB RelA/p65 and M-CSF expression via p38 MAPK in mesangial cells. Am J Physiol Renal Physiol. 2014;307(5):F571–80.",
"volume": "307",
"year": "2014"
},
{
"DOI": "10.4049/jimmunol.173.11.6965",
"author": "KN Anwar",
"doi-asserted-by": "publisher",
"first-page": "6965",
"issue": "11",
"journal-title": "J Immunol",
"key": "722_CR109",
"unstructured": "Anwar KN, Fazal F, Malik AB, Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol. 2004;173(11):6965–72.",
"volume": "173",
"year": "2004"
},
{
"DOI": "10.3390/microorganisms8071067",
"author": "J Yu",
"doi-asserted-by": "publisher",
"first-page": "1067",
"issue": "7",
"journal-title": "Microorganisms.",
"key": "722_CR110",
"unstructured": "Yu J, Sun X, Goie JYG, Zhang Y. Regulation of host immune responses against influenza A virus infection by mitogen-activated protein kinases (MAPKs). Microorganisms. 2020;8(7):1067.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.jsbmb.2006.06.030",
"author": "YX Chen",
"doi-asserted-by": "publisher",
"first-page": "179",
"issue": "4–5",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "722_CR111",
"unstructured": "Chen YX, Li ZB, Diao F, Cao DM, Fu CC, Lu J. Up-regulation of RhoB by glucocorticoids and its effects on the cell proliferation and NF-kappaB transcriptional activity. J Steroid Biochem Mol Biol. 2006;101(4–5):179–87.",
"volume": "101",
"year": "2006"
},
{
"DOI": "10.1038/s41419-022-04954-y",
"author": "A Kumar",
"doi-asserted-by": "publisher",
"first-page": "520",
"issue": "6",
"journal-title": "Cell Death Dis",
"key": "722_CR112",
"unstructured": "Kumar A, Mishra S, Kumar A, Raut AA, Sato S, Takaoka A, et al. Essential role of Rnd1 in innate immunity during viral and bacterial infections. Cell Death Dis. 2022;13(6):520.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1155/2015/671839",
"author": "X Tang",
"doi-asserted-by": "publisher",
"journal-title": "Oxid Med Cell Longev",
"key": "722_CR113",
"unstructured": "Tang X, Guo D, Lin C, Shi Z, Qian R, Fu W, et al. hCLOCK causes rho-kinase-mediated endothelial dysfunction and NF-kappaB-mediated inflammatory responses. Oxid Med Cell Longev. 2015;2015: 671839.",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.4049/jimmunol.1502677",
"author": "M Yoneda",
"doi-asserted-by": "publisher",
"first-page": "630",
"issue": "2",
"journal-title": "J Immunol",
"key": "722_CR114",
"unstructured": "Yoneda M, Hyun J, Jakubski S, Saito S, Nakajima A, Schiff ER, et al. Hepatitis B virus and DNA stimulation trigger a rapid innate immune response through NF-kappaB. J Immunol. 2016;197(2):630–43.",
"volume": "197",
"year": "2016"
},
{
"DOI": "10.1128/JVI.00946-16",
"author": "S Dam",
"doi-asserted-by": "publisher",
"first-page": "7980",
"issue": "17",
"journal-title": "J Virol",
"key": "722_CR115",
"unstructured": "Dam S, Kracht M, Pleschka S, Schmitz ML. The influenza A virus genotype determines the antiviral function of NF-kappaB. J Virol. 2016;90(17):7980–90.",
"volume": "90",
"year": "2016"
},
{
"DOI": "10.1038/s41580-018-0067-1",
"author": "M Dogterom",
"doi-asserted-by": "publisher",
"first-page": "38",
"issue": "1",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "722_CR116",
"unstructured": "Dogterom M, Koenderink GH. Actin-microtubule crosstalk in cell biology. Nat Rev Mol Cell Biol. 2019;20(1):38–54.",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.1128/jvi.01929-22",
"author": "JX Lou",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "J Virol",
"key": "722_CR117",
"unstructured": "Lou JX, Liu YY, Bai JS, Cheng Y, Zhang J, Liu CC, et al. Kinesin-1 regulates endocytic trafficking of classical swine fever virus along acetylated microtubules. J Virol. 2023;97(1): e0192922.",
"volume": "97",
"year": "2023"
},
{
"DOI": "10.1128/jvi.02205-21",
"author": "DL Li",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "J Virol",
"key": "722_CR118",
"unstructured": "Li DL, Yang MH, Liu LK, Meng C, Li MQ, Liu HP. Invasion and propagation of white spot syndrome virus: hijacking of the cytoskeleton, intracellular transport machinery, and nuclear import transporters. J Virol. 2022;96(12): e0220521.",
"volume": "96",
"year": "2022"
},
{
