High-dose intravenous vitamin C decreases rates of mechanical ventilation and cardiac arrest in severe COVID-19
et al., Internal and Emergency Medicine, doi:10.1007/s11739-022-02954-6, Mar 2022
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
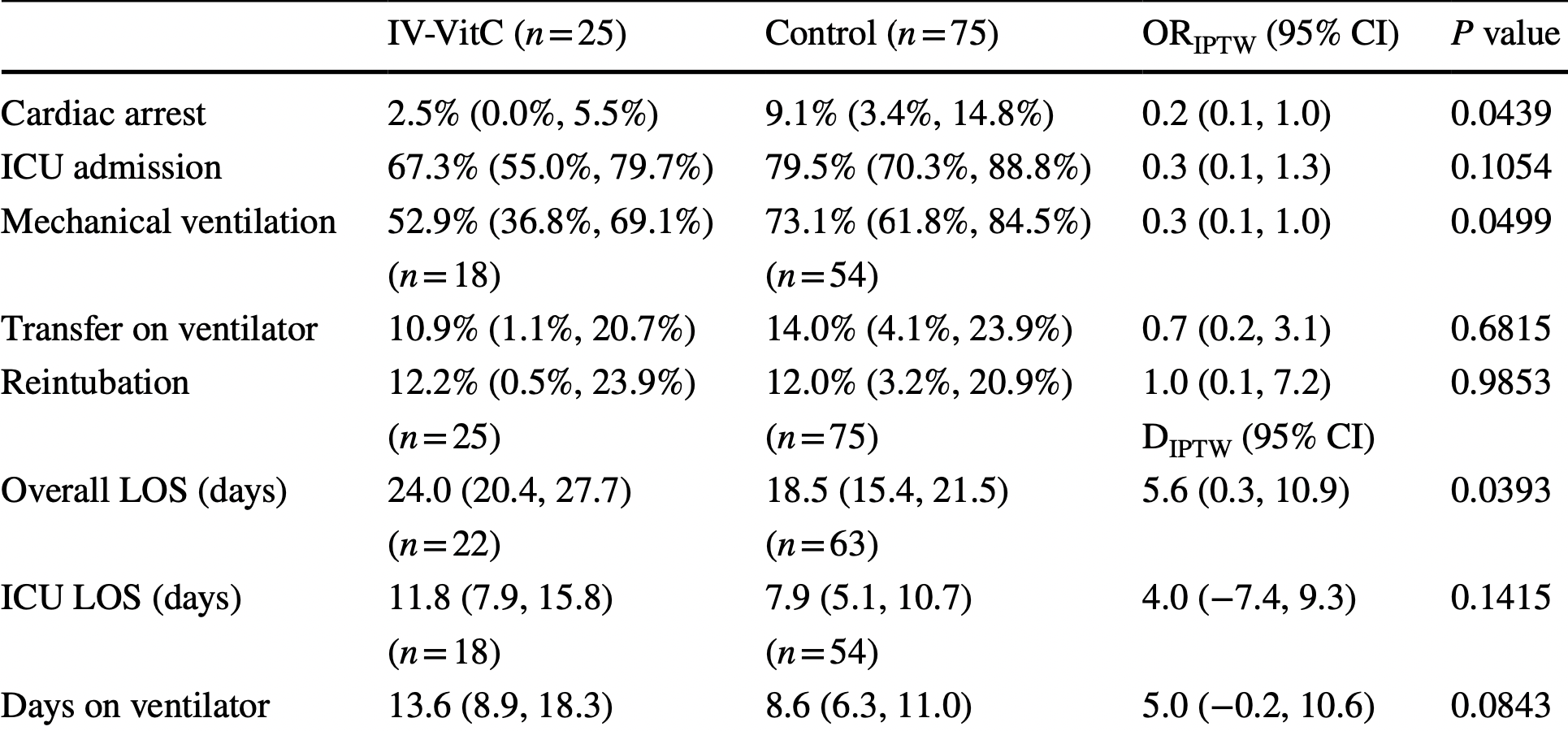
Retrospective 100 severe condition hospitalized patients in the USA, 25 treated with high-dose IV vitamin C, showing lower mechanical ventilation and cardiac arrest, and increased length of survival with treatment. 3g IV vitamin C every 6h for 7 days.
