Effect of Vitamin C on Clinical Outcomes of Critically Ill Patients With COVID-19: An Observational Study and Subsequent Meta-Analysis
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.814587, Feb 2022
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
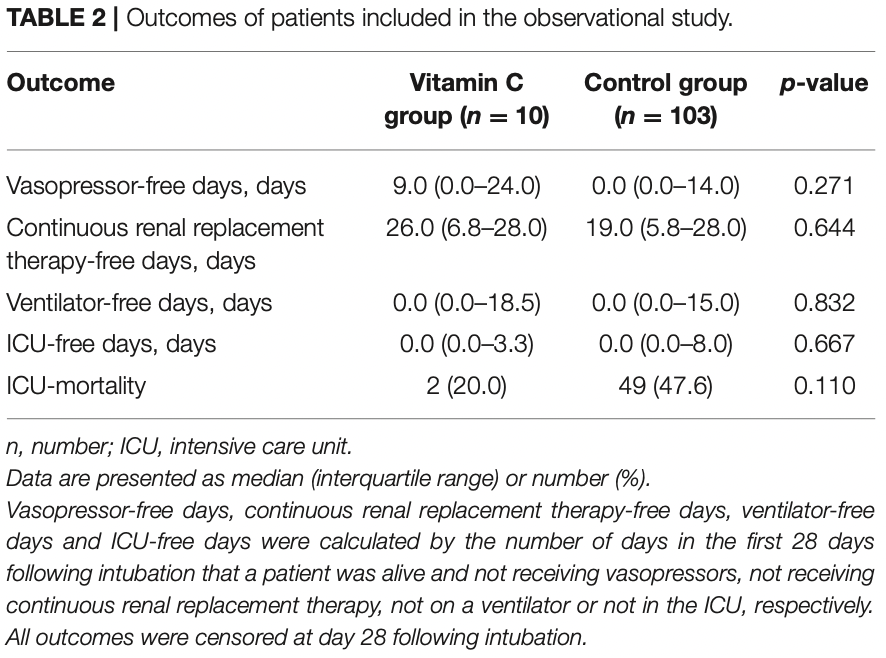
Retrospective 113 consecutive mechanically ventilated COVID+ ICU patients in Greece, 10 receiving high-dose IV vitamin C, showing lower mortality with treatment, without statistical significance (p=0.11).
The associated meta analysis includes only 11 studies, while there are currently 74 studies, 45 with mortality results. Authors only include critical patients, however not all studies with critical patients are included, for example1-4. The meta analysis also uses unadjusted results, while PSM, Cox proportional hazards, or KM results are reported by5-8. For7 authors use 28 day mortality, while the study reports longer term in-hospital mortality.
This is the 41st of 74 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000068.
21 studies are RCTs, which show efficacy with p=0.0012.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
|
risk of death, 58.0% lower, RR 0.42, p = 0.11, treatment 2 of 10 (20.0%), control 49 of 103 (47.6%), NNT 3.6.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Majidi et al., The Effect of Vitamin C on Pathological Parameters and Survival Duration of Critically Ill Coronavirus Disease 2019 Patients: A Randomized Clinical Trial, Frontiers in Immunology, doi:10.3389/fimmu.2021.717816.
2.
Yüksel et al., Effects of high dose vitamin c on patient outcomes in ARDS patients admitted to intensive care with COVID-19; multi-center retrospective study, Intensive Care Medicine Experimental, 9:S1, 001458, doi:10.1186/s40635-021-00413-8.
3.
Hamidi-Alamdari et al., Methylene blue for treatment of hospitalized COVID-19 patients: a randomized, controlled, open-label clinical trial, phase 2, Clinical and Translational Investigation, doi:10.24875/RIC.21000028.
4.
Özgünay et al., The use of vitamin C in the intensive care unit during the COVID-19 pandemic, The European Research Journal, doi:10.18621/eurj.938778.
5.
Al Sulaiman et al., Ascorbic Acid as an Adjunctive Therapy in Critically Ill Patients with COVID-19: A Multicenter Propensity Score Matched Study, Research Square, doi:10.21203/rs.3.rs-354711/v1.
6.
Zheng et al., No significant benefit of moderate-dose vitamin C on severe COVID-19 cases, Open Medicine, doi:10.1515/med-2021-0361.
Gavrielatou et al., 11 Feb 2022, retrospective, Greece, peer-reviewed, 10 authors, study period 21 October, 2020 - 8 March, 2021, average treatment delay 5.5 days.
