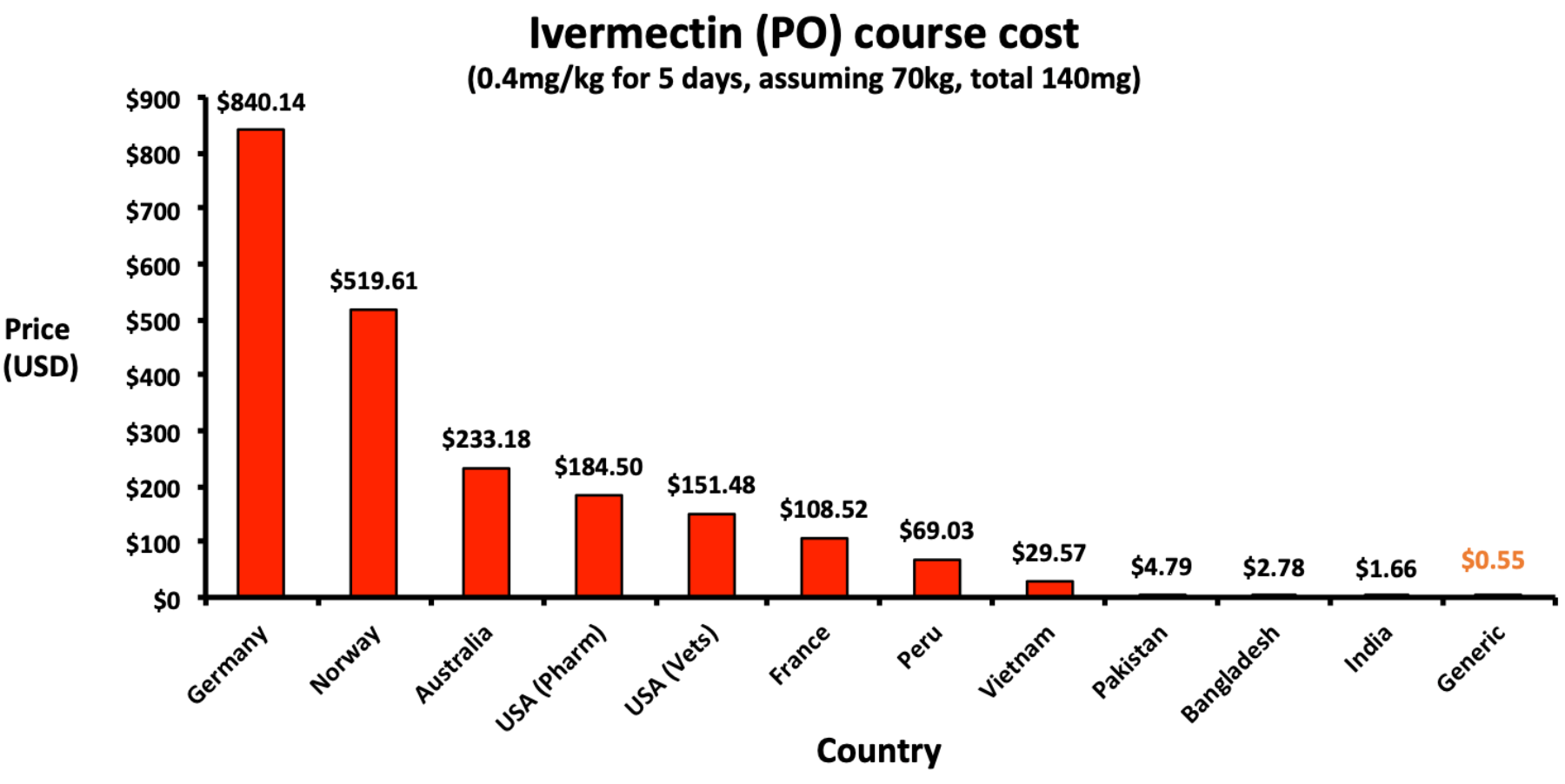
Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19
et al., medRxiv, doi:10.1101/2021.06.01.21258147, Jun 2021
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Analysis of the manufacturing cost of several COVID-19 medications, showing a cost of $0.55 per course of ivermectin, including excipients, formulation, tax, and profit.
