Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis
et al., Nutrients, doi:10.3390/nu14194217, Oct 2022
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
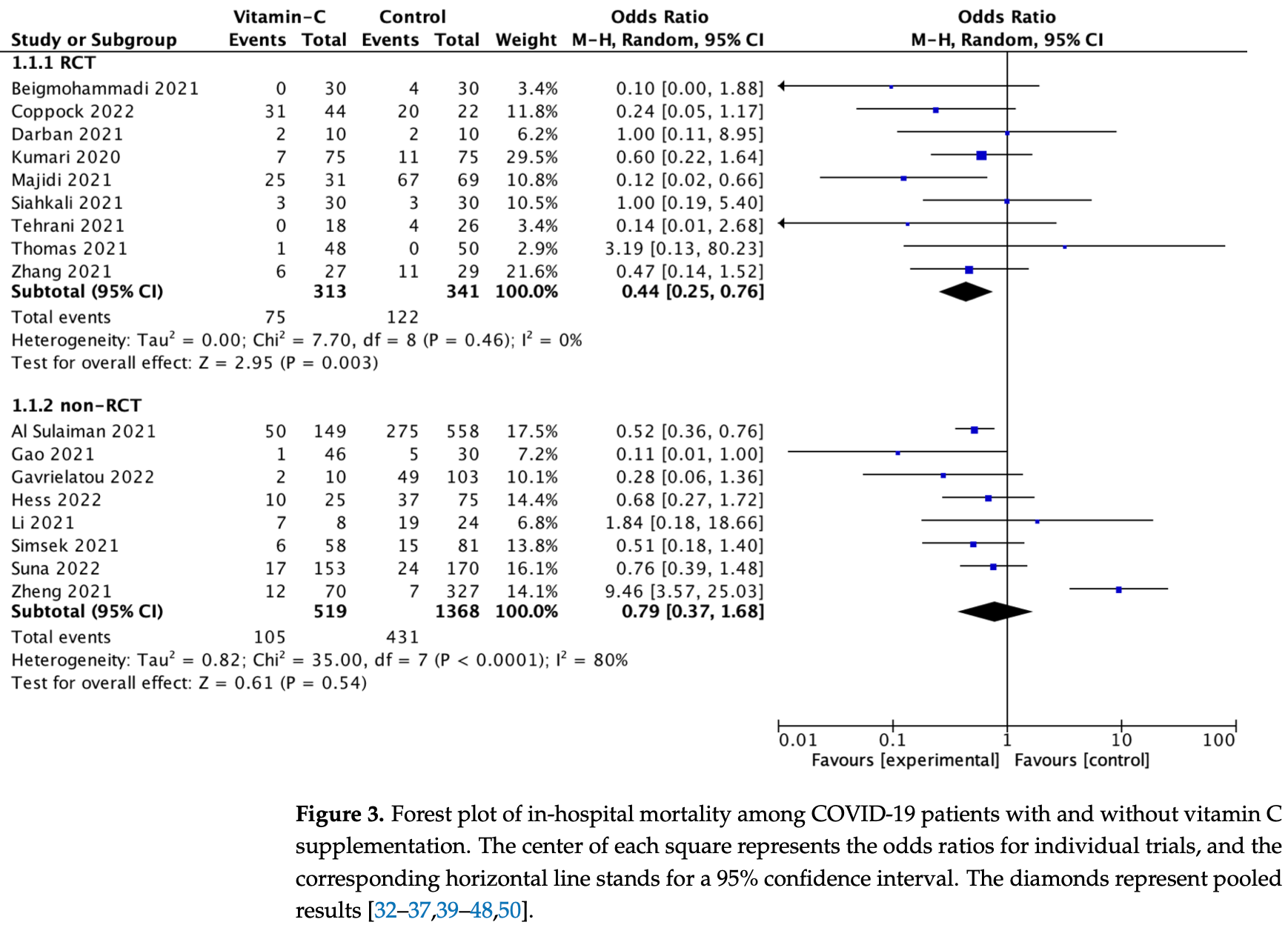
Systematic review and meta analysis of 19 studies showing lower mortality with vitamin C treatment, statistically significant for RCTs but not for non-RCT studies, and longer ICU length of stay.
7 meta-analyses show significant improvements with vitamin C for mortality1-5,
progression6,
severity1,5, and
cases7.
