Remdesivir and molnupiravir had comparable efficacy in lung transplant recipients with mild-to-moderate COVID-19: a single center experience
et al., Frontiers in Transplantation, doi:10.3389/frtra.2024.1408289, Jul 2024
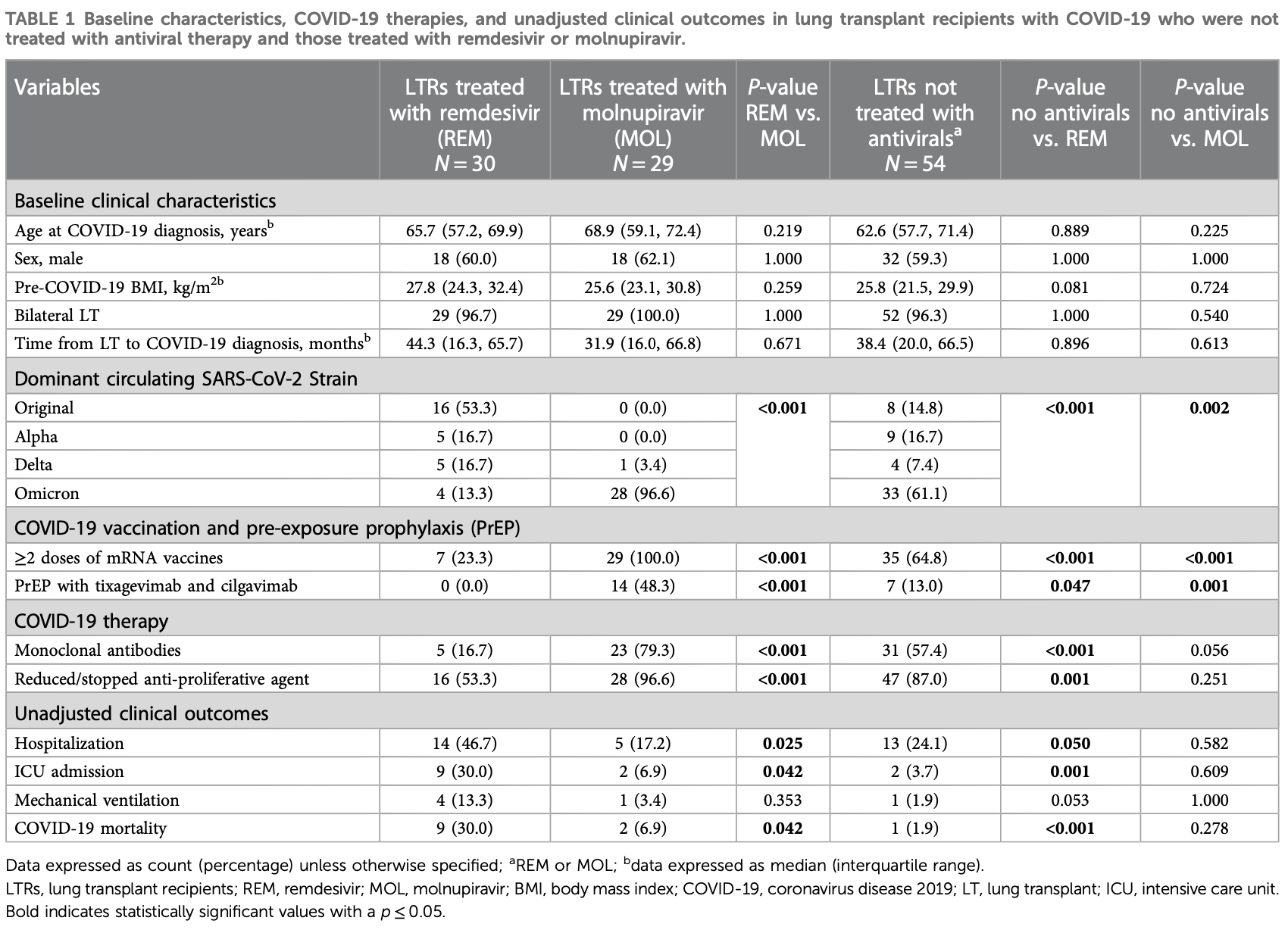
Retrospective 113 lung transplant recipients with mild-to-moderate COVID-19 showing higher mortality with remdesivir and molnupiravir in unadjusted analysis, with statistical significance for remdesivir. mAb PrEP and treatment and the dominant variant favored molnupiravir compared with the control group, however they favored the control group compared with remdesivir.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Study covers molnupiravir and remdesivir.
|
risk of death, 272.4% higher, RR 3.72, p = 0.28, treatment 2 of 29 (6.9%), control 1 of 54 (1.9%).
|
|
risk of mechanical ventilation, 86.2% higher, RR 1.86, p = 1.00, treatment 1 of 29 (3.4%), control 1 of 54 (1.9%).
|
|
risk of ICU admission, 86.2% higher, RR 1.86, p = 0.61, treatment 2 of 29 (6.9%), control 2 of 54 (3.7%).
|
|
risk of hospitalization, 28.4% lower, RR 0.72, p = 0.58, treatment 5 of 29 (17.2%), control 13 of 54 (24.1%), NNT 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Razia et al., 4 Jul 2024, retrospective, Italy, peer-reviewed, 6 authors, study period March 2020 - August 2022.