1.
Reich, S., Methodological Analysis of Bias Risks in Adaptive Multi-Arm Platform Trials: A Case-Series from Three COVID-19 Studies, Center for Open Science, doi:10.31222/osf.io/h5kc8_v1.
2.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
3.
Zhang et al., Rho-GTPases subfamily: cellular defectors orchestrating viral infection, Cellular & Molecular Biology Letters, doi:10.1186/s11658-025-00722-w.
4.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
5.
Ulloa-Aguilar et al., The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection, Cells, doi:10.3390/cells13181591.
6.
Enyeji et al., Effective Treatment of COVID-19 Infection with Repurposed Drugs: Case Reports, Viral Immunology, doi:10.1089/vim.2024.0034.
7.
Wimalawansa, S., Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity, Heliyon, doi:10.1016/j.heliyon.2024.e34691.
8.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
9.
Mehraeen et al., Treatments for Olfactory Dysfunction in COVID-19: A Systematic Review, International Archives of Otorhinolaryngology, doi:10.1055/s-0044-1786046.
10.
Scheim et al., Back to the Basics of SARS-CoV-2 Biochemistry: Microvascular Occlusive Glycan Bindings Govern Its Morbidities and Inform Therapeutic Responses, Viruses, doi:10.3390/v16040647.
11.
Yagisawa et al., Global trends in clinical trials of ivermectin for COVID-19—Part 2, The Japanese Journal of Antibiotics, doi:10.11553/antibiotics.77.1_45.
12.
Liu et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
13.
Scheim (B) et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
14.
Yemeke et al., Impact of the COVID-19 pandemic on the quality of medical products in Zimbabwe: a qualitative study based on key informant interviews with health system stakeholders, BMJ Open, doi:10.1136/bmjopen-2022-068923.
15.
Kory, P., The Global War on Ivermectin, International Covid Summit III, European Parliament, Brussels, covid19criticalcare.com/wp-content/uploads/2023/05/GLOBAL-WAR-ON-IVERMECTIN-PARLIAMENT.pdf.
16.
Babalola et al., The Place of Ivermectin in the Management of Covid-19: State of the Evidence, Medical Research Archives, doi:10.18103/mra.v11i4.3778.
17.
Loo et al., Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-023-03520-1.
18.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
19.
Kory (B), P., The Criminal Censorship of Ivermectin's Efficacy By The High-Impact Medical Journals - Part 1, Pierre Kory’s Medical Musings, pierrekory.substack.com/p/the-criminal-censorship-of-ivermectins.
20.
Al-kuraishy et al., Central effects of Ivermectin in alleviation of Covid-19-induced dysautonomia, Current Drug Targets, doi:10.2174/1389450123666220810102406.
21.
Schwartz, E., Does ivermectin have a place in the treatment of mild Covid-19?, New Microbes and New Infections, doi:10.1016/j.nmni.2022.100989.
22.
Marques et al., Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols, Brazilian Journal of Biology, doi:10.1590/1519-6984.258325.
23.
Semiz, S., SIT1 transporter as a potential novel target in treatment of COVID-19, Biomolecular Concepts, doi:10.1515/bmc-2021-0017.
24.
Zaidi et al., The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review, The Journal of Antibiotics, doi:10.1038/s41429-021-00491-6.
25.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
26.
Low et al., Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166294.
27.
Fordham et al., The uses and abuses of systematic reviews, OSF Preprints, doi:10.31219/osf.io/mp4f2.
28.
Kow et al., Pitfalls in Reporting Sample Size Calculation Across Randomized Controlled Trials Involving Ivermectin for the treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001441.
29.
Santin et al., Ivermectin: a multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes and New Infections, doi:10.1016/j.nmni.2021.100924.
30.
Adegboro et al., A review of the anti-viral effects of ivermectin, African Journal of Clinical and Experimental Microbiology, doi:10.4314/ajcem.v22i3.2.
31.
Turkia, M., A Continuation of a Timeline of Ivermectin-Related Events in the COVID-19 Pandemic [June 30, 2021], ResearchGate, doi:10.13140/RG.2.2.16973.36326.
32.