Currently there are 74 vitamin C for COVID-19 studies, showing 18% lower mortality [9‑27%], 9% lower ventilation [-12‑27%], 14% lower ICU admission [2‑24%], 19% lower hospitalization [7‑30%], and 3% fewer cases [-16‑19%].
|
risk of death, 56.0% lower, RR 0.44, p = 0.004, RCTs.
|
|
risk of death, 21.0% lower, RR 0.79, p = 0.55, non-RCTs.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Bhowmik et al., Impact of high-dose vitamin C on the mortality, severity, and duration of hospital stay in COVID-19 patients: A meta-analysis, Health Science Reports, doi:10.1002/hsr2.762.
2.
Olczak-Pruc et al., Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis, Nutrients, doi:10.3390/nu14194217.
3.
Kow et al., The effect of vitamin C on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials, Inflammopharmacology, doi:10.1007/s10787-023-01200-5.
4.
Kow (B) et al., Impact of uricosurics on mortality outcomes in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials, International Journal of Pharmacy Practice, doi:10.1093/ijpp/riae003.
5.
Qin et al., Effects of Vitamin C Supplements on Clinical Outcomes and Hospitalization Duration for Patients with Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis, Nutrition Reviews, doi:10.1093/nutrit/nuae154.
Olczak-Pruc et al., 10 Oct 2022, peer-reviewed, 8 authors.
Contact: lukasz.szarpak@gmail.com (corresponding author), lukasz.szarpak@bcm.edu.
Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis
Nutrients, doi:10.3390/nu14194217
Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe respiratory syndrome coronavirus 2 (SARS-CoV-2), millions of people have died, and the medical system has faced significant difficulties. Our purpose was to perform a meta-analysis to estimate the effect of vitamin C on in-hospital mortality and the ICU or hospital length of stay for patients diagnosed with COVID-19. We conducted a systematic review with meta-analysis in the following databases: PubMed, Web of Science, Scopus and Cochrane Central Register of Controlled Trials. We included studies that evaluated the effect of vitamin C supplementation, compared with standard treatment in COVID-19 patients who are ≥18 y of age. Nineteen trials were included in the meta-analysis. In-hospital mortality with and without vitamin C supplementation was 24.1% vs. 33.9% (OR = 0.59; 95%CI: 0.37 to 0.95; p = 0.03), respectively. Sub-analysis showed that, in randomized clinical trials, in-hospital mortality varied and amounted to 23.9% vs. 35.8% (OR = 0.44; 95%CI: 0.25 to 0.76; p = 0.003), respectively. In the non-randomized trials, in-hospital mortality was 24.2% vs. 33.5% (OR = 0.72; 95%CI: 0.38 to 1.39; p = 0.33), respectively. The ICU length of stay was longer in patients treated with vitamin C vs. standard therapy, 11.1 (7.3) vs. 8.3 (4.7) days (MD = 1.91; 95%CI: 0.89 to 2.93; p < 0.001), respectively. Acute kidney injury in patients treated with and without vitamin C varied and amounted to 27.8% vs. 45.0% (OR = 0.56; 95%CI: 0.40 to 0.78; p < 0.001), respectively. There were no differences in the frequency of other adverse events among patients' treatment with and without vitamin C (all p > 0.05). The use of vitamin C reduces hospital mortality. The length of stay in the ICU is longer among patients treated with vitamin C. In terms of patient safety, vitamin C has an acceptable profile. Low doses of vitamin C are effective and safe. Despite some evidence of the usefulness of vitamin C in modifying the course of COVID-19, it is too early to modify guidelines and recommendations. Further studies, in particular randomized clinical trials, are necessary.
Conflicts of Interest: The authors declare no conflict of interest.