"DOI": "10.1128/JVI.79.2.1191-1206.2005",
"author": "PP Naranatt",
"doi-asserted-by": "publisher",
"first-page": "1191",
"issue": "2",
"journal-title": "J Virol",
"key": "722_CR119",
"unstructured": "Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol. 2005;79(2):1191–206.",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.1128/JVI.05666-11",
"author": "JI Quetglas",
"doi-asserted-by": "publisher",
"first-page": "1758",
"issue": "3",
"journal-title": "J Virol",
"key": "722_CR120",
"unstructured": "Quetglas JI, Hernaez B, Galindo I, Munoz-Moreno R, Cuesta-Geijo MA, Alonso C. Small rho GTPases and cholesterol biosynthetic pathway intermediates in African swine fever virus infection. J Virol. 2012;86(3):1758–67.",
"volume": "86",
"year": "2012"
},
{
"DOI": "10.1038/s41590-021-01091-0",
"author": "MS Diamond",
"doi-asserted-by": "publisher",
"first-page": "165",
"issue": "2",
"journal-title": "Nat Immunol",
"key": "722_CR121",
"unstructured": "Diamond MS, Kanneganti TD. Innate immunity: the first line of defense against SARS-CoV-2. Nat Immunol. 2022;23(2):165–76.",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1152/ajplung.00118.2021",
"author": "DT Linfield",
"doi-asserted-by": "publisher",
"first-page": "L189",
"issue": "1",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "722_CR122",
"unstructured": "Linfield DT, Gao N, Raduka A, Harford TJ, Piedimonte G, Rezaee F. RSV attenuates epithelial cell restitution by inhibiting actin cytoskeleton-dependent cell migration. Am J Physiol Lung Cell Mol Physiol. 2021;321(1):L189–203.",
"volume": "321",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2019.02500",
"author": "V Upasani",
"doi-asserted-by": "publisher",
"first-page": "2500",
"journal-title": "Front Immunol",
"key": "722_CR123",
"unstructured": "Upasani V, Vo HTM, Ung S, Heng S, Laurent D, Choeung R, et al. Impaired antibody-independent immune response of B cells in patients with acute dengue infection. Front Immunol. 2019;10:2500.",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.4049/jimmunol.1602167",
"author": "M Burbage",
"doi-asserted-by": "publisher",
"first-page": "1682",
"issue": "5",
"journal-title": "J Immunol",
"key": "722_CR124",
"unstructured": "Burbage M, Keppler SJ, Montaner B, Mattila PK, Batista FD. The small Rho GTPase TC10 modulates B cell immune responses. J Immunol. 2017;199(5):1682–95.",
"volume": "199",
"year": "2017"
},
{
"DOI": "10.1038/ni1470",
"author": "A Hodges",
"doi-asserted-by": "publisher",
"first-page": "569",
"issue": "6",
"journal-title": "Nat Immunol",
"key": "722_CR125",
"unstructured": "Hodges A, Sharrocks K, Edelmann M, Baban D, Moris A, Schwartz O, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8(6):569–77.",
"volume": "8",
"year": "2007"
},
{
"DOI": "10.1084/jem.20141143",
"author": "M Burbage",
"doi-asserted-by": "publisher",
"first-page": "53",
"issue": "1",
"journal-title": "J Exp Med",
"key": "722_CR126",
"unstructured": "Burbage M, Keppler SJ, Gasparrini F, Martinez-Martin N, Gaya M, Feest C, et al. Cdc42 is a key regulator of B cell differentiation and is required for antiviral humoral immunity. J Exp Med. 2015;212(1):53–72.",
"volume": "212",
"year": "2015"
},
{
"DOI": "10.1038/cddis.2017.170",
"author": "Z Zhu",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Cell Death Dis",
"key": "722_CR127",
"unstructured": "Zhu Z, Li C, Du X, Wang G, Cao W, Yang F, et al. Foot-and-mouth disease virus infection inhibits LGP2 protein expression to exaggerate inflammatory response and promote viral replication. Cell Death Dis. 2017;8(4): e2747.",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1016/j.micinf.2023.105280",
"author": "E Yau",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "Microbes Infect",
"key": "722_CR128",
"unstructured": "Yau E, Yang L, Chen Y, Umstead TM, Stanley AE, Halstead ES, et al. SP-R210 isoforms of Myosin18A modulate endosomal sorting and recognition of influenza A virus infection in macrophages. Microbes Infect. 2024;26(3): 105280.",
"volume": "26",
"year": "2024"
},
{
"DOI": "10.1016/j.cellsig.2020.109599",
"author": "S Alarifi",
"doi-asserted-by": "publisher",
"journal-title": "Cell Signal",