Jagiasi et al., Variation in therapeutic strategies for the management of severe COVID-19 in India- A nationwide cross-sectional survey, The International Journal of Clinical Practice, doi:10.1111/ijcp.14574.
33.
Lind et al., Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis, Journal of General Internal Medicine, doi:10.1007/s11606-021-06948-6.
34.
Wang et al., Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19, medRxiv, doi:10.1101/2021.06.01.21258147.
35.
Kory (C) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001377.
36.
DiNicolantonio et al., Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors, Open Heart, doi:10.1136/openhrt-2021-001655.
37.
Turkia (B), M., A timeline of ivermectin-related events in the COVID-19 pandemic, Research Gate, www.researchgate.net/publication/350610718_A_Timeline_of_Ivermectin-Related_Events_in_the_COVID-19_Pandemic_April_3_2021.
38.
Wehbe et al., Repurposing Ivermectin for COVID-19: Molecular Aspects and Therapeutic Possibilities, Front. Immunol., doi:10.3389/fimmu.2021.663586.
39.
Yagisawa (B) et al., Global trends in clinical studies of ivermectin in COVID-19, The Japanese Journal of Antibiotics, 74-1, Mar 2021, jja-contents.wdc-jp.com/pdf/JJA74/74-1-open/74-1_44-95.pdf.
40.
Jans et al., The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.10.042.
41.
Kory (D) et al., Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Frontiers in Pharmacology, doi:10.3389/fphar.2021.643369.
42.
Formiga et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J. Control Release, doi:10.1016/j.jconrel.2020.10.009.
43.
Scheim (D), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
44.
Turkia (C), M., FLCCC Alliance MATH+ ascorbic acid and I-MASK+ ivermectin protocols for COVID-19 — a brief review, ResearchGate, www.researchgate.net/profile/Mika_Turkia/publication/345694745_FLCCC_Alliance_MATH_ascorbic_acid_and_I-MASK_ivermectin_protocols_for_COVID-19_-_A_Brief_Review/links/5fab010f4585150781078260/FLCCC-Alliance-MATH-ascorbic-acid-and-I-MASK-ivermectin-protocols-for-COVID-19-A-Brief-Review.pdf.
45.
Jans (B) et al., Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?, Cells 2020, 9:9, 2100, doi:10.3390/cells9092100.
46.
Elkholy et al., Ivermectin: A Closer Look at a Potential Remedy, Cureus, doi:10.7759/cureus.10378.
47.
DiNicolantonio (B) et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
48.
Vora et al., White paper on Ivermectin as a potential therapy for COVID-19, Indian Journal of Tuberculosis, doi:10.1016/j.ijtb.2020.07.031.
Wang et al., 3 Jun 2021, preprint, 4 authors.
Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19
doi:10.1101/2021.06.01.21258147
Background Currently, only dexamethasone, tocilizumab and sarilumab have conclusively been shown to reduce mortality of COVID-19. Safe and effective treatments will need to be both affordable and widely available globally to be used alongside vaccination programmes. This analysis will estimate and compare potential generic minimum costs of a selection of approved COVID-19 drug candidates with available international list prices.
Methods: We searched for repurposed drugs that have been approved by at least one of the WHO, FDA or NICE, or at least given emergency use authorisation or recommended for off-label prescription. Drug prices were searched for, for dexamethasone, budesonide, baricitinib, tocilizumab, casirivimab and imdevimab, and sarilumab using active pharmaceutical ingredients (API) data extracted from global shipping records. This was compared with national pricing data from a range of low, medium, and high-income countries. Annual API export volumes from India were used to estimate the current availability of each drug. Results: Repurposed therapies can be generically manufactured for some treatments at
Conflicts of interest: None to declare.
