Pilot Trial of High-dose vitamin C in critically ill COVID-19 patients (preprint 8/10/2020)
et al., Annals of Intensive Care, doi:10.1186/s13613-020-00792-3, Aug 2020
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small RCT for high-dose vitamin C for ICU patients showing reduced (but not statistically significant) mortality. Dosage was 12g of vitamin C/50ml every 12 hours for 7 days at a rate of 12ml/hour.
This is the 1st of 20 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0016.
This is the 2nd of 73 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000076.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
This may explain in part the very high mortality seen in this study.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
|
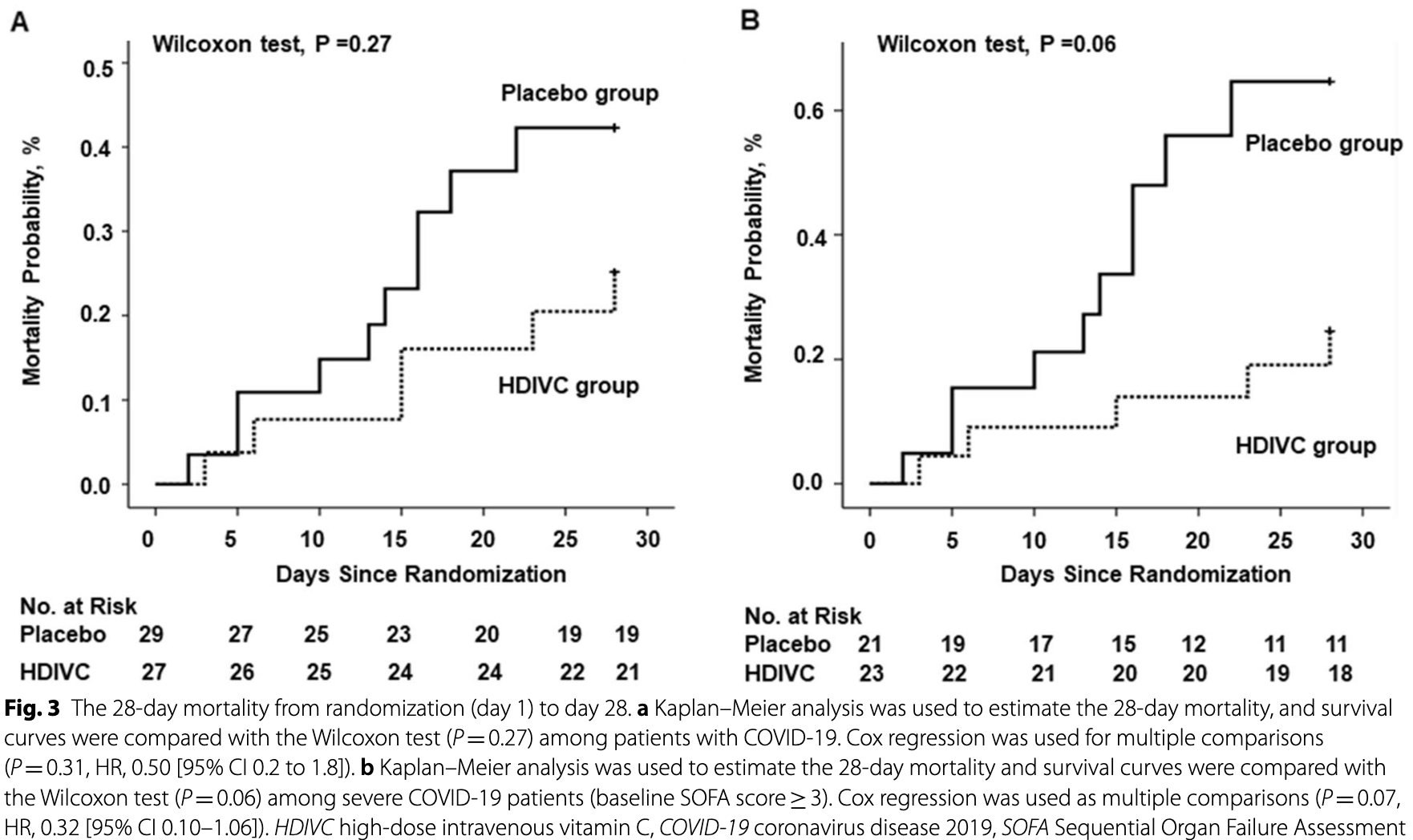
risk of death, 50.0% lower, RR 0.50, p = 0.20, treatment 6 of 27 (22.2%), control 11 of 29 (37.9%), NNT 6.4, adjusted per study, ICU mortality.
