The use of IV vitamin C for patients with COVID-19: a case series
et al., Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2020.1794819, Aug 2020
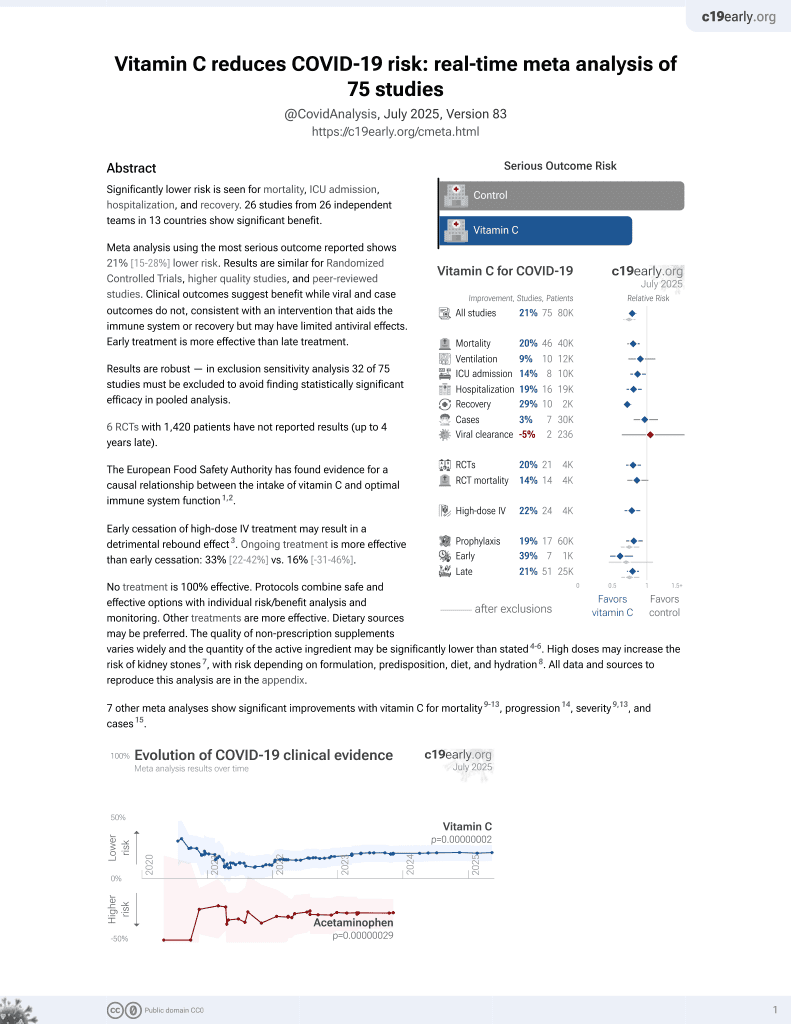
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
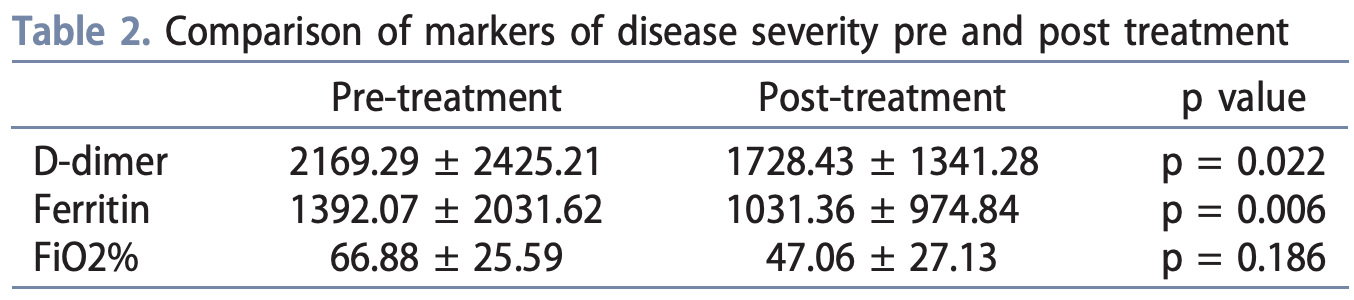
Case study of 17 patients receiving IV vitamin C for COVID-19, finding a significant decrease in inflammatory markers, including ferritin and D-dimer, and a trend to decreasing FiO2 requirements, after vitamin C administration. There was no control group.