Effect of Vitamin C on Clinical Outcomes of Critically Ill Patients With COVID-19: An Observational Study and Subsequent Meta-Analysis
Frontiers in Medicine, doi:10.3389/fmed.2022.814587
of mortality among critically ill patients with COVID-19. Additional evidence is anticipated from relevant large randomized controlled trials which are currently underway.
AUTHOR CONTRIBUTIONS EG contributed to study design, collected data, and interpreted data. EX and NX contributed to study design and the execution of the meta-analysis and they wrote the first draft of the manuscript. EX also undertook statistical analyses. AM, EI, and AKa contributed to data collection. DZ contributed to study design and data interpretation. CR and AKo contributed to data interpretation and critically revised the manuscript. IS conceived of the study, designed the study, supervised the data collection and statistical analyses, and is the guarantor, and final responsibility for the decision to submit for publication. All authors read and approved the final manuscript.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed. 2022.814587/full#supplementary-material
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Copyright © 2022 Gavrielatou, Xourgia, Xixi, Mantelou,..
References
Beigmohammadi, Bitarafan, Hoseindokht, Abdollahi, Amoozadeh et al., The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial, Trials, doi:10.1186/s13063-021-05795-4
Carr, Rowe, The emerging role of vitamin c in the prevention and treatment of COVID-19, Nutrients, doi:10.3390/nu12113286
Carr, Vitamin C in pneumonia and sepsis
Chang, Liao, Guan, Guo, Zhao et al., Combined treatment with hydrocortisone, vitamin c, and thiamine for sepsis and septic shock: a randomized controlled trial, Chest, doi:10.1016/j.chest.2020.02.065
Darban, Malek, Memarian, Gohari, Kiani et al., Efficacy of High dose vitamin C, melatonin and zinc in iranian patients with acute respiratory syndrome due to coronavirus infection: a pilot randomized trial, J Cell Mol Anesth, doi:10.22037/jcma.v6i2.32182
Fowler, Truwit, Hite, Morris, Dewilde et al., Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the cITRIS-ALI randomized clinical trial, JAMA, doi:10.1001/jama.2019.11825
Fujii, Luethi, Young, Frei, Eastwood et al., Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial, JAMA, doi:10.1001/jama.2019.22176
Gao, Xu, Wang, Lv, Ma et al., The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study, Aging, doi:10.18632/aging.202557
Higgins, Thomas, Chandler, Cumpston, Li et al., Cochrane Handbook for Systematic Reviews of Interventions version
Holford, Carr, Zawari, Vizcaychipi, Vitamin C intervention for critical COVID-19: a pragmatic review of the current level of evidence, Life, doi:10.3390/life11111166
Hooper, Hager, Understanding vitamin c in critical illness: a focus on dose, route, and disease, Crit Care Med, doi:10.1097/CCM.0000000000003718
Iglesias, Vassallo, Patel, Sullivan, Cavanaugh et al., Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: the ORANGES trial, Chest, doi:10.1016/j.chest.2020.02.049
Jakobsen, Gluud, Wetterslev, Winkel, When and how should multiple imputation be used for handling missing data in randomised clinical trials -a practical guide with flowcharts, BMC Med Res Methodol, doi:10.1186/s12874-017-0442-1
Jamalimoghadamsiahkali, Zarezade, Koolaji, Seyedalinaghi, Zendehdel et al., Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial, Eur J Med Res, doi:10.1186/s40001-021-00490-1
Krishnan, Patel, Desai, Sule, Paik et al., Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia, J Clin Anesth, doi:10.1016/j.jclinane.2020.110005
Kumari, Dembra, Dembra, Bhawna, Gul et al., The role of vitamin c as adjuvant therapy in COVID-19, Cureus, doi:10.7759/cureus.11779
Li, Ching, Hipple, Lopez, Sahibzada et al., Use of intravenous vitamin c in critically ill patients with covid-19 infection, J Pharm Pract, doi:10.1177/08971900211015052
Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche et al., The PRISMA statement for reporting systematic reviews and metaanalyses of studies that evaluate healthcare interventions: explanation and elaboration, BMJ, doi:10.1136/bmj.b2700
Marik, Khangoora, Rivera, Hooper, Catravas, Hydrocortisone, Vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study, Chest, doi:10.1016/j.chest.2016.11.036
Milani, Macchi, Guz-Mark, Vitamin C in the treatment of COVID-19, Nutrients, doi:10.3390/nu13041172
