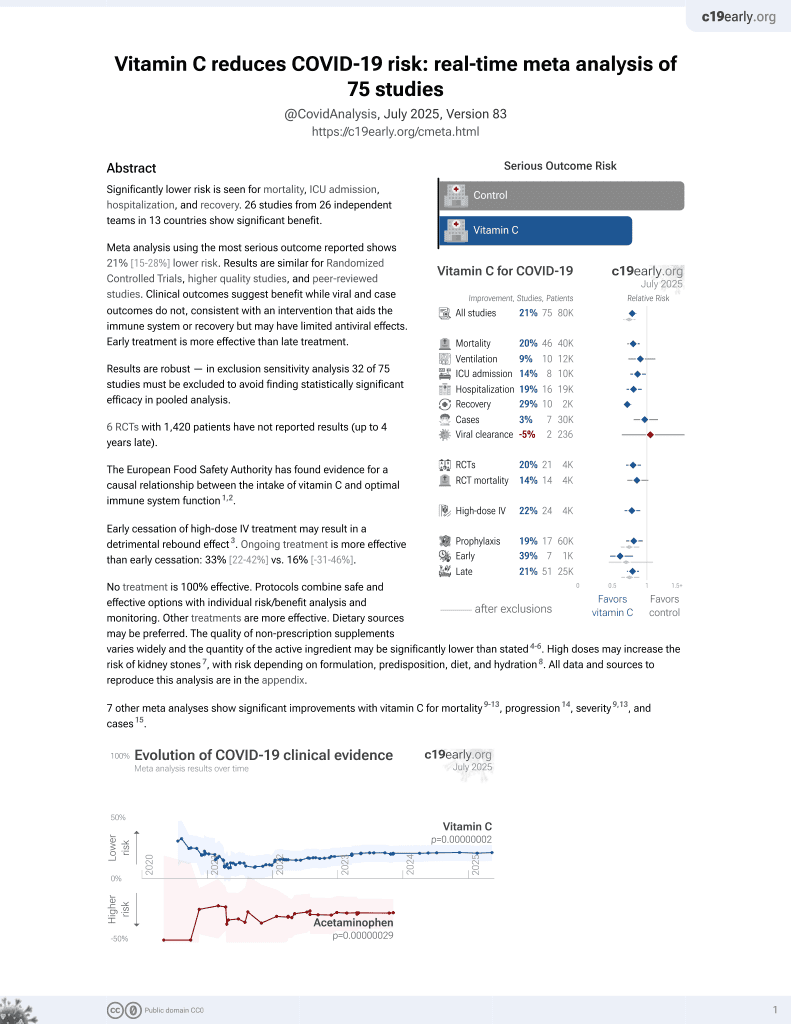
The effect of vitamin C on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials
et al., Inflammopharmacology, doi:10.1007/s10787-023-01200-5, Apr 2023
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Meta analysis of 11 vitamin C RCTs showing significantly lower COVID-19 mortality with treatment. The effect size is larger than in our analysis due to the authors' inclusion of 2 trials that we exclude due to combined treatments being likely to contribute more to the effect seen.
7 meta-analyses show significant improvements with vitamin C for mortality1-5,
progression6,
severity1,5, and
cases7.
Currently there are 74 vitamin C for COVID-19 studies, showing 18% lower mortality [9‑27%], 9% lower ventilation [-12‑27%], 14% lower ICU admission [2‑24%], 19% lower hospitalization [7‑30%], and 3% fewer cases [-16‑19%].