|
|
risk of death, 80.0% lower, RR 0.20, p = 0.04, treatment 5 of 27 (18.5%), control 11 of 29 (37.9%), NNT 5.2, adjusted per study, ICU mortality for SOFA ≥3.
|
|
risk of death, 50.0% lower, RR 0.50, p = 0.31, treatment 6 of 27 (22.2%), control 10 of 29 (34.5%), NNT 8.2, adjusted per study, 28 day mortality.
|
|
risk of death, 70.0% lower, RR 0.30, p = 0.07, treatment 5 of 27 (18.5%), control 10 of 29 (34.5%), NNT 6.3, adjusted per study, 28 day mortality for SOFA ≥3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zhang et al., 10 Aug 2020, Randomized Controlled Trial, China, peer-reviewed, 11 authors, study period 14 February, 2020 - 29 March, 2020, dosage 12000mg bid days 1-7.
Pilot trial of high-dose vitamin C in critically ill COVID-19 patients
Annals of Intensive Care, doi:10.1186/s13613-020-00792-3
Background: Few specific medications have been proven effective for the treatment of patients with severe coronavirus disease 2019 . Here, we tested whether high-dose vitamin C infusion was effective for severe COVID-19. Methods: This randomized, controlled, clinical trial was performed at 3 hospitals in Hubei, China. Patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the ICU were randomly assigned in as 1:1 ratio to either the high-dose intravenous vitamin C (HDIVC) or the placebo. HDIVC group received 12 g of vitamin C/50 ml every 12 h for 7 days at a rate of 12 ml/hour, and the placebo group received bacteriostatic water for injection in the same way within 48 h of arrival to ICU. The primary outcome was invasive mechanical ventilation-free days in 28 days (IMVFD28). Secondary outcomes were 28-day mortality, organ failure (Sequential Organ Failure Assessment (SOFA) score), and inflammation progression (interleukin-6). Results: Only 56 critical COVID-19 patients were ultimately recruited due to the early control of the outbreak. There was no difference in IMVFD28 between two groups (26.0 [9.0-28.0] in HDIVC vs 22.0 [8.50-28.0] in control, p = 0.57). HDIVC failed to reduce 28-day mortality (P = 0.27). During the 7-day treatment period, patients in the HDIVC group had a steady rise in the PaO 2 /FiO 2 (day 7: 229 vs. 151 mmHg, 95% CI 33 to 122, P = 0.01), which was not observed in the control group. IL-6 in the HDIVC group was lower than that in the control group (19.42 vs. 158.00; 95% CI -301.72 to -29.79; P = 0.04) on day 7.
Conclusion: This pilot trial showed that HDIVC failed to improve IMVFD28, but might show a potential signal of benefit in oxygenation for critically ill patients with COVID-19 improving PaO2/FiO2 even though.
Authors' contributions Concept and design: JZ, XR, YL. Acquisition, analysis, or interpretation of data: JZ, XR, YL, HX, GG, GL, ZM. Drafting of the manuscript: JZ, XR, YL, DB, ZP. Critical revision of the manuscript for important intellectual content: JZ, XR, YL, DB, ZP. Statistical analysis: JZ, YZ, FL. Obtained funding: HX, ZP. Administrative, technical, or material support: HX, XR, ZP. Supervision: HX, DB, ZP. All authors read and approved the final manuscript.