"key": "722_CR129",
"unstructured": "Alarifi S, Alkahtani S, Al-Qahtani AA, Stournaras C, Sourvinos G. Induction of interleukin-11 mediated by RhoA GTPase during human cytomegalovirus lytic infection. Cell Signal. 2020;70: 109599.",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.1128/mBio.01476-14",
"author": "BP Daniels",
"doi-asserted-by": "publisher",
"first-page": "e01476",
"issue": "5",
"journal-title": "MBio",
"key": "722_CR130",
"unstructured": "Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, Klein RS. Viral pathogen-associated molecular patterns regulate blood–brain barrier integrity via competing innate cytokine signals. MBio. 2014;5(5):e01476-e1514.",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.1128/JVI.03176-12",
"author": "W Jiang",
"doi-asserted-by": "publisher",
"first-page": "3039",
"issue": "6",
"journal-title": "J Virol",
"key": "722_CR131",
"unstructured": "Jiang W, Wang Q, Chen S, Gao S, Song L, Liu P, et al. Influenza A virus NS1 induces G0/G1 cell cycle arrest by inhibiting the expression and activity of RhoA protein. J Virol. 2013;87(6):3039–52.",
"volume": "87",
"year": "2013"
},
{
"DOI": "10.3390/ijms18030586",
"author": "Y Xu",
"doi-asserted-by": "publisher",
"first-page": "586",
"issue": "3",
"journal-title": "Int J Mol Sci",
"key": "722_CR132",
"unstructured": "Xu Y, Qi Y, Luo J, Yang J, Xie Q, Deng C, et al. Hepatitis B virus X protein stimulates proliferation, wound closure and inhibits apoptosis of HuH-7 cells via CDC42. Int J Mol Sci. 2017;18(3):586.",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.1016/j.molimm.2019.04.003",
"author": "L Fu",
"doi-asserted-by": "publisher",
"first-page": "87",
"journal-title": "Mol Immunol",
"key": "722_CR133",
"unstructured": "Fu L, Wang X, Zhai J, Qi W, Jing L, Ge Y, et al. Changes in apoptosis, proliferation and T lymphocyte subtype on thymic cells of SPF chickens infected with reticuloendotheliosis virus. Mol Immunol. 2019;111:87–94.",
"volume": "111",
"year": "2019"
},
{
"DOI": "10.1016/j.coviro.2020.03.001",
"author": "K Ayasoufi",
"doi-asserted-by": "publisher",
"first-page": "18",
"journal-title": "Curr Opin Virol",
"key": "722_CR134",
"unstructured": "Ayasoufi K, Pfaller CK. Seek and hide: the manipulating interplay of measles virus with the innate immune system. Curr Opin Virol. 2020;41:18–30.",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1016/j.csbj.2022.03.002",
"author": "S Li",
"doi-asserted-by": "publisher",
"first-page": "1244",
"journal-title": "Comput Struct Biotechnol J",
"key": "722_CR135",
"unstructured": "Li S, Zhou W, Li D, Pan T, Guo J, Zou H, et al. Comprehensive characterization of human-virus protein–protein interactions reveals disease comorbidities and potential antiviral drugs. Comput Struct Biotechnol J. 2022;20:1244–53.",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.2217/fmb-2020-0092",
"author": "J Wu",
"doi-asserted-by": "publisher",
"first-page": "445",
"journal-title": "Future Microbiol",
"key": "722_CR136",
"unstructured": "Wu J, Gu J, Shen L, Jia X, Yin Y, Chen Y, et al. The role of host cell Rab GTPases in influenza A virus infections. Future Microbiol. 2021;16:445–52.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.3390/ijms222011164",
"author": "V Gerlt",
"doi-asserted-by": "publisher",
"first-page": "11164",
"issue": "20",
"journal-title": "Int J Mol Sci",
"key": "722_CR137",
"unstructured": "Gerlt V, Mayr J, Del Sarto J, Ludwig S, Boergeling Y. Cellular Protein Phosphatase 2A Regulates Cell Survival Mechanisms in Influenza A Virus Infection. Int J Mol Sci. 2021;22(20):11164.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.3390/v11010046",
"author": "S Bedi",
"doi-asserted-by": "publisher",
"first-page": "46",
"issue": "1",
"journal-title": "Viruses",
"key": "722_CR138",
"unstructured": "Bedi S, Ono A. Friend or foe: the role of the cytoskeleton in influenza A virus assembly. Viruses. 2019;11(1):46.",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.3390/v12010117",
"doi-asserted-by": "crossref",
"key": "722_CR139",
"unstructured": "Simpson C, Yamauchi Y. Microtubules in influenza virus entry and egress. Viruses. 2020;12(1)."