Hiedra et al., 1 Aug 2020, peer-reviewed, 8 authors.
The use of IV vitamin C for patients with COVID-19: a case series
Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2020.1794819
Background: The coronavirus disease 2019 (COVID-19) pandemic has affected almost 2.5 million people worldwide with almost 170,000 deaths reported to date. So far, there is scarce evidence for the current treatment options available for COVID-19. Vitamin C has previously been used for treatment of severe sepsis and septic shock. We reviewed the feasibility of using vitamin C in the setting of COVID-19 in a series of patients. Methods: We sequentially identified a series of patients who were requiring at least 30% of FiO2 or more who received IV vitamin C as part of the COVID-19 treatment and analyzed their demographic and clinical characteristics. We compared inflammatory markers pre and post treatment including D-dimer and ferritin. Results: We identified a total of 17 patients who received IV vitamin C for COVID-19. The inpatient mortality rate in this series was 12% with 17.6% rates of intubation and mechanical ventilation. We noted a significant decrease in inflammatory markers, including ferritin and D-dimer, and a trend to decreasing FiO2 requirements, after vitamin C administration.
Conclusion: The use of IV vitamin C in patients with moderate to severe COVID-19 disease may be feasible.
Declaration of interest The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Alhazzani, Møller, Arabi, Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19), Intensive Care Med
Bhimraj, Morgan, Shumaker, Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19, Clinical Infectious Diseases
Caprio, Infante, Calanchini, Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects, Eat Weight Disord
Cheng, Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)?, Medicine in Drug Discovery
Fabbri, Infante, Ricordi, Editorial -Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections, Eur Rev Med Pharmacol Sci
Fortarezza, Pezzuto, COVID-19 nephropathy: what could pathologist say?, Nephropathol J
Fujii, Luethi, Young, Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the vitamins randomized clinical trial, Jama
Jayawardena, Sooriyaarachchi, Chourdakis, Enhancing immunity in viral infections, with special emphasis on COVID-19: A review, Diabetes & Metabolic Syndrome
Marik, Khangoora, Rivera, Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study, Chest
Mehta, Mcauley, Brown, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Mitchell, Vitamin-D and COVID-19: do deficient risk a poorer outcome?, Lancet Diabetes Endocrinol
Mousavi, Bereswill, Heimesaat, Immunomodulatory and antimicrobial effects of vitamin C, Eur J Microbiol Immunol (Bp)
Richardson, Hirsch, Narasimhan, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA
Stefan, Birkenfeld, Schulze, Obesity and impaired metabolic health in patients with COVID-19, Nat Rev Endocrinol
Truwit, Hite, Morris, Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial, Jama
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, Jama
DOI record:
{
"DOI": "10.1080/14787210.2020.1794819",
"ISSN": [
"1478-7210",
"1744-8336"
],
"URL": "http://dx.doi.org/10.1080/14787210.2020.1794819",
"alternative-id": [
"10.1080/14787210.2020.1794819"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-05-15"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2020-07-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2020-08-01"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1758-1994",
"affiliation": [
{
"name": "Department of Medicine, Einstein Medical Center Philadelphia, Philadelphia, PA, USA"
}
],
"authenticated-orcid": false,
"family": "Hiedra",
"given": "Raul",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7088-6677",
"affiliation": [
{
"name": "Department of Medicine, Einstein Medical Center Philadelphia, Philadelphia, PA, USA"
}
],
"authenticated-orcid": false,
"family": "Lo",
"given": "Kevin Bryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Einstein Medical Center Philadelphia, Philadelphia, PA, USA"
}
],
"family": "Elbashabsheh",
"given": "Mohammad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5687-4485",