References
Al Sulaiman, Aljuhani, Saleh, Badreldin, Al Harthi et al., Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: A propensity score matched study, Sci. Rep, doi:10.1038/s41598-021-96703-y
Ao, Li, Yuan, Wang, Nasr et al., Intravenous vitamin C use and risk of severity and mortality in COVID-19: A systematic review and meta-analysis, Nutr. Clin. Pract, doi:10.1002/ncp.10832
Bae, Kim, Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19, Molecules, doi:10.3390/molecules25225346
Beigmohammadi, Bitarafan, Hoseindokht, Abdollahi, Amoozadeh et al., The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: A randomized clinical trial, Trials, doi:10.1186/s13063-021-05795-4
Bhowmik, Barek, Aziz, Islam, Impact of high-dose vitamin C on the mortality, severity, and duration of hospital stay in COVID-19 patients: A meta-analysis, Health Sci. Rep, doi:10.1002/hsr2.762
Biancatelli, Berrill, Catravas, Marik, Quercetin, Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19), Front. Immunol, doi:10.3389/fimmu.2020.01451
Borges, Gennari-Felipe, Dias, Hatanaka, Melatonin et al., Potential Adjuvant Treatment for COVID-19 Patients, Front. Nutr, doi:10.3389/fnut.2021.821824
Brant, Angus, Is high-dose vitamin C beneficial for patients with sepsis?, JAMA, doi:10.1001/jama.2019.11643
Carr, Frei, Does vitamin C act as a pro-oxidant under physiological conditions?, FASEB J, doi:10.1096/fasebj.13.9.1007
Carr, Rowe, Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective, Nutrients, doi:10.3390/nu12071963
Cerullo, Negro, Parimbelli, Pecoraro, Perna et al., The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19, Front. Immunol, doi:10.3389/fimmu.2020.574029
Coppock, Violet, Vasquez, Belden, Foster et al., Pharmacologic Ascorbic Acid as Early Therapy for Hospitalized Patients with COVID-19: A Randomized Clinical Trial, Life, doi:10.3390/life12030453
Corrao, Mallaci Bocchio, Lo Monaco, Natoli, Cavezzi et al., Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc, Nutrients, doi:10.3390/nu13041261
Darban, Malek, Memarian, Gohari, Kiani et al., Efficacy of High Dose Vitamin C, Melatonin and Zinc in Iranian Patients with Acute Respiratory Syndrome due to Coronavirus Infection: A Pilot Randomized Trial, J. Cell. Mol. Anesth
Fashner, Ericson, Werner, Treatment of the common cold in children and adults, Am. Fam. Physician
Ferrón-Celma, Mansilla, Hassan, Garcia-Navarro, Comino et al., Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery, J. Surg. Res, doi:10.1016/j.jss.2008.04.024
Firouzi, Pahlavani, Navashenaq, Clayton, Beigmohammadi et al., The effect of Vitamin C and Zn supplementation on the immune system and clinical outcomes in COVID-19 patients, Clin. Nutr. Open Sci
Gao, Xu, Wang, Lv, Ma et al., The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study, Aging, doi:10.18632/aging.202557
Gavriatopoulou, Ntanasis-Stathopoulos, Korompoki, Fotiou, Migkou et al., Emerging treatment strategies for COVID-19 infection, Clin. Exp. Med, doi:10.1007/s10238-020-00671-y
Gavrielatou, Xourgia, Xixi, Mantelou, Ischaki et al., Effect of Vitamin C on Clinical Outcomes of Critically Ill Patients with COVID-19: An Observational Study and Subsequent Meta-Analysis, Front. Med, doi:10.3389/fmed.2022.814587
Gröber, Holick, The coronavirus disease (COVID-19)-A supportive approach with selected micronutrients, Int. J. Vitam. Nutr. Res, doi:10.1024/0300-9831/a000693
Hakamifard, Soltani, Maghsoudi, Rismanbaf, Aalinezhad et al., The effect of vitamin E and vitamin C in patients with COVID-19 pneumonia; a randomized controlled clinical trial, Immunopathol. Persa
Hemilä, De Man, Vitamin C and COVID-19, Front. Med, doi:10.3389/fmed.2020.559811
Hess, Halalau, Dokter, Paydawy, Karabon et al., High-dose intravenous vitamin C decreases rates of mechanical ventilation and cardiac arrest in severe COVID-19, Intern. Emerg. Med, doi:10.1007/s11739-022-02954-6
Higgins, Thompson, Deeks, Altman, Measuring inconsistency in meta-analyses, BMJ
Hozo, Djulbegovic, Hozo, Estimating the mean and variance from the median, range, and the size of a sample, BMC Med. Res. Methodol, doi:10.1186/1471-2288-5-13
Kaźmierczak-Bara Ńska, Boguszewska, Adamus-Grabicka, Karwowski, Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent, Nutrients, doi:10.3390/nu12051501
Kocot, Luchowska-Kocot, Kiełczykowska, Musik, Kurzepa, Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders?, Nutrients, doi:10.3390/nu9070659
Kumari, Dembra, Dembra, Bhawna, Gul et al., The Role of Vitamin C as Adjuvant Therapy in COVID-19, Cureus, doi:10.7759/cureus.11779
Lamontagne, Masse, Menard, Sprague, Pinto et al., Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit, N. Engl. J. Med, doi:10.1056/NEJMoa2200644
Li, Ching, Hipple, Lopez, Sahibzada et al., Use of Intravenous Vitamin C in Critically Ill Patients with COVID-19 Infection, J. Pharm. Pract, doi:10.1177/08971900211015052
Liugan, Carr, Vitamin C and neutrophil function: Findings from randomized controlled trials, Nutrients, doi:10.3390/nu11092102