|
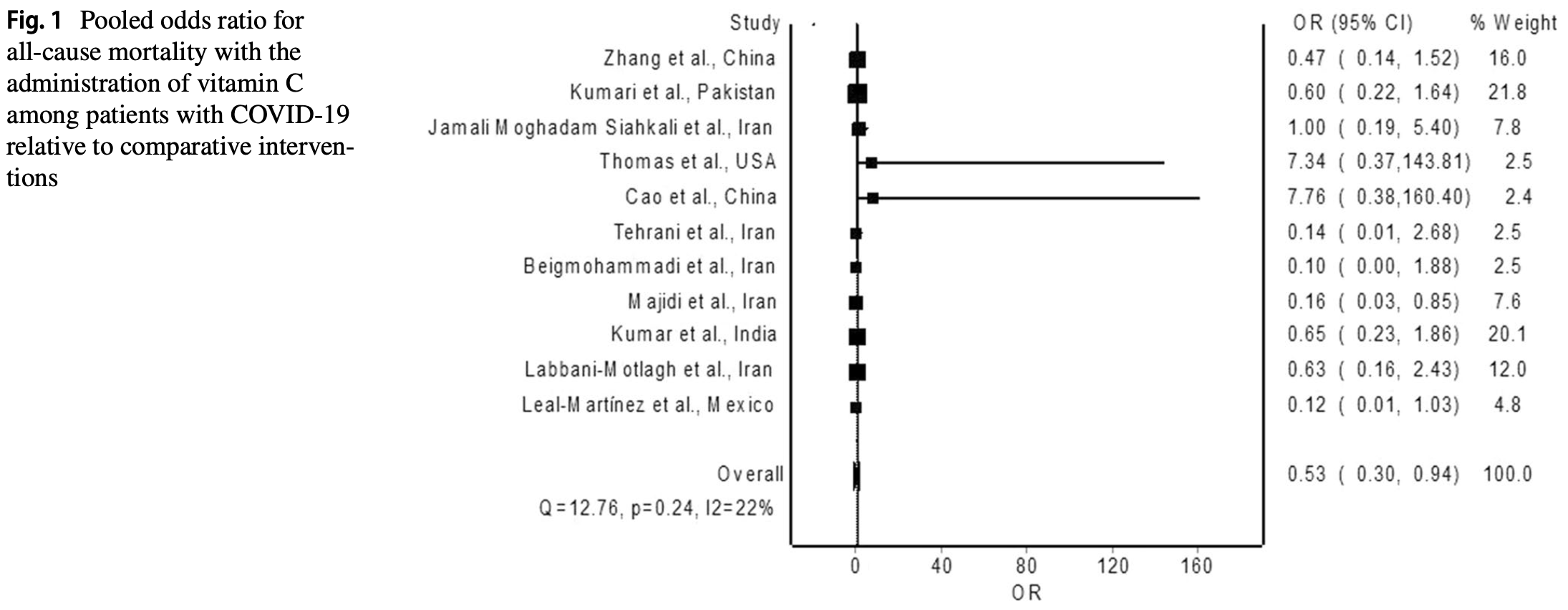
risk of death, 47.0% lower, OR 0.53, p = 0.03, RR approximated with OR.
|
|
risk of death, 53.0% lower, OR 0.47, p = 0.01, severe patients, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Bhowmik et al., Impact of high-dose vitamin C on the mortality, severity, and duration of hospital stay in COVID-19 patients: A meta-analysis, Health Science Reports, doi:10.1002/hsr2.762.
2.
Olczak-Pruc et al., Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis, Nutrients, doi:10.3390/nu14194217.
3.
Kow et al., The effect of vitamin C on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials, Inflammopharmacology, doi:10.1007/s10787-023-01200-5.
4.
Kow (B) et al., Impact of uricosurics on mortality outcomes in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials, International Journal of Pharmacy Practice, doi:10.1093/ijpp/riae003.
5.
Qin et al., Effects of Vitamin C Supplements on Clinical Outcomes and Hospitalization Duration for Patients with Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis, Nutrition Reviews, doi:10.1093/nutrit/nuae154.
Kow et al., 18 Apr 2023, peer-reviewed, 3 authors.
Contact: dineshsangarran.ramachandram@monash.edu, chiasiang_93@hotmail.com.
The effect of vitamin C on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials
Inflammopharmacology, doi:10.1007/s10787-023-01200-5
Background and Aims Vitamin C appears to be a viable treatment option for patients with COVID-19. Methods We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) of vitamin C versus comparative interventions in patients with COVID-19. The outcome of interest was all-cause mortality.
Results The meta-analysis of eleven trials using a random-effects model revealed significant reduction in the risk of allcause mortality with the administration of vitamin C among patients with COVID-19 relative to no vitamin C (pooled odds ratio = 0.53; 95% confidence interval 0.30-0.92). Subgroup analysis of studies that included patients with severe COVID-19 also produced findings of significant mortality reduction with the administration of vitamin C relative to no vitamin C (pooled odds ratio = 0.47; 95% confidence interval 0.26-0.84). Conclusion Overall, evidence from RCTs suggests a survival benefit for vitamin C in patients with severe COVID-19. However, we should await data from large-scale randomized trials to affirm its mortality benefits.
Author contributions CSK participated in the study conduct, and, data collection, as well as writing and reviewing the manuscript; DSR participated in data collection as well as writing and reviewing the manuscript; SSH performed the data analysis and interpretation; and all authors provided final approval of the manuscript for submission.