},
{
"DOI": "10.3390/v13102042",
"author": "A Suttitheptumrong",
"doi-asserted-by": "publisher",
"first-page": "2042",
"issue": "10",
"journal-title": "Viruses",
"key": "722_CR140",
"unstructured": "Suttitheptumrong A, Mahutchariyakul T, Rawarak N, Reamtong O, Boonnak K, Pattanakitsakul SN. Altered moesin and actin cytoskeleton protein rearrangements affect transendothelial permeability in human endothelial cells upon dengue virus infection and TNF-alpha treatment. Viruses. 2021;13(10):2042.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1080/1521654031000070645",
"author": "PE Boehmer",
"doi-asserted-by": "publisher",
"first-page": "13",
"issue": "1",
"journal-title": "IUBMB Life",
"key": "722_CR141",
"unstructured": "Boehmer PE, Nimonkar AV. Herpes virus replication. IUBMB Life. 2003;55(1):13–22.",
"volume": "55",
"year": "2003"
},
{
"DOI": "10.1371/journal.pone.0044072",
"author": "N Richerioux",
"doi-asserted-by": "publisher",
"issue": "8",
"journal-title": "PLoS ONE",
"key": "722_CR142",
"unstructured": "Richerioux N, Blondeau C, Wiedemann A, Remy S, Vautherot JF, Denesvre C. Rho-ROCK and Rac-PAK signaling pathways have opposing effects on the cell-to-cell spread of Marek’s Disease Virus. PLoS ONE. 2012;7(8): e44072.",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.3390/v10020092",
"doi-asserted-by": "crossref",
"key": "722_CR143",
"unstructured": "Miranda-Saksena M, Denes CE, Diefenbach RJ, Cunningham AL. Infection and transport of herpes simplex virus type 1 in neurons: role of the cytoskeleton. Viruses. 2018;10(2)."
},
{
"DOI": "10.1128/JVI.02848-06",
"author": "H Raghu",
"doi-asserted-by": "publisher",
"first-page": "7941",
"issue": "15",
"journal-title": "J Virol",
"key": "722_CR144",
"unstructured": "Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Caballero A, Sivakumar R, et al. Lipid rafts of primary endothelial cells are essential for Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J Virol. 2007;81(15):7941–59.",
"volume": "81",
"year": "2007"
},
{
"DOI": "10.1186/s12985-023-02229-2",
"author": "XM Li",
"doi-asserted-by": "publisher",
"first-page": "264",
"issue": "1",
"journal-title": "Virol J",
"key": "722_CR145",
"unstructured": "Li XM, Wang SP, Wang JY, Tang T, Wan B, Zeng L, et al. RhoA suppresses pseudorabies virus replication in vitro. Virol J. 2023;20(1):264.",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1016/j.virusres.2009.09.004",
"author": "HA Yi",
"doi-asserted-by": "publisher",
"first-page": "89",
"issue": "1–2",
"journal-title": "Virus Res",
"key": "722_CR146",
"unstructured": "Yi HA, Kim MS, Jang SY, Lee YM, Ahn JH, Lee CH. Cellular signals involved in cyclooxygenase-2 expression induced by human cytomegalovirus. Virus Res. 2009;146(1–2):89–96.",
"volume": "146",
"year": "2009"
},
{
"DOI": "10.1016/j.virol.2015.02.040",
"author": "X Liu",
"doi-asserted-by": "publisher",
"first-page": "568",
"journal-title": "Virology",
"key": "722_CR147",
"unstructured": "Liu X, Cohen JI. The role of PI3K/Akt in human herpesvirus infection: from the bench to the bedside. Virology. 2015;479–480:568–77.",
"volume": "479–480",
"year": "2015"
},
{
"DOI": "10.1016/j.tim.2007.08.003",
"author": "HW Favoreel",
"doi-asserted-by": "publisher",
"first-page": "426",
"issue": "9",
"journal-title": "Trends Microbiol",
"key": "722_CR148",
"unstructured": "Favoreel HW, Enquist LW, Feierbach B. Actin and Rho GTPases in herpesvirus biology. Trends Microbiol. 2007;15(9):426–33.",
"volume": "15",
"year": "2007"
},
{
"DOI": "10.1128/JVI.03634-14",
"author": "F Myster",
"doi-asserted-by": "publisher",
"first-page": "3630",
"issue": "7",
"journal-title": "J Virol",
"key": "722_CR149",
"unstructured": "Myster F, Palmeira L, Sorel O, Bouillenne F, DePauw E, Schwartz-Cornil I, et al. Viral semaphorin inhibits dendritic cell phagocytosis and migration but is not essential for gammaherpesvirus-induced lymphoproliferation in malignant catarrhal fever. J Virol. 2015;89(7):3630–47.",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.1007/s40011-021-01237-y",