This is the 45th of 73 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000076.
20 studies are RCTs, which show efficacy with p=0.0016.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 20.0% lower, HR 0.80, p = 0.54, treatment 10 of 25 (40.0%), control 37 of 75 (49.3%), NNT 11, time to event analysis, propensity score weighting.
|
|
risk of mechanical ventilation, 39.5% lower, RR 0.60, p = 0.05, treatment 18 of 25 (72.0%), control 54 of 75 (72.0%), odds ratio converted to relative risk, propensity score weighting.
|
|
risk of mechanical ventilation, 50.0% lower, HR 0.50, p = 0.03, treatment 18 of 25 (72.0%), control 54 of 75 (72.0%), time to event analysis, propensity score weighting.
|
|
risk of ICU admission, 27.2% lower, RR 0.73, p = 0.10, treatment 22 of 25 (88.0%), control 63 of 75 (84.0%), odds ratio converted to relative risk, propensity score weighting.
|
|
risk of ICU admission, 30.0% lower, HR 0.70, p = 0.19, treatment 22 of 25 (88.0%), control 63 of 75 (84.0%), time to event analysis, propensity score weighting.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hess et al., 29 Mar 2022, retrospective, USA, peer-reviewed, 9 authors, study period March 2020 - July 2020.
High-dose intravenous vitamin C decreases rates of mechanical ventilation and cardiac arrest in severe COVID-19
Internal and Emergency Medicine, doi:10.1007/s11739-022-02954-6
Intravenous vitamin C (IV-VitC) has been suggested as a treatment for severe sepsis and acute respiratory distress syndrome; however, there are limited studies evaluating its use in severe COVID-19. Efficacy and safety of high-dose IV-VitC (HDIVC) in patients with severe COVID-19 were evaluated. This observational cohort was conducted at a single-center, 530 bed, community teaching hospital and took place from March 2020 through July 2020. Inverse probability treatment weighting (IPTW) was utilized to compare outcomes in patients with severe COVID-19 treated with and without HDIVC. Patients were enrolled if they were older than 18 years of age and were hospitalized secondary to severe COVID-19 infection, indicated by an oxygenation index < 300. Primary study outcomes included mortality, mechanical ventilation, intensive care unit (ICU) admission, and cardiac arrest. From a total of 100 patients enrolled, 25 patients were in the HDIVC group and 75 patients in the control group. The average time to death was significantly longer for HDIVC patients (P = 0.0139), with an average of 22.9 days versus 13.7 days for control patients. Patients who received HDIVC also had significantly lower rates of mechanical ventilation (52.93% vs. 73.14%; OR IPTW = 0.27; P = 0.0499) and cardiac arrest (2.46% vs. 9.06%; OR IPTW = 0.23; P = 0.0439). HDIVC may be an effective treatment in decreasing the rates of mechanical ventilation and cardiac arrest in hospitalized patients with severe COVID-19. A longer hospital stay and prolonged time to death may suggest that HDIVC may protect against clinical deterioration in severe COVID-19.
Author contributions ALH and AH were responsible for drafting and coordinating the manuscript writing process, multiple critical revisions, and final approval of the manuscript. AH was responsible for the institutional review board approval and has overseen the entire study. JD was responsible for drafting part of the manuscript and critical revision of the manuscript. AK was responsible for the manuscript introduction and critical revision of the manuscript. ALH, JD, TP and RB are responsible for the data integrity. PK was responsible for the statistical analysis and for critical revision of the manuscript. PK drafted the results and tables. AB and SG provided critical revision of the manuscript. All authors read and approved the final manuscript.
Conflict of interest The authors have no competing interests to declare.