Majidi, Rabbani, Gholami, Gholamalizadeh, Bourbour et al., The Effect of Vitamin C on Pathological Parameters and Survival Duration of Critically Ill Coronavirus Disease 2019 Patients: A Randomized Clinical Trial, Front. Immunol, doi:10.3389/fimmu.2021.717816
Manning, Mitchell, Appadurai, Shakya, Pierce et al., Vitamin C promotes maturation of T-cells, Antioxid. Redox Signal, doi:10.1089/ars.2012.4988
Mcguinness, Higgins, Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments, Res. Synth. Methods, doi:10.1002/jrsm.1411
Migliorini, Vaishya, Eschweiler, Oliva, Hildebrand et al., Vitamins C and D and COVID-19 Susceptibility, Severity and Progression: An Evidence Based Systematic Review, Medicina, doi:10.3390/medicina58070941
Milani, Macchi, Guz-Mark, Vitamin C in the Treatment of COVID-19, Nutrients, doi:10.3390/nu13041172
Miranda-Massari, Toro, Loh, Rodriguez, Borges et al., The Effects of Vitamin C on the Multiple Pathophysiological Stages of COVID-19, Life
Moores, Vitamin C: A wound healing perspective, Br. J. Community Nurs, doi:10.12968/bjcn.2013.18.Sup12.S6
Muralidar, Ambi, Sekaran, Krishnan, The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2, Biochimie, doi:10.1016/j.biochi.2020.09.018
Padayatty, Katz, Wang, Eck, Kwon et al., Vitamin C as an antioxidant: Evaluation of its role in disease prevention, J. Am. Coll. Nutr, doi:10.1080/07315724.2003.10719272
Padayatty, Sun, Wang, Riordan, Hewitt et al., Vitamin C pharmacokinetics: Implications for oral and intravenous use, Ann. Intern. Med, doi:10.7326/0003-4819-140-7-200404060-00010
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 statement: An updated guideline for reporting systematic reviews, BMJ, doi:10.1136/bmj.n71
Pedrosa, Barros, Leite-Lais, Nutritional risk of vitamin D, vitamin C, zinc, and selenium deficiency on risk and clinical outcomes of COVID-19: A narrative review, Clin. Nutr. ESPEN, doi:10.1016/j.clnesp.2021.11.003
Pourkarim, Pourtaghi-Anvarian, Rezaee, Molnupiravir: A new candidate for COVID-19 treatment, Pharmacol. Res. Perspect, doi:10.1002/prp2.909
Rawat, Roy, Maitra, Gulati, Khanna et al., Vitamin C and COVID-19 treatment: A systematic review and meta-analysis of randomized controlled trials, Diabetes Metab. Syndr, doi:10.1016/j.dsx.2021.102324
Rs, Reddy, Batra, Srivastava, Syal, Vitamin C and its therapeutic potential in the management of COVID19, Clin. Nutr. ESPEN, doi:10.1016/j.clnesp.2022.05.026
Shahbaz, Fatima, Basharat, Bibi, Yu et al., Role of vitamin C in preventing of COVID-19 infection, progression and severity, AIMS Microbiol, doi:10.3934/microbiol.2022010
Shakoor, Feehan, Al Dhaheri, Ali, Platat et al., Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19?, Maturitas
Shilotri, Bhat, Effect of mega doses of vitamin C on bactericidal ativity of leukocytes, Am. J. Clin. Nutr
Siahkali, Zarezade, Koolaji, Seyed Alinaghi, Zendehdel et al., Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial, Eur. J. Med. Res
Simsek, Senkal, Yazici, Yonca, Tahmaz et al., Effects of high dose vitamin C administration in COVID-19 patients, Ann. Med. Res, doi:10.5455/annalsmedres.2020.10.1043
Sterne, Hernán, Reeves, Savović, Berkman et al., ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions, BMJ
Sterne, Savović, Page, Elbers, Blencowe et al., RoB 2: A revised tool for assessing risk of bias in randomised trials, BMJ, doi:10.1136/bmj.l4898
Suna, Melahat, Murat, Figen, Ayperi, Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia, Med. Clin, doi:10.1016/j.medcli.2021.04.010
Szarpak, Pruc, Gasecka, Jaguszewski, Michalski et al., Should we supplement zinc in COVID-19 patients? Evidence from a meta-analysis, Pol. Arch. Intern. Med, doi:10.20452/pamw.16048
Teafatiller, Agrawal, De Robles, Rahmatpanah, Subramanian et al., Vitamin C Enhances Antiviral Functions of Lung Epithelial Cells, Biomolecules, doi:10.3390/biom11081148
Tehrani, Yadegarynia, Abrishami, Moradi, Gharaei et al., An investigation into the Effects of Intravenous Vitamin C on Pulmonary CT Findings and Clinical Outcomes of Patients with COVID 19 Pneumonia A Randomized Clinical Trial, Urol. J
Thomas, Patel, Bittel, Wolski, Wang et al., Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients with SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.0369
Vollbracht, Kraft, Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue, Nutrients, doi:10.3390/nu13041154
Vollbracht, Kraft, Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C, Front. Pharmacol, doi:10.3389/fphar.2022.899198
Zhang, Rao, Li, Zhu, Liu et al., Pilot trial of high-dose vitamin C in critically ill COVID-19 patients, Ann. Intensive Care, doi:10.1186/s13613-020-00792-3
Zhao, Liu, Liu, Peng, Huang et al., High Dose Intravenous Vitamin C for Preventing The Disease Aggravation of Moderate COVID-19 Pneumonia. A Retrospective Propensity Matched Before-After Study, Front. Pharmacol, doi:10.3389/fphar.2021.638556
Zheng, Chen, Jiang, Guo, Luo et al., No significant benefit of moderate-dose vitamin C on severe COVID-19 cases, Open Med, doi:10.1515/med-2021-0361
DOI record:
{
"DOI": "10.3390/nu14194217",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu14194217",