Ethics approval and consent to participate This study is a multicenter, randomized trial, which was approved by the ethic committee of Zhongnan Hospital of Wuhan University (#2020001). It was registered on the website of ClinicalTrials.gov (ID: NCT04264533) before patient recruitment. Informed consents were obtained from the patients or family members.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Braciale, Sun, Kim, Regulating the adaptive immune response to respiratory virus infection, Nat Rev Immunol
Carr, Rosengrave, Bayer, Chambers, Mehrtens et al., Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes, Crit Care
Carr, Shaw, Fowler, Natarajan, Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock?, Crit Care
Chen, Wu, Guo, Cao, Huang et al., Clinical and immunological features of severe and moderate coronavirus disease 2019, J Clin Investig
Chen, Zhao, Qu, Chen, Xiong et al., Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients, Clinical infectious
Chiscano-Camon, Ruiz-Rodriguez, Ruiz-Sanmartin, Roca, Ferrer, Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome, Crit Care
Fisher, Kraskauskas, Martin, Farkas, Wegelin et al., Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid, Am J Physiol Lung Cell Mol Physiol
Fisher, Seropian, Kraskauskas, Thakkar, Voelkel et al., Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury, Crit Care Med
Fowler, Syed, Knowlson, Sculthorpe, Farthing et al., Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis, J Transl Med
Fowler, Truwit, Hite, Morris, Dewilde et al., Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial, JAMA
Fujii, Luethi, Young, Frei, Eastwood et al., Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial, JAMA
Grooth, Oudermans-Van Straaten, Early plasma vitamin C concentration, organ dysfunction and ICU mortality, Intensive Care Med
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Hartel, Strunk, Bucsky, Schultz, Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes, Cytokine
Hemila, Chalker, Vitamin C Can Shorten the Length of Stay in the ICU: A Meta-Analysis, Nutrients
Hemila, Chalker, Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis, J Intensive Care
Hu, Yuan, Wang, Li, Cai et al., Efficacy and safety of vitamin C for atrial fibrillation after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials, Int J Surg
Iii, Kim, Lepler, Malhotra, Debesa et al., Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome, World J Crit Care Med
Jin, Cai, Cheng, Cheng, Deng et al., A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version), Mil Med Res
Khoshnam-Rad, Khalili, Safety of vitamin C in sepsis: a neglected topic, Curr Opin Crit Care
Koekkoek, Van Zanten Ar, Antioxidant vitamins and trace elements in critical illness, Nutr Clin Pract
Li, Evidence is stronger than you think: a meta-analysis of vitamin C use in patients with sepsis, Crit Care
Liu, Li, Zhou, Guan, Xiang, Can we use interleukin-6 (IL-6) blockade for coronavirus disease (COVID-19)-induced cytokine release syndrome (CRS), J Autoimmun
Madhusudana, Shamsundar, Seetharaman, In vitro inactivation of the rabies virus by ascorbic acid, Int J Infect Dis
Munster, Koopmans, Van Doremalen, Van Riel, De, A novel coronavirus emerging in china -key questions for impact assessment, N Engl J Med
Nieman, Peters, Henson, Nevines, Thompson, Influence of vitamin C supplementation on cytokine changes following an ultramarathon, J Interferon Cytokine Res
Padayatty, Sun, Wang, Riordan, Hewitt et al., Vitamin C pharmacokinetics: implications for oral and intravenous use, Ann Intern Med
Pathan, Hemingway, Alizadeh, Stephens, Boldrick et al., Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock, Lancet
Peiris, Chu, Cheng, Chan, Hung et al., Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study, Lancet
Qin, Zhou, Hu, Zhang, Yang et al., Dysregulation of immune response in patients with COVID-19 in Wuhan, China, Clin Infect Dis
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan China, Intensive Care Med
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China Intensive Care Med
Tanaka, Matsuda, Miyagantani, Yukioka, Matsuda et al., Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study, Arch Surg
Tanaka, Narazaki, Kishimoto, Immunotherapeutic implications of IL-6 blockade for cytokine storm, Immunotherapy
Tian, Hu, Niu, Liu, Xu et al., Pulmonary pathology of earlyphase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer, J Thorac Oncol
Vassilakopoulos, Karatza, Katsaounou, Kollintza, Zakynthinos et al., Antioxidants attenuate the plasma cytokine response to exercise in humans, J Appl Physiol
Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA
Xu, Han, Li, Sun, Wang et al., Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir Med
Xu, Zheng, Liang, Gao, Gu, Vitamins for prevention of contrastinduced acute kidney injury: a systematic review and trial sequential analysis, Am J Cardiovasc Drugs
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1186/s13613-020-00792-3",
"ISSN": [
"2110-5820"
],