Contact: sofya.tokman@dignityhealth.org.
Remdesivir and molnupiravir had comparable efficacy in lung transplant recipients with mild-to-moderate COVID-19: a single center experience
Frontiers in Transplantation, doi:10.3389/frtra.2024.1408289
Introduction: Remdesivir (REM) and molnupiravir (MOL) are commonly used to treat lung transplant recipients (LTRs) with COVID-19; however, the clinical efficacy of these medications is yet to be compared. In this retrospective cohort study, we compared the clinical outcomes between LTRs with mild-tomoderate COVID-19 treated with REM and those treated with MOL. Methods and Results: Between March 2020 and August 2022, 195 LTRs developed COVID-19 at our center. After excluding 82 who presented with severe disease requiring hospitalization, the remaining 113 were included in the analysis: 54 did not receive antiviral treatment, 30 were treated with REM, and 29 were treated with MOL. Adjusted multivariable logistic regression analysis showed similar rates of hospitalization (adjusted odds ratio (aOR) 1.169, [95% confidence interval (95% CI) 0.105-12.997, p = 0.899], ICU admission (aOR 0.822, 95% CI 0.042-16.220, p = 0.898), mechanical ventilation (aOR 0.903, 95% CI 0.015-55.124, p = 0.961), and COVID-19related mortality (aOR 0.822, 95% CI 0.042-16.220, p = 0.898) between LTRs treated with REM and those treated with MOL for mild-to-moderate COVID-19, irrespective of SARS-CoV-2 strain. Conclusion: MOL may be a suitable alternative to REM to treat LTRs with mildto-moderate COVID-19, and the choice of antiviral therapy can be driven by practical considerations such as route of administration and drug availability.
Ethics statement The studies involving humans were approved by The Institutional Review Board of St. Joseph's Hospital and Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because the study was a retrospective data analysis study with no identifiable patient information.
Author contributions
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bhimraj, Morgan, Shumaker, Baden, Cheng et al., Infectious Diseases Society of America Guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciac724
Dauriat, Beaumont, Nguyen, Picard, Penhouet et al., Efficacy of three COVID-19 vaccine doses in lung transplant recipients: a multicentre cohort study, Eur Respir J, doi:10.1183/13993003.00502-2022
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe COVID-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Hallett, Greenberg, Boyarsky, Shah, Ou et al., SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients, J Heart Lung Transplant, doi:10.1016/j.healun.2021.07.026
Havlin, Svorcova, Dvorackova, Lastovicka, Lischke et al., Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients, J Heart Lung Transplant, doi:10.1016/j.healun.2021.05.004
Lo, Jordan, Arvey, Sudhamsu, Shrivastava-Ranjan et al., GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses, Sci Rep, doi:10.1038/srep43395
Narasimhan, Mahimainathan, Clark, Usmani, Cao et al., Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines, Vaccines, doi:10.3390/vaccines9070708
Peled, Lavee, Sternik, Segev, Wieder-Finesod, BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response, J Heart Lung Transplant, doi:10.1016/j.healun.2021.04.003
Razia, None
Sheahan, Sims, Graham, Menachery, Gralinski et al., Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses, Sci Transl Med, doi:10.1126/scitranslmed.aal3653
Sindu, Razia, Bay, Padiyar, Grief et al., Evolving impact of the COVID-19 pandemic on lung transplant recipients: a single-center experience, J Heart Lung Transplant, doi:10.1016/j.healun.2023.10.010
Warren, Jordan, Lo, Ray, Mackman et al., Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys, Nature, doi:10.1038/nature17180
DOI record:
{
"DOI": "10.3389/frtra.2024.1408289",
"ISSN": [
"2813-2440"
],
"URL": "http://dx.doi.org/10.3389/frtra.2024.1408289",
"abstract": "<jats:sec><jats:title>Introduction</jats:title><jats:p>Remdesivir (REM) and molnupiravir (MOL) are commonly used to treat lung transplant recipients (LTRs) with COVID-19; however, the clinical efficacy of these medications is yet to be compared. In this retrospective cohort study, we compared the clinical outcomes between LTRs with mild-to-moderate COVID-19 treated with REM and those treated with MOL.</jats:p></jats:sec><jats:sec><jats:title>Methods and Results</jats:title><jats:p>Between March 2020 and August 2022, 195 LTRs developed COVID-19 at our center. After excluding 82 who presented with severe disease requiring hospitalization, the remaining 113 were included in the analysis: 54 did not receive antiviral treatment, 30 were treated with REM, and 29 were treated with MOL. Adjusted multivariable logistic regression analysis showed similar rates of hospitalization (adjusted odds ratio (aOR) 1.169, [95% confidence interval (95% CI) 0.105–12.997, <jats:italic>p</jats:italic> = 0.899], ICU admission (aOR 0.822, 95% CI 0.042–16.220, <jats:italic>p</jats:italic> = 0.898), mechanical ventilation (aOR 0.903, 95% CI 0.015–55.124, <jats:italic>p</jats:italic> = 0.961), and COVID-19-related mortality (aOR 0.822, 95% CI 0.042–16.220, <jats:italic>p</jats:italic> = 0.898) between LTRs treated with REM and those treated with MOL for mild-to-moderate COVID-19, irrespective of SARS-CoV-2 strain.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>MOL may be a suitable alternative to REM to treat LTRs with mild-to-moderate COVID-19, and the choice of antiviral therapy can be driven by practical considerations such as route of administration and drug availability.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/frtra.2024.1408289"