"abstract": "<jats:p>Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe respiratory syndrome coronavirus 2 (SARS-CoV-2), millions of people have died, and the medical system has faced significant difficulties. Our purpose was to perform a meta-analysis to estimate the effect of vitamin C on in-hospital mortality and the ICU or hospital length of stay for patients diagnosed with COVID-19. We conducted a systematic review with meta-analysis in the following databases: PubMed, Web of Science, Scopus and Cochrane Central Register of Controlled Trials. We included studies that evaluated the effect of vitamin C supplementation, compared with standard treatment in COVID-19 patients who are ≥18 y of age. Nineteen trials were included in the meta-analysis. In-hospital mortality with and without vitamin C supplementation was 24.1% vs. 33.9% (OR = 0.59; 95%CI: 0.37 to 0.95; p = 0.03), respectively. Sub-analysis showed that, in randomized clinical trials, in-hospital mortality varied and amounted to 23.9% vs. 35.8% (OR = 0.44; 95%CI: 0.25 to 0.76; p = 0.003), respectively. In the non-randomized trials, in-hospital mortality was 24.2% vs. 33.5% (OR = 0.72; 95%CI: 0.38 to 1.39; p = 0.33), respectively. The ICU length of stay was longer in patients treated with vitamin C vs. standard therapy, 11.1 (7.3) vs. 8.3 (4.7) days (MD = 1.91; 95%CI: 0.89 to 2.93; p < 0.001), respectively. Acute kidney injury in patients treated with and without vitamin C varied and amounted to 27.8% vs. 45.0% (OR = 0.56; 95%CI: 0.40 to 0.78; p < 0.001), respectively. There were no differences in the frequency of other adverse events among patients’ treatment with and without vitamin C (all p > 0.05). The use of vitamin C reduces hospital mortality. The length of stay in the ICU is longer among patients treated with vitamin C. In terms of patient safety, vitamin C has an acceptable profile. Low doses of vitamin C are effective and safe. Despite some evidence of the usefulness of vitamin C in modifying the course of COVID-19, it is too early to modify guidelines and recommendations. Further studies, in particular randomized clinical trials, are necessary.</jats:p>",
"alternative-id": [
"nu14194217"
],
"author": [
{
"affiliation": [],
"family": "Olczak-Pruc",
"given": "Monika",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5648-4652",
"affiliation": [],
"authenticated-orcid": false,
"family": "Swieczkowski",
"given": "Damian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4167-1962",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ladny",
"given": "Jerzy R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2140-9732",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pruc",
"given": "Michal",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3597-2048",
"affiliation": [],
"authenticated-orcid": false,
"family": "Juarez-Vela",
"given": "Raul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rafique",
"given": "Zubaid",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2730-6742",
"affiliation": [],
"authenticated-orcid": false,
"family": "Peacock",
"given": "Frank W.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0973-5455",
"affiliation": [],
"authenticated-orcid": false,
"family": "Szarpak",
"given": "Lukasz",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
11
]
],
"date-time": "2022-10-11T04:50:01Z",
"timestamp": 1665463801000
},
"deposited": {
"date-parts": [
[
2022,
10,
11
]
],
"date-time": "2022-10-11T05:40:29Z",
"timestamp": 1665466829000
},
"indexed": {
"date-parts": [
[
2022,
10,
11
]
],
"date-time": "2022-10-11T06:15:47Z",
"timestamp": 1665468947790
},
"is-referenced-by-count": 0,
"issue": "19",
"issued": {
"date-parts": [
[
2022,
10,
10
]
]
},
"journal-issue": {
"issue": "19",
"published-online": {
"date-parts": [
[
2022,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T00:00:00Z",
"timestamp": 1665360000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/14/19/4217/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "4217",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
10,
10
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
10
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.biochi.2020.09.018",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1007/s10238-020-00671-y",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.20452/pamw.16048",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1002/prp2.909",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.3390/molecules25225346",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/j.maturitas.2020.08.003",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1080/07315724.2003.10719272",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.3390/nu9070659",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1096/fasebj.13.9.1007",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.3390/nu12051501",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1093/ajcn/30.7.1077",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.3390/nu11092102",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.jss.2008.04.024",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1089/ars.2012.4988",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.12968/bjcn.2013.18.Sup12.S6",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.3390/biom11081148",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.3390/life11121341",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.3389/fimmu.2020.574029",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"article-title": "Treatment of the common cold in children and adults",