Human and animal rights statement This study as approved by the Institutional Review Board, Beaumont Health. Informed consent Informed consent was waived as the curent study was had a retrospective design. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bhimraj, Morgan, Shumaker, Lavergne, Baden et al., Guidelines on the treatment and management of patients with COVID-19, Inf Dis Soc Am
Carr, Rosengrave, Bayer, Chambers, Mehrtens et al., Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes, Crit Care
Carr, Rowe, The emerging role of vitamin C in the prevention and treatment of COVID-19, Nutrients, doi:10.3390/nu12113286
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J Chronic Dis, doi:10.1016/0021-9681(87)90171-8
De Grooth, Manubulu-Choo, Zandvliet, Spoelstra-De Man, Girbes, Vitamin c pharmacokinetics in critically Ill patients: a randomized trial of four IV regimens, Chest
Fontana, oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19, Kidney Int Rep
Fowler, Truwit, Hite, Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial, JAMA, doi:10.1001/jama.2019.11825
Gibson, Qin, Puah, COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS, Med J Aust, doi:10.5694/mja2.50674
Hemilä, Chalker, Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis, J Intensive Care, doi:10.1186/s40560-020-0432-y
Hiedra, Lo, Elbashabsheh, The use of IV vitamin C for patients with COVID-19: a case series, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2020.1794819
Hoang, Show, Fang, Han, Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection, J Glob Antimicrob Resist, doi:10.1016/j.jgar.2020.09.025
Holford, Carr, Jovic, Ali, Whitaker et al., Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19, Nutrients
Kashiouris, Heureux, Cable, Fisher, Leichtle et al., The emerging role of vitamin C as a treatment for sepsis, Nutrients, doi:10.3390/nu12020292
Keddissi, Youness, Jones, Kinasewitz, Fluid management in acute respiratory distress syndrome: a narrative review, Can J Respir Ther, doi:10.29390/cjrt-2018-016
Mark, Khangoora, Rivera, Hooper, Catravas, Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock, Chest, doi:10.1016/j.chest.2016.11.036
Mehta, Kellum, Shah, Molitoris, Ronco et al., Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury, Crit Care, doi:10.1186/cc5713
Wu, Leung, Bushman, Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China, Nat Med, doi:10.1038/s41591-020-0822-7
Zhang, Rao, Li, Pilot trial of high-dose vitamin C in critically ill COVID-19 patients, Ann Intensive Care, doi:10.1186/s13613-020-00792-3
Zhao, Ling, Li, Peng, Huang et al., Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study, Ann Palliat Med, doi:10.21037/apm-20-1387
DOI record:
{
"DOI": "10.1007/s11739-022-02954-6",
"ISSN": [
"1828-0447",
"1970-9366"
],
"URL": "http://dx.doi.org/10.1007/s11739-022-02954-6",
"alternative-id": [
"2954"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "30 March 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "22 February 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "29 March 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors have no competing interests to declare."
},
{
"group": {
"label": "Human and animal rights statement",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "This study as approved by the Institutional Review Board, Beaumont Health."
},
{
"group": {
"label": "Informed consent",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Informed consent was waived as the curent study was had a retrospective design."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"affiliation": [],
"family": "Hess",
"given": "Andrea L.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1805-992X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Halalau",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dokter",
"given": "Jonathan J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paydawy",
"given": "Tania S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karabon",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bastani",
"given": "Aveh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baker",
"given": "Rebecca E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balla",
"given": "Abdalla Kara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galens",
"given": "Stephen A.",
"sequence": "additional"
}
],
"container-title": [
"Internal and Emergency Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T09:30:36Z",
"timestamp": 1648546236000
},
"deposited": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T12:01:27Z",