"author": "PK Gupta",
"doi-asserted-by": "publisher",
"first-page": "495",
"issue": "3",
"journal-title": "Proc Natl Acad Sci India Sect B Biol Sci",
"key": "722_CR150",
"unstructured": "Gupta PK, Saxena A. HIV/AIDS: current updates on the disease, treatment and prevention. Proc Natl Acad Sci India Sect B Biol Sci. 2021;91(3):495–510.",
"volume": "91",
"year": "2021"
},
{
"DOI": "10.1016/j.tim.2019.06.002",
"author": "B Chen",
"doi-asserted-by": "publisher",
"first-page": "878",
"issue": "10",
"journal-title": "Trends Microbiol",
"key": "722_CR151",
"unstructured": "Chen B. Molecular mechanism of HIV-1 entry. Trends Microbiol. 2019;27(10):878–91.",
"volume": "27",
"year": "2019"
},
{
"DOI": "10.3390/v10020063",
"author": "A Ospina Stella",
"doi-asserted-by": "publisher",
"first-page": "63",
"issue": "2",
"journal-title": "Viruses",
"key": "722_CR152",
"unstructured": "Ospina Stella A, Turville S. All-round manipulation of the actin cytoskeleton by HIV. Viruses. 2018;10(2):63.",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1016/j.it.2022.06.001",
"author": "N Linden",
"doi-asserted-by": "publisher",
"first-page": "617",
"issue": "8",
"journal-title": "Trends Immunol",
"key": "722_CR153",
"unstructured": "Linden N, Jones RB. Potential multi-modal effects of provirus integration on HIV-1 persistence: lessons from other viruses. Trends Immunol. 2022;43(8):617–29.",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1128/JVI.78.17.9458-9473.2004",
"author": "V Krishnan",
"doi-asserted-by": "publisher",
"first-page": "9458",
"issue": "17",
"journal-title": "J Virol",
"key": "722_CR154",
"unstructured": "Krishnan V, Zeichner SL. Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency. J Virol. 2004;78(17):9458–73.",
"volume": "78",
"year": "2004"
},
{
"DOI": "10.1073/pnas.2014783118",
"doi-asserted-by": "crossref",
"key": "722_CR155",
"unstructured": "Ma G, Yasunaga JI, Shimura K, Takemoto K, Watanabe M, Amano M, et al. Human retroviral antisense mRNAs are retained in the nuclei of infected cells for viral persistence. Proc Natl Acad Sci USA. 2021;118(17)."
},
{
"DOI": "10.1016/S0960-9822(02)70792-6",
"author": "X Lu",
"doi-asserted-by": "publisher",
"first-page": "1677",
"issue": "12",
"journal-title": "Curr Biol",
"key": "722_CR156",
"unstructured": "Lu X, Wu X, Plemenitas A, Yu H, Sawai ET, Abo A, et al. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr Biol. 1996;6(12):1677–84.",
"volume": "6",
"year": "1996"
},
{
"DOI": "10.1186/s12977-017-0328-7",
"author": "MB Lucera",
"doi-asserted-by": "publisher",
"first-page": "4",
"issue": "1",
"journal-title": "Retrovirology",
"key": "722_CR157",
"unstructured": "Lucera MB, Fleissner Z, Tabler CO, Schlatzer DM, Troyer Z, Tilton JC. HIV signaling through CD4 and CCR5 activates Rho family GTPases that are required for optimal infection of primary CD4+ T cells. Retrovirology. 2017;14(1):4.",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1038/jcbfm.2009.214",
"author": "Y Zhong",
"doi-asserted-by": "publisher",
"first-page": "522",
"issue": "3",
"journal-title": "J Cereb Blood Flow Metab",
"key": "722_CR158",
"unstructured": "Zhong Y, Hennig B, Toborek M. Intact lipid rafts regulate HIV-1 Tat protein-induced activation of the Rho signaling and upregulation of P-glycoprotein in brain endothelial cells. J Cereb Blood Flow Metab. 2010;30(3):522–33.",
"volume": "30",
"year": "2010"
},
{
"DOI": "10.1038/s41579-023-00919-w",
"author": "AC Langedijk",
"doi-asserted-by": "publisher",
"first-page": "734",
"issue": "11",
"journal-title": "Nat Rev Microbiol",
"key": "722_CR159",
"unstructured": "Langedijk AC, Bont LJ. Respiratory syncytial virus infection and novel interventions. Nat Rev Microbiol. 2023;21(11):734–49.",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1093/jac/dkh355",
"author": "PJ Budge",
"doi-asserted-by": "publisher",
"first-page": "299",
"issue": "2",
"journal-title": "J Antimicrob Chemother",
"key": "722_CR160",