References
Ahlqvist, Mcgeough, Senanayake, Armstrong, Yadaw et al., Progress Toward a Large-Scale Synthesis of Molnupiravir (MK-4482, EIDD-2801) from Cytidine, ACS Omega, doi:10.1021/acsomega.1c00772
Barber, Dzintars, Estimated cost-based generic prices for molnupiravir for the treatment of COVID-19 infection
Bhuyan, In India, black markets for tocilizumab spring up as demand for the Covid-19 drug surges
Eaton, Covid-19: WHO warns against "vaccine nationalism" or face further virus mutations, BMJ, doi:10.1136/bmj.n292
Gotham, Barber, Hill, Estimation of cost-based prices for injectable medicines in the WHO Essential Medicines List, BMJ Open
Group, Yu, Bafadhel, Dorward, Hayward et al., Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial, doi:10.1101/2021.04.10.21254672
Hill, Barber, Gotham, Estimated costs of production and potential prices for the WHO Essential Medicines List, BMJ Global Health
Hill, Garratt, Levi, Falconer, Ellis et al., Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection
Hill, Simmons, Gotham, Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C, J Virus Erad
Hill, Wang, Levi, Minimum costs to manufacture new treatments for COVID-19
Horby, Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(21)00149-5
Horby, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label
Horby, Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label
Horby, Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2022926
Horby, Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(20)32013-4
Investigators, Gordon, Mouncey, Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2100433
Kalil, Patterson, Mehta, Baricitinib plus remdesivir for hospitalized adults with Covid-19, New England Journal of Medicine
Khan, Stewart, Fabbri, Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19, Thorax. Published Online First, doi:10.1136/thoraxjnl-2020-215266
Klutz, Holtmann, Lobedann, Cost evaluation of antibody production processes in different operation modes, Chemical Engineering Science
Love, The production of generic drugs in India, BMJ
Mahase, Covid-19: Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports, BMJ
Moon, Jambert, Childs, A win-win solution?: A critical analysis of tiered pricing to improve access to medicines in developing countries, Global Health, doi:10.1186/1744-8603-7-39
Mullard, Flooded by the torrent: the COVID-19 drug pipeline, Lancet, doi:10.1016/S0140-6736(20)30894-1
New, Revise Biosimilar Guidelines, Scientists Demand; WHO Says Not Now
Pan, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med, doi:10.1056/NEJMoa2023184
Popp, Stegemann, Metzendorf, Gould, Kranke et al., Ivermectin for preventing and treating COVID-19, Cochrane Database of Systematic Reviews, doi:10.1002/14651858.CD015017.pub2
Ramakrishnan, Jr, Langford, Mahdi, Jeffers et al., Inhaled in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respiratory Medicine
Recovery Collaborative, Dexamethasone in hospitalized patients with Covid-19, New England Journal of Medicine
Simoens, Vulto, A health economic guide to market access of biosimilars, Expert Opin Biol Ther, doi:10.1080/14712598.2021.1849132
Stebbing, Phelan, Griffin, COVID-19: combining antiviral and antiinflammatory treatments, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30132-8
Tardif, Bouabdallaoui, Allier, randomised, double-blinded, adaptive, placebocontrolled, multicentre trial
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody cocktail clinical outcomes study in Covid-19 outpatients
Wire, Governments Spent at Least €93bn on COVID-19 Vaccines and Therapeutics During the Last 11 Months
DOI record:
{
"DOI": "10.1101/2021.06.01.21258147",
"URL": "http://dx.doi.org/10.1101/2021.06.01.21258147",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Currently, only dexamethasone, tocilizumab and sarilumab have conclusively been shown to reduce mortality of COVID-19. Safe and effective treatments will need to be both affordable and widely available globally to be used alongside vaccination programmes. This analysis will estimate and compare potential generic minimum costs of a selection of approved COVID-19 drug candidates with available international list prices.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We searched for repurposed drugs that have been approved by at least one of the WHO, FDA or NICE, or at least given emergency use authorisation or recommended for off-label prescription. Drug prices were searched for, for dexamethasone, budesonide, baricitinib, tocilizumab, casirivimab and imdevimab, and sarilumab using active pharmaceutical ingredients (API) data extracted from global shipping records. This was compared with national pricing data from a range of low, medium, and high-income countries. Annual API export volumes from India were used to estimate the current availability of each drug.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Repurposed therapies can be generically manufactured for some treatments at very low per-course costs, ranging from $2.58 for IV dexamethasone (or $0.19 orally) and $4.34 for inhaled budesonide. No export price data was available for baricitinib, tocilizumab, casirivimab and imdevimab or sarilumab, but courses of these treatments are priced highly, ranging from $6.67 for baricitinib to $875.5 for sarilumab. When comparing international list prices, we found wide variations between countries.