"timestamp": 1648555287000
},
"indexed": {
"date-parts": [
[
2022,
3,
30
]
],
"date-time": "2022-03-30T04:47:31Z",
"timestamp": 1648615651880
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1828-0447"
},
{
"type": "electronic",
"value": "1970-9366"
}
],
"issued": {
"date-parts": [
[
2022,
3,
29
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T00:00:00Z",
"timestamp": 1648512000000
}
},
{
"URL": "https://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T00:00:00Z",
"timestamp": 1648512000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11739-022-02954-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s11739-022-02954-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11739-022-02954-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
3,
29
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
29
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "2954_CR1",
"unstructured": "https://coronavirus.jhu.edu/map.html"
},
{
"DOI": "10.1056/NEJMoa2007764",
"author": "JH Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "2954_CR2",
"unstructured": "Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Leutkemeyer A (2020) Remdesivir for the treatment of Covid-19—final report. N Engl J Med 383:1813–1826. https://doi.org/10.1056/NEJMoa2007764",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.5694/mja2.50674",
"author": "PG Gibson",
"doi-asserted-by": "publisher",
"first-page": "54",
"journal-title": "Med J Aust",
"key": "2954_CR3",
"unstructured": "Gibson PG, Qin L, Puah SH (2020) COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre- COVID-19 ARDS. Med J Aust 213:54–56. https://doi.org/10.5694/mja2.50674",
"volume": "213",
"year": "2020"
},
{
"DOI": "10.1186/s13054-017-1891-y",
"author": "AC Carr",
"doi-asserted-by": "publisher",
"first-page": "300",
"journal-title": "Crit Care",
"key": "2954_CR4",
"unstructured": "Carr AC, Rosengrave P, Bayer S, Chambers S, Mehrtens J, Shaw GM (2017) Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care 21:300",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.1001/jama.2019.11825",
"author": "AA Fowler",
"doi-asserted-by": "publisher",
"first-page": "1261",
"journal-title": "JAMA",
"key": "2954_CR5",
"unstructured": "Fowler AA, Truwit JD, Hite RD (2019) Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 322:1261–1270. https://doi.org/10.1001/jama.2019.11825",
"volume": "322",
"year": "2019"
},
{
"DOI": "10.1016/j.jgar.2020.09.025",
"author": "BX Hoang",
"doi-asserted-by": "publisher",
"first-page": "256",
"journal-title": "J Glob Antimicrob Resist",
"key": "2954_CR6",
"unstructured": "Hoang BX, Show G, Fang W, Han B (2020) Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist 23:256–262. https://doi.org/10.1016/j.jgar.2020.09.025",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.3390/nu12020292",
"author": "MG Kashiouris",
"doi-asserted-by": "publisher",
"first-page": "292",
"issue": "2",
"journal-title": "Nutrients",
"key": "2954_CR7",
"unstructured": "Kashiouris MG, L’Heureux M, Cable CA, Fisher BJ, Leichtle SA, Fowler AA (2020) The emerging role of vitamin C as a treatment for sepsis. Nutrients 12(2):292. https://doi.org/10.3390/nu12020292",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2016.11.036",
"author": "PE Mark",
"doi-asserted-by": "publisher",
"first-page": "1229",
"issue": "6",
"journal-title": "Chest",
"key": "2954_CR8",
"unstructured": "Mark PE, Khangoora V, Rivera R, Hooper MH, Catravas J (2017) Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock. Chest 151(6):1229–1238. https://doi.org/10.1016/j.chest.2016.11.036",
"volume": "151",
"year": "2017"
},
{
"key": "2954_CR9",
"unstructured": "Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia (n.d.). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04264533"
},
{
"DOI": "10.1186/s13613-020-00792-3",
"author": "J Zhang",
"doi-asserted-by": "publisher",
"first-page": "5",
"journal-title": "Ann Intensive Care",
"key": "2954_CR10",
"unstructured": "Zhang J, Rao X, Li Y et al (2021) Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care 11:5. https://doi.org/10.1186/s13613-020-00792-3",
"volume": "11",
"year": "2021"
},
{
"key": "2954_CR11",
"unstructured": "Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Gallaghe, Muller WJ, O’Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y (2020) Guidelines on the treatment and management of patients with COVID-19. Inf Dis Soc Am. https://www.idsociety.org/COVID19guidelines"
},
{
"DOI": "10.1038/s41591-020-0822-7",
"author": "JT Wu",
"doi-asserted-by": "publisher",