"unstructured": "Budge PJ, Graham BS. Inhibition of respiratory syncytial virus by RhoA-derived peptides: implications for the development of improved antiviral agents targeting heparin-binding viruses. J Antimicrob Chemother. 2004;54(2):299–302.",
"volume": "54",
"year": "2004"
},
{
"DOI": "10.1016/j.ejcb.2023.151336",
"author": "N Gao",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "Eur J Cell Biol",
"key": "722_CR161",
"unstructured": "Gao N, Raduka A, Rezaee F. Vitamin D(3) protects against respiratory syncytial virus-induced barrier dysfunction in airway epithelial cells via PKA signaling pathway. Eur J Cell Biol. 2023;102(3): 151336.",
"volume": "102",
"year": "2023"
},
{
"DOI": "10.1111/bph.12658",
"author": "M Biro",
"doi-asserted-by": "publisher",
"first-page": "5491",
"issue": "24",
"journal-title": "Br J Pharmacol",
"key": "722_CR162",
"unstructured": "Biro M, Munoz MA, Weninger W. Targeting Rho-GTPases in immune cell migration and inflammation. Br J Pharmacol. 2014;171(24):5491–506.",
"volume": "171",
"year": "2014"
},
{
"DOI": "10.1159/000151729",
"author": "K Iesato",
"doi-asserted-by": "publisher",
"first-page": "434",
"issue": "4",
"journal-title": "Respiration",
"key": "722_CR163",
"unstructured": "Iesato K, Tatsumi K, Saito K, Ogasawara T, Sakao S, Tada Y, et al. Tiotropium bromide attenuates respiratory syncytial virus replication in epithelial cells. Respiration. 2008;76(4):434–41.",
"volume": "76",
"year": "2008"
},
{
"author": "PF Torrence",
"first-page": "226",
"issue": "4",
"journal-title": "Drug News Perspect",
"key": "722_CR164",
"unstructured": "Torrence PF. RSV infections: developments in the search for new drugs. Drug News Perspect. 2000;13(4):226–33.",
"volume": "13",
"year": "2000"
},
{
"DOI": "10.1007/978-3-031-40086-5_9",
"author": "RJ Sugrue",
"doi-asserted-by": "publisher",
"first-page": "227",
"journal-title": "Subcell Biochem",
"key": "722_CR165",
"unstructured": "Sugrue RJ, Tan BH. Defining the assembleome of the respiratory syncytial virus. Subcell Biochem. 2023;106:227–49.",
"volume": "106",
"year": "2023"
},
{
"DOI": "10.3390/v10030120",
"author": "ACD Matos",
"doi-asserted-by": "publisher",
"first-page": "120",
"issue": "3",
"journal-title": "Viruses",
"key": "722_CR166",
"unstructured": "Matos ACD, Rehfeld IS, Guedes M, Lobato ZIP. Bovine vaccinia: insights into the disease in cattle. Viruses. 2018;10(3):120.",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1126/science.1122411",
"author": "F Valderrama",
"doi-asserted-by": "publisher",
"first-page": "377",
"issue": "5759",
"journal-title": "Science",
"key": "722_CR167",
"unstructured": "Valderrama F, Cordeiro JV, Schleich S, Frischknecht F, Way M. Vaccinia virus-induced cell motility requires F11L-mediated inhibition of RhoA signaling. Science. 2006;311(5759):377–81.",
"volume": "311",
"year": "2006"
},
{
"DOI": "10.1186/s12917-015-0417-6",
"author": "PY Lin",
"doi-asserted-by": "publisher",
"first-page": "103",
"journal-title": "BMC Vet Res",
"key": "722_CR168",
"unstructured": "Lin PY, Chang CD, Chen YC, Shih WL. RhoA/ROCK1 regulates Avian Reovirus S1133-induced switch from autophagy to apoptosis. BMC Vet Res. 2015;11:103.",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1016/S1016-8478(23)14014-3",
"author": "HJ Liu",
"doi-asserted-by": "publisher",
"first-page": "396",
"issue": "4",
"journal-title": "Mol Cells",
"key": "722_CR169",
"unstructured": "Liu HJ, Lin PY, Wang LR, Hsu HY, Liao MH, Shih WL. Activation of small GTPases RhoA and Rac1 is required for avian reovirus p10-induced syncytium formation. Mol Cells. 2008;26(4):396–403.",
"volume": "26",
"year": "2008"
},
{
"DOI": "10.1016/j.antiviral.2018.06.011",
"author": "Y Yin",
"doi-asserted-by": "publisher",
"first-page": "92",
"journal-title": "Antiviral Res",
"key": "722_CR170",
"unstructured": "Yin Y, Chen S, Hakim MS, Wang W, Xu L, Dang W, et al. 6-Thioguanine inhibits rotavirus replication through suppression of Rac1 GDP/GTP cycling. Antiviral Res. 2018;156:92–101.",