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Successful management of COVID-19 will require equitable access to treatment for all populations, not just those able to pay high prices. Dexamethasone and budesonide are widely available and affordable, whilst monoclonal antibodies and IV treatment courses are more expensive.</jats:p></jats:sec><jats:sec><jats:title>Key Points</jats:title><jats:list list-type=\"bullet\"><jats:list-item><jats:p>Re-purposed drugs must be affordable worldwide to compliment COVID-19 vaccine programmes.</jats:p></jats:list-item><jats:list-item><jats:p>Estimated costs/course were: dexamethasone (Oral $0.22, IV $2.58), budesonide ($4.34), baricitnib ($6.67), tocilizumab ($410.59), sarilumab ($875.70). Casirivimab and imdevimab = no data available.</jats:p></jats:list-item><jats:list-item><jats:p>High drug prices will limit access.</jats:p></jats:list-item></jats:list></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
10,
18
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7635-9461",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wang",
"given": "Junzheng",
"sequence": "first"
},
{
"affiliation": [],
"family": "Levi",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ellis",
"given": "Leah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hill",
"given": "Andrew",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
4
]
],
"date-time": "2021-06-04T03:25:14Z",
"timestamp": 1622777114000
},
"deposited": {
"date-parts": [
[
2022,
12,
29
]
],
"date-time": "2022-12-29T19:26:22Z",
"timestamp": 1672341982000
},
"group-title": "Health Economics",
"indexed": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T23:52:47Z",
"timestamp": 1709337167721
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2021,
6,
3
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.06.01.21258147",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
6,
3
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
6,
3
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1136/bmj.n292",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.1"
},
{
"DOI": "10.1016/S2055-6640(20)30018-2",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.2"
},
{
"DOI": "10.1056/NEJMoa2022926",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.3"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.4"
},
{
"DOI": "10.1016/S0140-6736(20)32013-4",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.5"
},
{
"DOI": "10.1016/S0140-6736(21)00149-5",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.6"
},
{
"DOI": "10.1101/2021.05.18.21257267",
"doi-asserted-by": "crossref",
"key": "2021102011301112000_2021.06.01.21258147v3.7",
"unstructured": "RECOVERY Collaborative Group. Horby P et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. [Preprint]. MedRxiv. 18th May 2021. doi: https://doi.org/10.1101/2021.05.18.21257267"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.8"
},
{
"DOI": "10.1093/ofid/ofab358",
"doi-asserted-by": "crossref",
"key": "2021102011301112000_2021.06.01.21258147v3.9",
"unstructured": "Hill A , Garratt A , Levi J , Falconer J , Ellis L , McCann K et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. [Revised manuscript]. Open Forum Infectious Diseases. 2021"
},
{
"DOI": "10.1002/14651858.CD015017.pub2",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.10"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with Covid-19",
"doi-asserted-by": "crossref",
"first-page": "795",
"issue": "9",
"journal-title": "New England Journal of Medicine",
"key": "2021102011301112000_2021.06.01.21258147v3.11",
"volume": "384",
"year": "2021"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.12",
"unstructured": "US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes drug combination for treatment of COVID-19. [Press Release]. 19th November 2020. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19 [Accessed 01/10/2021]"
},
{
"DOI": "10.1136/bmj.n2422",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.13"
},
{
"DOI": "10.1101/2021.06.15.21258542",
"doi-asserted-by": "crossref",
"key": "2021102011301112000_2021.06.01.21258147v3.14",
"unstructured": "RECOVERY Collaborative Group, Horby P et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. [Preprint]. MedRxiv.16th June 2021. doi: https://doi.org/10.1101/2021.06.15.21258542"
},
{
"DOI": "10.1101/2021.05.19.21257469",
"doi-asserted-by": "crossref",
"key": "2021102011301112000_2021.06.01.21258147v3.15",
"unstructured": "Weinreich DM , Sivapalasingam S , Norton T , Ali S , Gao H , Bhore R , et al. REGEN-COV antibody cocktail clinical outcomes study in Covid-19 outpatients. [Preprint]. MedRxiv. 6th July 2021. doi: https://doi.org/10.1101/2021.05.19.21257469"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.16",
"unstructured": "National Institute for Health and Care Excellence (NICE). COVID-19 rapid guideline: Managing COVID-19. Section 7.2: Casirivimab and imdevimab - hospital use. Version 13.2 published on 13.10.2021. Available from https://files.magicapp.org/guideline/ee9a60f9-6475-4f1c-9a31-d8046a513991/published_guideline_5679-13_2.pdf [Accessed 14/10/2021]."