"URL": "http://dx.doi.org/10.1186/s13613-020-00792-3",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Few specific medications have been proven effective for the treatment of patients with severe coronavirus disease 2019 (COVID-19). Here, we tested whether high-dose vitamin C infusion was effective for severe COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This randomized, controlled, clinical trial was performed at 3 hospitals in Hubei, China. Patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the ICU were randomly assigned in as 1:1 ratio to either the high-dose intravenous vitamin C (HDIVC) or the placebo. HDIVC group received 12 g of vitamin C/50 ml every 12 h for 7 days at a rate of 12 ml/hour, and the placebo group received bacteriostatic water for injection in the same way within 48 h of arrival to ICU. The primary outcome was invasive mechanical ventilation-free days in 28 days (IMVFD28). Secondary outcomes were 28-day mortality, organ failure (Sequential Organ Failure Assessment (SOFA) score), and inflammation progression (interleukin-6).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Only 56 critical COVID-19 patients were ultimately recruited due to the early control of the outbreak. There was no difference in IMVFD28 between two groups (26.0 [9.0–28.0] in HDIVC vs 22.0 [8.50–28.0] in control, <jats:italic>p</jats:italic> = 0.57). HDIVC failed to reduce 28-day mortality (<jats:italic>P</jats:italic> = 0.27). During the 7-day treatment period, patients in the HDIVC group had a steady rise in the PaO<jats:sub>2</jats:sub>/FiO<jats:sub>2</jats:sub> (day 7: 229 vs. 151 mmHg, 95% CI 33 to 122, <jats:italic>P</jats:italic> = 0.01), which was not observed in the control group. IL-6 in the HDIVC group was lower than that in the control group (19.42 vs. 158.00; 95% CI -301.72 to -29.79; <jats:italic>P</jats:italic> = 0.04) on day 7.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>This pilot trial showed that HDIVC failed to improve IMVFD28, but might show a potential signal of benefit in oxygenation for critically ill patients with COVID-19 improving PaO2/FiO2 even though.</jats:p>\n </jats:sec>",
"alternative-id": [
"792"
],
"article-number": "5",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "14 October 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "18 December 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "9 January 2021"
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "This study is a multicenter, randomized trial, which was approved by the ethic committee of Zhongnan Hospital of Wuhan University (#2020001). It was registered on the website of ClinicalTrials.gov (ID: NCT04264533) before patient recruitment. Informed consents were obtained from the patients or family members."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Zhang",
"given": "Jing",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rao",
"given": "Xin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yiming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Yuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Fang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Guangling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Guoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meng",
"given": "Zhongji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Backer",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xiang",
"given": "Hui",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3873-9607",
"affiliation": [],
"authenticated-orcid": false,
"family": "Peng",
"given": "Zhiyong",
"sequence": "additional"
}
],
"container-title": "Annals of Intensive Care",
"container-title-short": "Ann. Intensive Care",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
1,
9
]
],
"date-time": "2021-01-09T08:10:48Z",
"timestamp": 1610179848000
},
"deposited": {
"date-parts": [
[
2021,
1,
12
]
],
"date-time": "2021-01-12T21:18:05Z",
"timestamp": 1610486285000
},
"funder": [
{
"DOI": "10.13039/501100018806",
"award": [
"2020FCA024",
"2020FCA020"
],
"doi-asserted-by": "crossref",
"name": "Science and Technology Department of Hubei Province"
},
{
"DOI": "10.13039/501100012226",
"award": [
"2042020kfxg18"
],
"doi-asserted-by": "publisher",
"name": "Fundamental Research Funds for the Central Universities"
},
{
"DOI": "10.13039/501100012476",
"award": [
"2042020kfxg13"
],
"doi-asserted-by": "publisher",
"name": "Fundamental Research Funds for Central Universities of the Central South University"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T18:36:30Z",
"timestamp": 1712601390852
},
"is-referenced-by-count": 157,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
1,
9
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
9
]
],
"date-time": "2021-01-09T00:00:00Z",
"timestamp": 1610150400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
9
]
],
"date-time": "2021-01-09T00:00:00Z",
"timestamp": 1610150400000
}
}
],
"link": [
{
"URL": "http://link.springer.com/content/pdf/10.1186/s13613-020-00792-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/article/10.1186/s13613-020-00792-3/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/content/pdf/10.1186/s13613-020-00792-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2021,
1,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
1,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.jtho.2020.02.010",
"author": "S Tian",
"doi-asserted-by": "publisher",
"first-page": "700",
"issue": "5",
"journal-title": "J Thorac Oncol",
"key": "792_CR1",
"unstructured": "Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–4.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2000929",
"author": "VJ Munster",
"doi-asserted-by": "publisher",
"first-page": "692",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "792_CR2",
"unstructured": "Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in china - key questions for impact assessment. N Engl J Med. 2020;382(8):692–4.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "WJ Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "792_CR3",
"unstructured": "Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "crossref",
"key": "792_CR4",
"unstructured": "Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y et al: Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020."