"author": "Fashner",
"first-page": "153",
"journal-title": "Am. Fam. Physician",
"key": "ref19",
"volume": "86",
"year": "2012"
},
{
"DOI": "10.3390/nu12071963",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1001/jama.2019.11643",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.7326/0003-4819-140-7-200404060-00010",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.3389/fimmu.2020.01451",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.3390/nu13041172",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.3390/nu13041154",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1136/bmj.n71",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1136/bmj.l4898",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1136/bmj.i4919",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1002/jrsm.1411",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1186/1471-2288-5-13",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1136/bmj.327.7414.557",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1038/s41598-021-96703-y",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1186/s13063-021-05795-4",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.3390/life12030453",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"article-title": "Efficacy of High Dose Vitamin C, Melatonin and Zinc in Iranian Patients with Acute Respiratory Syndrome due to Coronavirus Infection: A Pilot Randomized Trial",
"author": "Darban",
"first-page": "164",
"journal-title": "J. Cell. Mol. Anesth.",
"key": "ref35",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.18632/aging.202557",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3389/fmed.2022.814587",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"article-title": "The effect of vitamin E and vitamin C in patients with COVID-19 pneumonia; a randomized controlled clinical trial",
"author": "Hakamifard",
"first-page": "e08",
"journal-title": "Immunopathol. Persa.",
"key": "ref38",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1007/s11739-022-02954-6",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.7759/cureus.11779",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1177/08971900211015052",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.3389/fimmu.2021.717816",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1186/s40001-021-00490-1",
"article-title": "Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial",
"author": "Siahkali",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "Eur. J. Med. Res.",
"key": "ref43",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.5455/annalsmedres.2020.10.1043",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1016/j.medcli.2021.04.010",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"article-title": "An investigation into the Effects of Intravenous Vitamin C on Pulmonary CT Findings and Clinical Outcomes of Patients with COVID 19 Pneumonia A Randomized Clinical Trial",
"author": "Tehrani",
"first-page": "6863",
"journal-title": "Urol. J.",
"key": "ref46",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.0369",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1186/s13613-020-00792-3",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.3389/fphar.2021.638556",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1515/med-2021-0361",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1056/NEJMoa2200644",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1016/j.clnesp.2021.11.003",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.3934/microbiol.2022010",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.3389/fnut.2021.821824",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.3389/fphar.2022.899198",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1024/0300-9831/a000693",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1002/ncp.10832",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.3390/medicina58070941",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.1016/j.dsx.2021.102324",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.3389/fmed.2020.559811",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1002/hsr2.762",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.3390/nu13041261",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1016/j.clnesp.2022.05.026",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.1016/j.nutos.2022.06.006",
"doi-asserted-by": "publisher",
"key": "ref64"
}
],
"reference-count": 64,
"references-count": 64,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/14/19/4217"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis",
"type": "journal-article",
"volume": "14"
}