Declarations Conflict of interest All authors have no conflicts of interest to declare. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Beigmohammadi, Bitarafan, Hoseindokht, Abdollahi, Amoozadeh et al., The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial, Trials
Cao, Wei, Zou, Jiang, Wang et al., Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial, J Allergy Clin Immunol
Jamalimoghadamsiahkali, Zarezade, Koolaji, Seyedalinaghi, Zendehdel et al., Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial, Eur J Med Res
Kumar, Bhushan, Supriya, Ganapule, Lohani et al., Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial, J Family Med Prim Care
Kumari, Dembra, Dembra, Bhawna, Gul et al., The role of vitamin C as adjuvant therapy in COVID-19, Cureus
Labbani-Motlagh, Amini, Aliannejad, Sadeghi, Shafiee et al., High-dose intravenous vitamin C in early stages of severe acute respiratory syndrome coronavirus 2 infection: a double-blind, randomized, controlled clinical trial, J Res Pharm Pract
Leal-Martínez, Abarca-Bernal, García-Pérez, González-Tolosa, Cruz-Cázares et al., Effect of a nutritional support system to increase survival and reduce mortality in patients with COVID-19 in stage III and comorbidities: a blinded randomized controlled clinical trial, Int J Environ Res Public Health
Majidi, Rabbani, Gholami, Gholamalizadeh, Bourbour et al., The effect of vitamin C on pathological parameters and survival duration of critically Ill coronavirus disease 2019 patients: A randomized clinical trial, Front Immunol
Tehrani, Yadegarynia, Abrishami, Moradi, Gharaei et al., An investigation into the Effects of intravenous vitamin C on pulmonary CT Findings and clinical outcomes of patients with COVID 19 pneumonia a randomized clinical trial, Urol J
Thomas, Patel, Bittel, Wolski, Wang et al., Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory Patients With SARS-CoV-2 infection: the COVID A to Z randomized clinical trial, JAMA Netw Open
Zhang, Rao, Li, Zhu, Liu et al., Pilot trial of high-dose vitamin C in critically ill COVID-19 patients, Ann Intensive Care
DOI record:
{
"DOI": "10.1007/s10787-023-01200-5",
"ISSN": [
"0925-4692",
"1568-5608"
],
"URL": "http://dx.doi.org/10.1007/s10787-023-01200-5",
"alternative-id": [
"1200"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "8 February 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "18 March 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "18 April 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "All authors have no conflicts of interest to declare."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8186-2926",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kow",
"given": "Chia Siang",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hasan",
"given": "Syed Shahzad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramachandram",
"given": "Dinesh Sangarran",
"sequence": "additional"
}
],
"container-title": "Inflammopharmacology",
"container-title-short": "Inflammopharmacol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
4,
18
]
],
"date-time": "2023-04-18T12:02:54Z",
"timestamp": 1681819374000
},
"deposited": {
"date-parts": [
[
2023,
4,
18
]
],
"date-time": "2023-04-18T13:21:37Z",
"timestamp": 1681824097000
},
"indexed": {
"date-parts": [
[
2023,
4,
19
]
],
"date-time": "2023-04-19T06:33:40Z",
"timestamp": 1681886020832
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
4,
18
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
18
]
],
"date-time": "2023-04-18T00:00:00Z",
"timestamp": 1681776000000
}
},
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
18
]
],
"date-time": "2023-04-18T00:00:00Z",
"timestamp": 1681776000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s10787-023-01200-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s10787-023-01200-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s10787-023-01200-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2023,
4,
18
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
18
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1186/s13063-021-05795-4",
"author": "MT Beigmohammadi",
"doi-asserted-by": "publisher",
"first-page": "802",
"journal-title": "Trials",
"key": "1200_CR1",
"unstructured": "Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Soltani D (2021) The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial. Trials 22:802",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2020.05.019",
"author": "Y Cao",
"doi-asserted-by": "publisher",
"first-page": "137",
"journal-title": "J Allergy Clin Immunol",
"key": "1200_CR2",