},
{
"DOI": "10.1056/NEJMoa2100433",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.17"
},
{
"author": "RECOVERY Collaborative Group",
"first-page": "693",
"issue": "8",
"journal-title": "Dexamethasone in hospitalized patients with Covid-19 New England Journal of Medicine",
"key": "2021102011301112000_2021.06.01.21258147v3.18",
"volume": "384",
"year": "2021"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.19",
"unstructured": "Panjiva Database. Available from: https://panjiva.com [Accessed 10/04/2021]"
},
{
"DOI": "10.1136/bmj.d1694",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.20"
},
{
"DOI": "10.1136/bmjopen-2018-027780",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.21"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.22"
},
{
"DOI": "10.1101/2021.04.10.21254672",
"doi-asserted-by": "crossref",
"key": "2021102011301112000_2021.06.01.21258147v3.23",
"unstructured": "PRINCIPLE Collaborative Group, Yu L-M , Bafadhel M , Dorward J , Hayward G , Saville BR , Gbinigie O et al. Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial. [Preprint]. 12th April 2021. Medrxiv. https://doi.org/10.1101/2021.04.10.21254672"
},
{
"DOI": "10.1016/j.ces.2015.10.029",
"doi-asserted-by": "crossref",
"key": "2021102011301112000_2021.06.01.21258147v3.24",
"unstructured": "Klutz S , Holtmann L , Lobedann M et al. Cost evaluation of antibody production processes in different operation modes. Chemical Engineering Science, 141, 63–74."
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.25",
"unstructured": "Bhuyan A. In India, black markets for tocilizumab spring up as demand for the Covid-19 drug surges. Scroll.in. 7th July 2021. Available from https://scroll.in/article/966644/in-india-black-markets-for-tocilizumab-spring-up-as-demand-for-the-covid-19-drug-surges [Accessed 10/07/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.26",
"unstructured": "National Institute for Health and Care Excellence (NICE). COVID-19 prescribing briefing: corticosteroids. February 2021. Available from: https://www.nice.org.uk/guidance/ng159/resources/covid19-prescribing-briefing-corticosteroids-pdf-8839913581 [Accessed 10/02/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.27",
"unstructured": "Randomised Evaluation of COVID-19 Therapy (RECOVERY) Trial Protocol. Version 7.0. 18th June 2020. Available from: https://www.recoverytrial.net/files/recovery-protocol-v7-0-2020-06-18.pdf [Accessed 10/02/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.28",
"unstructured": "Department of Health and Social Care. COVID-19 Therapeutic Alert: Interleukin-6 inhibitors (tocilizumab or sarilumab) for patients admitted to ICU with COVID-19 pneumonia (adults)Reference CEM/CMO/2021/001. 8th January 2021. Available from: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103134 [Accessed 10/03/2021]"
},
{
"DOI": "10.1016/S2055-6640(20)30691-9",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.29"
},
{
"DOI": "10.1186/1744-8603-7-39",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.30"
},
{
"DOI": "10.1590/1413-81232017228.16262017",
"doi-asserted-by": "crossref",
"key": "2021102011301112000_2021.06.01.21258147v3.31",
"unstructured": "United Nation Secretary General’s High-Level Panel on Access to Medicines. Report of the United Nation Secretary General’s High-Level Panel on Access to Medicines: Promoting innovation and access to health technologies. 14th September 2016. p. 22. Available from: http://www.unsgaccessmeds.org/final-report. [Accessed 10/04/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.32",
"unstructured": "World Trade Organisation, Council for Trade-Related Aspects of Intellectual Property Rights. Waiver from certain provisions of the TRIPS agreement for the prevention, containment and treatment of COVID-19 -Communication from India and South Africa. 2nd October 2020. IP/C/W/669. Available from https://docs.wto.org/dol2fe/Pages/SS/directdoc.aspx?filename=q:/IP/C/W669.pdf&Open=True [Accessed 10/04/2021]"
},
{
"DOI": "10.1136/thoraxjnl-2020-215266",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.33"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.34",