"first-page": "506",
"journal-title": "Nat Med",
"key": "2954_CR12",
"unstructured": "Wu JT, Leung K, Bushman M et al (2020) Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med 26:506–510. https://doi.org/10.1038/s41591-020-0822-7",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"author": "ME Charlson",
"doi-asserted-by": "publisher",
"first-page": "373",
"issue": "5",
"journal-title": "J Chronic Dis",
"key": "2954_CR13",
"unstructured": "Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383. https://doi.org/10.1016/0021-9681(87)90171-8 (PMID: 3558716)",
"volume": "40",
"year": "1987"
},
{
"DOI": "10.1186/cc5713",
"author": "RL Mehta",
"doi-asserted-by": "publisher",
"first-page": "31",
"issue": "2",
"journal-title": "Crit Care",
"key": "2954_CR14",
"unstructured": "Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11(2):31. https://doi.org/10.1186/cc5713 (PMID: 17331245; PMCID: PMC2206446)",
"volume": "11",
"year": "2007"
},
{
"DOI": "10.1016/j.chest.2018.02.025",
"author": "HJ de Grooth",
"doi-asserted-by": "publisher",
"first-page": "1368",
"issue": "6",
"journal-title": "Chest",
"key": "2954_CR15",
"unstructured": "de Grooth HJ, Manubulu-Choo WP, Zandvliet AS, Spoelstra-de Man AME, Girbes AR et al (2018) Vitamin c pharmacokinetics in critically Ill patients: a randomized trial of four IV regimens. Chest 153(6):1368–1377",
"volume": "153",
"year": "2018"
},
{
"DOI": "10.1186/s40560-020-0432-y",
"author": "H Hemilä",
"doi-asserted-by": "publisher",
"first-page": "15",
"issue": "8",
"journal-title": "J Intensive Care",
"key": "2954_CR16",
"unstructured": "Hemilä H, Chalker E (2020) Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care 7(8):15. https://doi.org/10.1186/s40560-020-0432-y (PMID: 32047636; PMCID: PMC7006137)",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.3390/nu12123760",
"author": "P Holford",
"doi-asserted-by": "publisher",
"first-page": "3760",
"issue": "12",
"journal-title": "Nutrients",
"key": "2954_CR17",
"unstructured": "Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD (2020) Vitamin C—an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients 12(12):3760",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.ekir.2020.07.008",
"author": "F Fontana",
"doi-asserted-by": "publisher",
"first-page": "1815",
"issue": "10",
"journal-title": "Kidney Int Rep",
"key": "2954_CR18",
"unstructured": "Fontana F et al (2020) oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Rep 5(10):1815–1822",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.29390/cjrt-2018-016",
"author": "JI Keddissi",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Can J Respir Ther",
"key": "2954_CR19",
"unstructured": "Keddissi JI, Youness HA, Jones KR, Kinasewitz GT (2018) Fluid management in acute respiratory distress syndrome: a narrative review. Can J Respir Ther 55:1–8. https://doi.org/10.29390/cjrt-2018-016",
"volume": "55",
"year": "2018"
},
{
"DOI": "10.21037/apm-20-1387",
"doi-asserted-by": "publisher",
"key": "2954_CR20",
"unstructured": "Zhao B, Ling Y, Li J, Peng Y, Huang J, Wang Y, Qu H, Gao Y, Li Y, Hu B, Lu S, Lu H, Zhang W, Mao E (2020) Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study. Ann Palliat Med. https://doi.org/10.21037/apm-20-1387. Epub ahead of print. PMID: 33222462"
},
{
"DOI": "10.1080/14787210.2020.1794819",
"author": "R Hiedra",
"doi-asserted-by": "publisher",
"first-page": "1259",
"issue": "12",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "2954_CR21",
"unstructured": "Hiedra R, Lo KB, Elbashabsheh M et al (2020) The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev Anti Infect Ther 18(12):1259–1261. https://doi.org/10.1080/14787210.2020.1794819",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.3390/nu12113286",
"author": "AC Carr",
"doi-asserted-by": "publisher",
"first-page": "3286",
"issue": "11",
"journal-title": "Nutrients",
"key": "2954_CR22",
"unstructured": "Carr AC, Rowe S (2020) The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 12(11):3286. https://doi.org/10.3390/nu12113286 (PMID: 33121019; PMCID: PMC7693980)",
"volume": "12",
"year": "2020"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s11739-022-02954-6"
}
},
"score": 1,
"short-container-title": [
"Intern Emerg Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Emergency Medicine",
"Internal Medicine"
],
"subtitle": [],
"title": [
"High-dose intravenous vitamin C decreases rates of mechanical ventilation and cardiac arrest in severe COVID-19"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}