"volume": "156",
"year": "2018"
},
{
"DOI": "10.1007/s11033-020-05980-9",
"author": "D Fan",
"doi-asserted-by": "publisher",
"first-page": "9739",
"issue": "12",
"journal-title": "Mol Biol Rep",
"key": "722_CR171",
"unstructured": "Fan D, Wu N, Zhang J, Wang Z, Wang P, Gao N, et al. Effect of the Rho GTPase inhibitor-1 on the entry of dengue serotype 2 virus into EAhy926 cells. Mol Biol Rep. 2020;47(12):9739–47.",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.3390/ijms23094738",
"author": "D Yu",
"doi-asserted-by": "publisher",
"first-page": "4738",
"issue": "9",
"journal-title": "Int J Mol Sci",
"key": "722_CR172",
"unstructured": "Yu D, Wang L, Wang Y. Recent advances in application of computer-aided drug design in anti-influenza A virus drug discovery. Int J Mol Sci. 2022;23(9):4738.",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1080/07391102.2023.2259496",
"doi-asserted-by": "crossref",
"key": "722_CR173",
"unstructured": "Mufti IU, Sufyan M, Shahid I, Alzahrani AR, Shahzad N, IM MA, et al. Computer-aided identification of dengue virus NS2B/NS3 protease inhibitors: an integrated molecular modelling approach for screening of phytochemicals. J Biomol Struct Dyn. 2023:1–12."
},
{
"DOI": "10.1007/s11030-024-10850-8",
"author": "F Finke",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Mol Divers",
"key": "722_CR174",
"unstructured": "Finke F, Hungerland J, Solov’yov IA, Schuhmann F. Different receptor models show differences in ligand binding strength and location: a computational drug screening for the tick-borne encephalitis virus. Mol Divers. 2024;29:281.",
"volume": "29",
"year": "2024"
},
{
"DOI": "10.1080/07391102.2020.1794974",
"author": "MM Rahman",
"doi-asserted-by": "publisher",
"first-page": "6231",
"issue": "16",
"journal-title": "J Biomol Struct Dyn",
"key": "722_CR175",
"unstructured": "Rahman MM, Saha T, Islam KJ, Suman RH, Biswas S, Rahat EU, et al. Virtual screening, molecular dynamics and structure–activity relationship studies to identify potent approved drugs for Covid-19 treatment. J Biomol Struct Dyn. 2021;39(16):6231–41.",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1016/j.drup.2024.101053",
"author": "S Du",
"doi-asserted-by": "publisher",
"journal-title": "Drug Resist Updat",
"key": "722_CR176",
"unstructured": "Du S, Hu X, Menendez-Arias L, Zhan P, Liu X. Target-based drug design strategies to overcome resistance to antiviral agents: opportunities and challenges. Drug Resist Updat. 2024;73: 101053.",
"volume": "73",
"year": "2024"
},
{
"DOI": "10.1684/vir.2020.0864",
"author": "S Burrel",
"doi-asserted-by": "publisher",
"first-page": "325",
"issue": "5",
"journal-title": "Virologie (Montrouge)",
"key": "722_CR177",
"unstructured": "Burrel S, Topalis D, Boutolleau D. Herpes simplex virus resistance to antivirals. Virologie (Montrouge). 2020;24(5):325–42.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.drudis.2020.11.014",
"author": "H Patel",
"doi-asserted-by": "publisher",
"first-page": "503",
"issue": "2",
"journal-title": "Drug Discov Today",
"key": "722_CR178",
"unstructured": "Patel H, Kukol A. Integrating molecular modelling methods to advance influenza A virus drug discovery. Drug Discov Today. 2021;26(2):503–10.",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.3390/ijms23010393",
"author": "S Kralj",
"doi-asserted-by": "publisher",
"first-page": "393",
"issue": "1",
"journal-title": "Int J Mol Sci",
"key": "722_CR179",
"unstructured": "Kralj S, Jukic M, Bren U. Commercial SARS-CoV-2 targeted, protease inhibitor focused and protein–protein interaction inhibitor focused molecular libraries for virtual screening and drug design. Int J Mol Sci. 2021;23(1):393.",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1016/bs.apcsb.2019.11.008",
"author": "A Bagchi",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Adv Protein Chem Struct Biol",
"key": "722_CR180",
"unstructured": "Bagchi A. Latest trends in structure based drug design with protein targets. Adv Protein Chem Struct Biol. 2020;121:1–23.",