"unstructured": "World Health Organisation. Therapeutics and COVID-19: living guideline. 31st March 2021. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1 [Accessed 01/04/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.35",
"unstructured": "ThePharmaLetter. US govt procuring $1.2 billion of Merck’s COVID-19 candidate. 9th June 2021. Available from https://www.thepharmaletter.com/article/us-govt-procuring-1-2-billion-of-merck-s-covid-19-candidate [Accessed 01/07/2021]"
},
{
"DOI": "10.1136/bmjgh-2017-000571",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.36"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.37",
"unstructured": "World Health Organisation. Commitment and call to action: Global collaboration to accelerate new COVID-19 health technologies. 24th April 2020. [Statement]. Available from: https://www.who.int/news-room/detail/24-04-2020-commitment-and-call-to-action-global-collaboration-to-accelerate-new-covid-19-health-technologies [Accessed 10/03/2021]"
},
{
"DOI": "10.1016/S0140-6736(20)30894-1",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.38"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.39",
"unstructured": "Institute of Medicine (US) Forum on Medical and Public Health Preparedness for Catastrophic Events. Medical Countermeasures Dispensing: Emergency Use Authorization and the Postal Model, Workshop Summary. Washington (DC): National Academies Press (US); 2010. Emergency Use Authorization. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53122 [Accessed 10/03/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.40",
"unstructured": "Business Wire. Governments Spent at Least €93bn on COVID-19 Vaccines and Therapeutics During the Last 11 Months. 11th January 2021. Available from: https://www.businesswire.com/news/home/20210110005098/en [Accessed 10/03/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.41",
"unstructured": "Barber M , Dzintars G. Estimated cost-based generic prices for molnupiravir for the treatment of COVID-19 infection. 1st October 2021. Available from https://scholar.harvard.edu/melissabarber/publications/estimated-cost-based-generic-prices-molnupiravir-treatment-covid-19 [Accessed 10/10/2021]"
},
{
"DOI": "10.1021/acsomega.1c00772",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.42"
},
{
"DOI": "10.1080/14712598.2021.1849132",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.43"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.44",
"unstructured": "Association for Accessible Medicines. 2020 Generic Drug & Biosimilars Access & Savings in the U.S. Report. 2020. Available from https://accessiblemeds.org/sites/default/files/2020-09/AAM-2020-Generics-Biosimilars-Access-Savings-Report-US-Web.pdf [Accessed 10/04/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.45",
"unstructured": "New W. Revise Biosimilar Guidelines, Scientists Demand; WHO Says Not Now. 25th April 2019. Health Policy Watch. Available from https://healthpolicy-watch.news/revise-biosimilar-guidelines-scientists-demand-who-says-not-now/ [Accessed 10/04/2021]"
},
{
"DOI": "10.1016/S1473-3099(20)30132-8",
"doi-asserted-by": "publisher",
"key": "2021102011301112000_2021.06.01.21258147v3.46"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.47",
"unstructured": "US Food and Drugs Administration. Emergency Use Authorization 099. 24th June 2021. [Letter]. Available from https://www.fda.gov/media/150319/download [Accessed 10/10/2021]"
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.48",
"unstructured": "National Institute for Health and Care Excellence (NICE). Treatments being monitored by RAPID C-19. Available from https://www.nice.org.uk/covid-19/rapid-c-19-treatments-currently-monitored. [Accessed 10/10/2021]."
},
{
"key": "2021102011301112000_2021.06.01.21258147v3.49",
"unstructured": "US Food and Drugs Administration. Letter to Regeneron Inc. 21st November 2020. [Letter]. Available from https://www.fda.gov/media/143891/download. [Accessed 10/10/2021]."
}
],
"reference-count": 49,
"references-count": 49,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.1093/ofid/ofab581",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.06.01.21258147"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19",
"type": "posted-content"
}