"affiliation": [
{
"name": "Department of Medicine, Einstein Medical Center Philadelphia, Philadelphia, PA, USA"
}
],
"authenticated-orcid": false,
"family": "Gul",
"given": "Fahad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy Department, Einstein Medical Center Philadelphia, Philadelphia, PA, USA"
}
],
"family": "Wright",
"given": "Robert Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Einstein Medical Center Philadelphia, Philadelphia, PA, USA"
}
],
"family": "Albano",
"given": "Jeri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Einstein Medical Center Philadelphia, Philadelphia, PA, USA"
}
],
"family": "Azmaiparashvili",
"given": "Zurab",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care and Sleep Medicine, Einstein Medical Center Philadelphia, Philadelphia, PA, USA"
}
],
"family": "Patarroyo Aponte",
"given": "Gabriel",
"sequence": "additional"
}
],
"container-title": "Expert Review of Anti-infective Therapy",
"container-title-short": "Expert Review of Anti-infective Therapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
14
]
],
"date-time": "2020-07-14T15:22:29Z",
"timestamp": 1594740149000
},
"deposited": {
"date-parts": [
[
2021,
5,
8
]
],
"date-time": "2021-05-08T14:36:48Z",
"timestamp": 1620484608000
},
"indexed": {
"date-parts": [
[
2024,
5,
5
]
],
"date-time": "2024-05-05T00:50:54Z",
"timestamp": 1714870254752
},
"is-referenced-by-count": 98,
"issue": "12",
"issued": {
"date-parts": [
[
2020,
8,
1
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2020,
12,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/14787210.2020.1794819",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1259-1261",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2020,
8,
1
]
]
},
"published-online": {
"date-parts": [
[
2020,
8,
1
]
]
},
"published-print": {
"date-parts": [
[
2020,
12,
1
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"key": "cit0001",
"unstructured": "World Health Organization. Coronavirus disease; 2019. (COVID-19): situation report, 93."
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "cit0002"
},
{
"key": "cit0003",
"unstructured": "NIH COVID-19 treatment guidelines. https://covid19treatmentguidelines.nih.gov/accessed [cited 2020 Apr 23]"
},
{
"DOI": "10.1093/cid/ciaa478",
"doi-asserted-by": "crossref",
"key": "cit0004",
"unstructured": "Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clinical Infectious Diseases. 2020 Apr 27."
},
{
"author": "Alhazzani W",
"first-page": "1",
"journal-title": "Intensive Care Med",
"key": "cit0005",
"year": "2020"
},
{
"DOI": "10.1016/j.dsx.2020.04.015",
"doi-asserted-by": "crossref",
"key": "cit0006",
"unstructured": "Jayawardena R, Sooriyaarachchi P, Chourdakis M, et al. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020 Apr 16."
},
{
"DOI": "10.1556/1886.2019.00016",
"doi-asserted-by": "publisher",
"key": "cit0007"
},
{
"DOI": "10.1038/s41574-020-0364-6",
"doi-asserted-by": "publisher",
"key": "cit0008"
},
{
"DOI": "10.34172/jnp.2020.32",
"doi-asserted-by": "publisher",
"key": "cit0009"
},
{
"DOI": "10.1016/j.chest.2016.11.036",
"doi-asserted-by": "publisher",
"key": "cit0010"
},
{
"author": "Truwit JD",
"first-page": "125",
"issue": "13",
"journal-title": "Jama",
"key": "cit0011",
"volume": "322",
"year": "2019"
},
{
"DOI": "10.1001/jama.2019.22176",
"doi-asserted-by": "publisher",
"key": "cit0012"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "cit0013"
},
{
"DOI": "10.1016/j.medidd.2020.100028",
"doi-asserted-by": "publisher",
"key": "cit0014"
},
{
"DOI": "10.1016/S2213-8587(20)30183-2",
"doi-asserted-by": "publisher",
"key": "cit0015"
},
{
"author": "Fabbri A",
"first-page": "4048",
"issue": "7",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "cit0016",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1007/s40519-016-0312-6",
"doi-asserted-by": "publisher",
"key": "cit0017"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "cit0018"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/14787210.2020.1794819"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The use of IV vitamin C for patients with COVID-19: a case series",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "18"
}