],
"author": [
{
"affiliation": [],
"family": "Razia",
"given": "Deepika",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sindu",
"given": "Devika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cherrier",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grief",
"given": "Katherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walia",
"given": "Rajat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tokman",
"given": "Sofya",
"sequence": "additional"
}
],
"container-title": "Frontiers in Transplantation",
"container-title-short": "Front. Transplant.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2024,
7,
4
]
],
"date-time": "2024-07-04T04:48:50Z",
"timestamp": 1720068530000
},
"deposited": {
"date-parts": [
[
2024,
7,
4
]
],
"date-time": "2024-07-04T04:48:54Z",
"timestamp": 1720068534000
},
"indexed": {
"date-parts": [
[
2024,
7,
5
]
],
"date-time": "2024-07-05T00:16:51Z",
"timestamp": 1720138611512
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
7,
4
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
4
]
],
"date-time": "2024-07-04T00:00:00Z",
"timestamp": 1720051200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/frtra.2024.1408289/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2024,
7,
4
]
]
},
"published-online": {
"date-parts": [
[
2024,
7,
4
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/j.healun.2023.10.010",
"article-title": "Evolving impact of the COVID-19 pandemic on lung transplant recipients: a single-center experience",
"author": "Sindu",
"doi-asserted-by": "publisher",
"first-page": "442",
"journal-title": "J Heart Lung Transplant",
"key": "B1",
"volume": "43",
"year": "2024"
},
{
"DOI": "10.1183/13993003.00502-2022",
"article-title": "Efficacy of three COVID-19 vaccine doses in lung transplant recipients: a multicentre cohort study",
"author": "Dauriat",
"doi-asserted-by": "publisher",
"first-page": "2200502",
"journal-title": "Eur Respir J",
"key": "B2",
"volume": "61",
"year": "2023"
},
{
"DOI": "10.1016/j.healun.2021.07.026",
"article-title": "SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients",
"author": "Hallett",
"doi-asserted-by": "publisher",
"first-page": "1579",
"journal-title": "J Heart Lung Transplant",
"key": "B3",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1016/j.healun.2021.05.004",
"article-title": "Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients",
"author": "Havlin",
"doi-asserted-by": "publisher",
"first-page": "754",
"journal-title": "J Heart Lung Transplant",
"key": "B4",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.3390/vaccines9070708",
"article-title": "Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines",
"author": "Narasimhan",
"doi-asserted-by": "publisher",
"journal-title": "Vaccines (Basel)",
"key": "B5",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.healun.2021.04.003",
"article-title": "BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response",
"author": "Peled",
"doi-asserted-by": "publisher",
"first-page": "759",
"journal-title": "J Heart Lung Transplant",
"key": "B6",
"volume": "40",
"year": "2021"
},
{
"article-title": "Infectious Diseases Society of America Guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19)",
"author": "Bhimraj",
"first-page": "e250",
"key": "B7",
"volume-title": "Clin Infect Dis",
"year": "2022"
},
{
"DOI": "10.1038/srep43395",
"article-title": "GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses",
"author": "Lo",
"doi-asserted-by": "publisher",
"first-page": "43395",
"journal-title": "Sci Rep",
"key": "B8",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1126/scitranslmed.aal3653",
"article-title": "Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses",
"author": "Sheahan",
"doi-asserted-by": "publisher",
"journal-title": "Sci Transl Med",
"key": "B9",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1038/nature17180",
"article-title": "Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys",
"author": "Warren",
"doi-asserted-by": "publisher",
"first-page": "381",
"journal-title": "Nature",
"key": "B10",
"volume": "531",
"year": "2016"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe COVID-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "B11",
"volume": "386",
"year": "2022"
},
{
"key": "B12",
"volume-title": "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines",
"year": "2024"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/frtra.2024.1408289/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Remdesivir and molnupiravir had comparable efficacy in lung transplant recipients with mild-to-moderate COVID-19: a single center experience",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "3"
}