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "792_CR5",
"unstructured": "Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "crossref",
"key": "792_CR6",
"unstructured": "Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL et al: Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020."
},
{
"DOI": "10.1038/nri3166",
"author": "TJ Braciale",
"doi-asserted-by": "publisher",
"first-page": "295",
"issue": "4",
"journal-title": "Nat Rev Immunol",
"key": "792_CR7",
"unstructured": "Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12(4):295–305.",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1172/JCI137244",
"author": "G Chen",
"doi-asserted-by": "publisher",
"first-page": "2620",
"issue": "5",
"journal-title": "J Clin Investig",
"key": "792_CR8",
"unstructured": "Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig. 2020;130(5):2620–9.",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"author": "Z Xu",
"doi-asserted-by": "publisher",
"first-page": "420",
"issue": "4",
"journal-title": "Lancet Respir Med",
"key": "792_CR9",
"unstructured": "Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.2139/ssrn.3541136",
"doi-asserted-by": "crossref",
"key": "792_CR10",
"unstructured": "Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W et al: Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 2020."
},
{
"DOI": "10.1016/S0140-6736(03)13412-5",
"author": "JS Peiris",
"doi-asserted-by": "publisher",
"first-page": "1767",
"issue": "9371",
"journal-title": "Lancet",
"key": "792_CR11",
"unstructured": "Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–72.",
"volume": "361",
"year": "2003"
},
{
"DOI": "10.1186/s13054-015-1131-2",
"author": "AC Carr",
"doi-asserted-by": "publisher",
"first-page": "418",
"journal-title": "Crit Care",
"key": "792_CR12",
"unstructured": "Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. 2015;19:418.",
"volume": "19",
"year": "2015"
},
{
"DOI": "10.1177/0884533616653832",
"author": "WA Koekkoek",
"doi-asserted-by": "publisher",
"first-page": "457",
"issue": "4",
"journal-title": "Nutr Clin Pract",
"key": "792_CR13",
"unstructured": "Koekkoek WA, van Zanten AR. Antioxidant vitamins and trace elements in critical illness. Nutr Clin Pract. 2016;31(4):457–74.",
"volume": "31",
"year": "2016"
},
{
"DOI": "10.1089/10799900050198480",
"author": "DC Nieman",
"doi-asserted-by": "publisher",
"first-page": "1029",
"issue": "11",
"journal-title": "J Interferon Cytokine Res",
"key": "792_CR14",
"unstructured": "Nieman DC, Peters EM, Henson DA, Nevines EI, Thompson MM. Influence of vitamin C supplementation on cytokine changes following an ultramarathon. J Interferon Cytokine Res. 2000;20(11):1029–35.",
"volume": "20",
"year": "2000"
},
{
"DOI": "10.1152/japplphysiol.00735.2002",
"doi-asserted-by": "crossref",
"key": "792_CR15",
"unstructured": "Vassilakopoulos T, Karatza MH, Katsaounou P, Kollintza A, Zakynthinos S, Roussos C: Antioxidants attenuate the plasma cytokine response to exercise in humans. J Appl Physiol (1985) 2003, 94(3):1025–1032."
},
{
"DOI": "10.1186/s13054-017-1891-y",
"author": "AC Carr",
"doi-asserted-by": "publisher",
"first-page": "300",
"issue": "1",
"journal-title": "Crit Care",
"key": "792_CR16",
"unstructured": "Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21(1):300.",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.7326/0003-4819-140-7-200404060-00010",
"author": "SJ Padayatty",
"doi-asserted-by": "publisher",
"first-page": "533",
"issue": "7",
"journal-title": "Ann Intern Med",
"key": "792_CR17",
"unstructured": "Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533–7.",
"volume": "140",
"year": "2004"
},
{
"DOI": "10.1186/s13054-020-03249-y",
"author": "L Chiscano-Camon",
"doi-asserted-by": "publisher",
"first-page": "522",
"issue": "1",
"journal-title": "Crit Care",
"key": "792_CR18",
"unstructured": "Chiscano-Camon L, Ruiz-Rodriguez JC, Ruiz-Sanmartin A, Roca O, Ferrer R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care. 2020;24(1):522.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1186/1479-5876-12-32",
"author": "AA Fowler",
"doi-asserted-by": "publisher",
"first-page": "32",
"journal-title": "J Transl Med",
"key": "792_CR19",
"unstructured": "Fowler AA, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32.",
"volume": "12",
"year": "2014"
},
{
"DOI": "10.1001/jama.2019.22176",
"doi-asserted-by": "crossref",
"key": "792_CR20",
"unstructured": "Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, Deane AM, Shehabi Y, Hajjar LA, Oliveira G et al: Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020."