"volume": "121",
"year": "2020"
},
{
"DOI": "10.1038/s41401-021-00851-w",
"author": "CR Wu",
"doi-asserted-by": "publisher",
"first-page": "3021",
"issue": "12",
"journal-title": "Acta Pharmacol Sin",
"key": "722_CR181",
"unstructured": "Wu CR, Yin WC, Jiang Y, Xu HE. Structure genomics of SARS-CoV-2 and its Omicron variant: drug design templates for COVID-19. Acta Pharmacol Sin. 2022;43(12):3021–33.",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-21610-9",
"author": "R Kumar",
"doi-asserted-by": "publisher",
"first-page": "17811",
"issue": "1",
"journal-title": "Sci Rep",
"key": "722_CR182",
"unstructured": "Kumar R, Chander Y, Khandelwal N, Verma A, Rawat KD, Shringi BN, et al. ROCK1/MLC2 inhibition induces decay of viral mRNA in BPXV infected cells. Sci Rep. 2022;12(1):17811.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1073/pnas.2014242118",
"doi-asserted-by": "crossref",
"key": "722_CR183",
"unstructured": "Chichili VPR, Chew TW, Shankar S, Er SY, Chin CF, Jobichen C, et al. Structural basis for p50RhoGAP BCH domain-mediated regulation of Rho inactivation. Proc Natl Acad Sci USA. 2021;118(21)."
},
{
"DOI": "10.1007/978-1-4939-9089-4_2",
"author": "P Shinn",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "Methods Mol Biol",
"key": "722_CR184",
"unstructured": "Shinn P, Chen L, Ferrer M, Itkin Z, Klumpp-Thomas C, McKnight C, et al. High-throughput screening for drug combinations. Methods Mol Biol. 2019;1939:11–35.",
"volume": "1939",
"year": "2019"
},
{
"DOI": "10.1016/j.isci.2019.07.003",
"author": "LD Jasenosky",
"doi-asserted-by": "publisher",
"first-page": "1279",
"journal-title": "iScience.",
"key": "722_CR185",
"unstructured": "Jasenosky LD, Cadena C, Mire CE, Borisevich V, Haridas V, Ranjbar S, et al. The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus. iScience. 2019;19:1279–90.",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1016/j.jep.2019.111901",
"author": "H Xu",
"doi-asserted-by": "publisher",
"journal-title": "J Ethnopharmacol",
"key": "722_CR186",
"unstructured": "Xu H, He L, Chen J, Hou X, Fan F, Wu H, et al. Different types of effective fractions from Radix Isatidis revealed a multiple-target synergy effect against respiratory syncytial virus through RIG-I and MDA5 signaling pathways, a pilot study to testify the theory of superposition of traditional Chinese Medicine efficacy. J Ethnopharmacol. 2019;239: 111901.",
"volume": "239",
"year": "2019"
},
{
"DOI": "10.1128/CMR.00168-19",
"doi-asserted-by": "crossref",
"key": "722_CR187",
"unstructured": "Kumar N, Sharma S, Kumar R, Tripathi BN, Barua S, Ly H, et al. Host-directed antiviral therapy. Clin Microbiol Rev. 2020;33(3)."
},
{
"DOI": "10.1038/s41419-023-05633-2",
"author": "X Liu",
"doi-asserted-by": "publisher",
"first-page": "118",
"issue": "2",
"journal-title": "Cell Death Dis.",
"key": "722_CR188",
"unstructured": "Liu X, Jiang Y, Zhou H, Zhao X, Li M, Bao Z, et al. Dasabuvir suppresses esophageal squamous cell carcinoma growth in vitro and in vivo through targeting ROCK1. Cell Death Dis. 2023;14(2):118.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41598-018-30460-3",
"author": "SH Lee",
"doi-asserted-by": "publisher",
"first-page": "12469",
"issue": "1",
"journal-title": "Sci Rep.",
"key": "722_CR189",
"unstructured": "Lee SH, Moon JS, Pak BY, Kim GW, Lee W, Cho H, et al. HA1077 displays synergistic activity with daclatasvir against hepatitis C virus and suppresses the emergence of NS5A resistance-associated substitutions in mice. Sci Rep. 2018;8(1):12469.",
"volume": "8",
"year": "2018"
}
],
"reference-count": 189,
"references-count": 189,
"relation": {},
"resource": {
"primary": {
"URL": "https://cmbl.biomedcentral.com/articles/10.1186/s11658-025-00722-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Rho-GTPases subfamily: cellular defectors orchestrating viral infection",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "30"
}