"unstructured": "Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, Huang L, Meng F, Huang L, Wang N, Zhou X, Luo H, Mao Z, Chen X, Xie J, Liu J, Cheng H, Zhao J, Huang G, Wang W, Zhou J (2020) Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 146:137-146.e3",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1186/s40001-021-00490-1",
"author": "S JamaliMoghadamSiahkali",
"doi-asserted-by": "publisher",
"first-page": "20",
"journal-title": "Eur J Med Res",
"key": "1200_CR3",
"unstructured": "JamaliMoghadamSiahkali S, Zarezade B, Koolaji S, SeyedAlinaghi S, Zendehdel A, Tabarestani M, Sekhavati Moghadam E, Abbasian L, Dehghan Manshadi SA, Salehi M, Hasannezhad M, Ghaderkhani S, Meidani M, Salahshour F, Jafari F, Manafi N, Ghiasvand F (2021) Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res 26:20",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.4103/jfmpc.jfmpc_2437_21",
"author": "V Kumar",
"doi-asserted-by": "publisher",
"first-page": "4758",
"journal-title": "J Family Med Prim Care",
"key": "1200_CR4",
"unstructured": "Kumar V, Bhushan D, Supriya S, Ganapule AA, Lohani P, Shyama PS, Majhi PK, Anand U, Kumar R, Bhadani UK (2022) Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial. J Family Med Prim Care 11:4758–4765",
"volume": "11",
"year": "2022"
},
{
"author": "P Kumari",
"journal-title": "Cureus",
"key": "1200_CR5",
"unstructured": "Kumari P, Dembra S, Dembra P, Bhawna F, Gul A, Ali B, Sohail H, Kumar B, Memon MK, Rizwan A (2020) The role of vitamin C as adjuvant therapy in COVID-19. Cureus 12:e11779",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.4103/jrpp.jrpp_30_22",
"author": "Z Labbani-Motlagh",
"doi-asserted-by": "publisher",
"first-page": "64",
"journal-title": "J Res Pharm Pract",
"key": "1200_CR12",
"unstructured": "Labbani-Motlagh Z, Amini S, Aliannejad R, Sadeghi A, Shafiee G, Heshmat R, Jafary M, Talaschian M, Akhtari M, Jamshidi A, Mahmoudi M, Sadeghi K (2022) High-dose intravenous vitamin C in early stages of severe acute respiratory syndrome coronavirus 2 infection: a double-blind, randomized, controlled clinical trial. J Res Pharm Pract 11:64-72",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3390/ijerph19031172",
"author": "F Leal-Martínez",
"doi-asserted-by": "publisher",
"journal-title": "Int J Environ Res Public Health",
"key": "1200_CR11",
"unstructured": "Leal-Martínez F, Abarca-Bernal L, García-Pérez A, González-Tolosa D, Cruz-Cázares G, Montell-García M, Ibarra A (2022) Effect of a nutritional support system to increase survival and reduce mortality in patients with COVID-19 in stage III and comorbidities: a blinded randomized controlled clinical trial. Int J Environ Res Public Health 19:1172",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2021.717816",
"author": "N Majidi",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "1200_CR6",
"unstructured": "Majidi N, Rabbani F, Gholami S, Gholamalizadeh M, BourBour F, Rastgoo S, Hajipour A, Shadnoosh M, Akbari ME, Bahar B, Ashoori N, Alizadeh A, Samipoor F, Moslem A, Doaei S, Suzuki K (2021) The effect of vitamin C on pathological parameters and survival duration of critically Ill coronavirus disease 2019 patients: A randomized clinical trial. Front Immunol 12:717816",
"volume": "12",
"year": "2021"
},
{
"author": "S Tehrani",
"first-page": "460",
"journal-title": "Urol J",
"key": "1200_CR8",
"unstructured": "Tehrani S, Yadegarynia D, Abrishami A, Moradi H, Gharaei B, Rauofi M, Maghsoudi Nejad F, Sali S, Khabiri N, Abolghasemi S (2022) An investigation into the Effects of intravenous vitamin C on pulmonary CT Findings and clinical outcomes of patients with COVID 19 pneumonia a randomized clinical trial. Urol J 19:460–465",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.0369",
"author": "S Thomas",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw Open",
"key": "1200_CR9",
"unstructured": "Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, Il’Giovine ZJ, Mehra R, McWilliams C, Nissen SE, Desai MY (2021) Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory Patients With SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open 4:e210369",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1186/s13613-020-00792-3",
"author": "J Zhang",
"doi-asserted-by": "publisher",
"first-page": "5",
"journal-title": "Ann Intensive Care",
"key": "1200_CR10",
"unstructured": "Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, Luo G, Meng Z, De Backer D, Xiang H, Peng Z (2021) Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care 11:5",
"volume": "11",
"year": "2021"
}
],
"reference-count": 11,
"references-count": 11,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s10787-023-01200-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology",
"Immunology"
],
"subtitle": [],
"title": "The effect of vitamin C on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}