},
{
"DOI": "10.1001/jama.2019.11825",
"author": "AA Fowler",
"doi-asserted-by": "publisher",
"first-page": "1261",
"issue": "13",
"journal-title": "JAMA",
"key": "792_CR21",
"unstructured": "Fowler AA, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR, Natarajan R, Brophy DF, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261–70.",
"volume": "322",
"year": "2019"
},
{
"DOI": "10.5492/wjccm.v6.i1.85",
"author": "AA Fowler Iii",
"doi-asserted-by": "publisher",
"first-page": "85",
"issue": "1",
"journal-title": "World J Crit Care Med",
"key": "792_CR22",
"unstructured": "Fowler Iii AA, Kim C, Lepler L, Malhotra R, Debesa O, Natarajan R, Fisher BJ, Syed A, DeWilde C, Priday A, et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Crit Care Med. 2017;6(1):85–90.",
"volume": "6",
"year": "2017"
},
{
"DOI": "10.1016/j.ijsu.2016.12.009",
"author": "X Hu",
"doi-asserted-by": "publisher",
"first-page": "58",
"journal-title": "Int J Surg",
"key": "792_CR23",
"unstructured": "Hu X, Yuan L, Wang H, Li C, Cai J, Hu Y, Ma C. Efficacy and safety of vitamin C for atrial fibrillation after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. Int J Surg. 2017;37:58–64.",
"volume": "37",
"year": "2017"
},
{
"DOI": "10.1001/archsurg.135.3.326",
"author": "H Tanaka",
"doi-asserted-by": "publisher",
"first-page": "326",
"issue": "3",
"journal-title": "Arch Surg",
"key": "792_CR24",
"unstructured": "Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135(3):326–31.",
"volume": "135",
"year": "2000"
},
{
"DOI": "10.1186/s13054-018-2191-x",
"author": "J Li",
"doi-asserted-by": "publisher",
"first-page": "258",
"issue": "1",
"journal-title": "Crit Care",
"key": "792_CR25",
"unstructured": "Li J. Evidence is stronger than you think: a meta-analysis of vitamin C use in patients with sepsis. Crit Care. 2018;22(1):258.",
"volume": "22",
"year": "2018"
},
{
"DOI": "10.1007/s40256-018-0274-3",
"author": "Y Xu",
"doi-asserted-by": "publisher",
"first-page": "373",
"issue": "5",
"journal-title": "Am J Cardiovasc Drugs",
"key": "792_CR26",
"unstructured": "Xu Y, Zheng X, Liang B, Gao J, Gu Z. Vitamins for prevention of contrast-induced acute kidney injury: a systematic review and trial sequential analysis. Am J Cardiovasc Drugs. 2018;18(5):373–86.",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1186/s40560-020-0432-y",
"author": "H Hemila",
"doi-asserted-by": "publisher",
"first-page": "15",
"journal-title": "J Intensive Care",
"key": "792_CR27",
"unstructured": "Hemila H, Chalker E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care. 2020;8:15.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/nu11040708",
"doi-asserted-by": "crossref",
"key": "792_CR28",
"unstructured": "Hemila H, Chalker E: Vitamin C Can Shorten the Length of Stay in the ICU: A Meta-Analysis. Nutrients 2019, 11(4)."
},
{
"DOI": "10.1016/j.ijid.2003.09.002",
"author": "SN Madhusudana",
"doi-asserted-by": "publisher",
"first-page": "21",
"issue": "1",
"journal-title": "Int J Infect Dis",
"key": "792_CR29",
"unstructured": "Madhusudana SN, Shamsundar R, Seetharaman S. In vitro inactivation of the rabies virus by ascorbic acid. Int J Infect Dis. 2004;8(1):21–5.",
"volume": "8",
"year": "2004"
},
{
"author": "YH Jin",
"first-page": "4",
"issue": "1",
"journal-title": "Mil Med Res",
"key": "792_CR30",
"unstructured": "Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7(1):4.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1097/MCC.0000000000000622",
"author": "N Khoshnam-Rad",
"doi-asserted-by": "publisher",
"first-page": "329",
"issue": "4",
"journal-title": "Curr Opin Crit Care",
"key": "792_CR31",
"unstructured": "Khoshnam-Rad N, Khalili H. Safety of vitamin C in sepsis: a neglected topic. Curr Opin Crit Care. 2019;25(4):329–33.",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"author": "Q Ruan",
"doi-asserted-by": "publisher",
"first-page": "846",
"issue": "5",
"journal-title": "China Intensive Care Med",
"key": "792_CR32",
"unstructured": "Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China Intensive Care Med. 2020;46(5):846–8.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1152/ajplung.00300.2011",
"author": "BJ Fisher",
"doi-asserted-by": "publisher",
"first-page": "L20",
"issue": "1",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "792_CR33",
"unstructured": "Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA 3rd, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012;303(1):L20-32.",
"volume": "303",
"year": "2012"
},
{
"DOI": "10.1097/CCM.0b013e3182120cb8",
"author": "BJ Fisher",
"doi-asserted-by": "publisher",
"first-page": "1454",
"issue": "6",
"journal-title": "Crit Care Med",
"key": "792_CR34",
"unstructured": "Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Fowler AA 3rd, Natarajan R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med. 2011;39(6):1454–60.",
"volume": "39",
"year": "2011"
},
{
"key": "792_CR35",
"unstructured": "De Grooth HMS-dM, A.M.; Oudermans-van Straaten, H.M. : Early plasma vitamin C concentration, organ dysfunction and ICU mortality. Intensive Care Med 2014:40, S199."
},
{
"DOI": "10.1101/2020.02.29.20029520",
"doi-asserted-by": "crossref",
"key": "792_CR36",
"unstructured": "Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, Men D, Huang Q, Liu Y, Yang B et al: Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020."
},
{
"DOI": "10.1007/s00134-020-05991-x",
"author": "Q Ruan",
"doi-asserted-by": "publisher",
"first-page": "846",
"issue": "5",
"journal-title": "Intensive Care Med",
"key": "792_CR37",
"unstructured": "Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan China. Intensive Care Med. 2020;46(5):846–8.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/j.jaut.2020.102452",
"author": "B Liu",
"doi-asserted-by": "publisher",
"first-page": "102452",
"journal-title": "J Autoimmun",
"key": "792_CR38",
"unstructured": "Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease (COVID-19)-induced cytokine release syndrome (CRS). J Autoimmun. 2020;111:102452.",
"volume": "111",
"year": "2020"
},
{
"DOI": "10.2217/imt-2016-0020",
"author": "T Tanaka",
"doi-asserted-by": "publisher",
"first-page": "959",
"issue": "8",
"journal-title": "Immunotherapy",
"key": "792_CR39",
"unstructured": "Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–70.",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1016/S0140-6736(03)15326-3",
"author": "N Pathan",
"doi-asserted-by": "publisher",
"first-page": "203",
"issue": "9404",
"journal-title": "Lancet",
"key": "792_CR40",
"unstructured": "Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, McCabe C, Welch SB, Whitney A, O’Gara P, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363(9404):203–9.",
"volume": "363",
"year": "2004"
},
{
"DOI": "10.1073/pnas.2005615117",
"doi-asserted-by": "crossref",
"key": "792_CR41",
"unstructured": "Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X et al: Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020."
},
{
"DOI": "10.1016/j.cyto.2004.02.004",
"author": "C Hartel",
"doi-asserted-by": "publisher",
"first-page": "101",
"issue": "4–5",
"journal-title": "Cytokine",
"key": "792_CR42",
"unstructured": "Hartel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27(4–5):101–6.",
"volume": "27",
"year": "2004"
}
],
"reference-count": 42,
"references-count": 42,
"relation": {},
"resource": {
"primary": {
"URL": "https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-020-00792-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine"
],
"subtitle": [],
"title": "Pilot trial of high-dose vitamin C in critically ill COVID-19 patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "11"
}