Papoutsi, Giannakoulis, Xourgia, Routsi, Kotanidou et al., Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies, Crit Care, doi:10.1186/s13054-021-03540-6
Patel, Haar, Handslip, Auepanwiriyakul, Lee et al., Natural history, trajectory, and management of mechanically ventilated COVID-19 patients in the United Kingdom, Intensive Care Med, doi:10.1007/s00134-021-06389-z
Rawat, Roy, Maitra, Gulati, Khanna et al., Vitamin C and COVID-19 treatment: a systematic review and meta-analysis of randomized controlled trials, Diabetes Metab Syndr, doi:10.1016/j.dsx.2021.102324
Recovery Collaborative Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Sato, Hasegawa, Prasitlumkum, Ueoka, Nishida et al., Effect of IV high-dose vitamin c on mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials, Crit Care Med, doi:10.1097/CCM.0000000000005263
Schenck, Oromendia, Torres, Berlin, Choi et al., Rapidly improving ARDS in therapeutic randomized controlled trials, Chest, doi:10.1016/j.chest.2018.09.031
Sevransky, Rothman, Hager, Bernard, Brown et al., Effect of vitamin C, thiamine, and hydrocortisone on ventilator-and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial, JAMA, doi:10.1001/jama.2020.24505
Siempos, Xourgia, Ntaidou, Zervakis, Magira et al., Effect of Early vs. Delayed or no intubation on clinical outcomes of patients with COVID-19: an observational study, Front Med, doi:10.3389/fmed.2020.614152
Sterne, Savović, Page, Elbers, Blencowe et al., RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ, doi:10.1136/bmj.l4898
Sulaiman, Aljuhani, Saleh, Badreldin, Harthi et al., Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: a propensity score matched study, Sci Rep, doi:10.1038/s41598-021-96703-y
Yanase, Fujii, Naorungroj, Belletti, Luethi et al., Harm of IV high-dose vitamin C therapy in adult patients: a scoping review, Crit Care Med, doi:10.1097/CCM.0000000000004396
Zhang, Rao, Li, Zhu, Liu et al., Pilot trial of high-dose vitamin C in critically ill COVID-19 patients, Ann Intensive Care, doi:10.1186/s13613-020-00792-3
Zheng, Chen, Jiang, Guo, Luo et al., No significant benefit of moderate-dose vitamin C on severe COVID-19 cases, Open Med Wars Pol, doi:10.1515/med-2021-0361
DOI record:
{
"DOI": "10.3389/fmed.2022.814587",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2022.814587",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Whether vitamin C provides any benefit when administered in critically ill patients, including those with coronavirus disease (COVID-19), is controversial. We endeavored to estimate the effect of administration of vitamin C on clinical outcomes of critically ill patients with COVID-19 by performing an observational study and subsequent meta-analysis.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Firstly, we conducted an observational study of critically ill patients with laboratory-confirmed COVID-19 who consecutively underwent invasive mechanical ventilation in an academic intensive care unit (ICU) during the second pandemic wave. We compared all-cause mortality of patients receiving vitamin C (“vitamin C” group) or not (“control” group) on top of standard-of-care. Subsequently, we systematically searched PubMed and CENTRAL for relevant studies, which reported on all-cause mortality (primary outcome) and/or morbidity of critically ill patients with COVID-19 receiving vitamin C or not treatment. Pooled risk ratio (RR) and 95% confidence intervals (CI) were calculated using a random effects model. The meta-analysis was registered with PROSPERO.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>In the observational study, baseline characteristics were comparable between the two groups. Mortality was 20.0% (2/10) in the vitamin C group vs. 47.6% (49/103; <jats:italic>p</jats:italic> = 0.11) in the control group. Subsequently, the meta-analysis included 11 studies (6 observational; five randomized controlled trials) enrolling 1,807 critically ill patients with COVID-19. Mortality of patients receiving vitamin C on top of standard-of-care was not lower than patients receiving standard-of-care alone (25.8 vs. 34.7%; RR 0.85, 95% CI 0.57–1.26; <jats:italic>p</jats:italic> = 0.42).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>After combining results of our observational cohort with those of relevant studies into a meta-analysis of data from 1,807 patients, we found that administration vitamin C as opposed to standard-of-care alone might not be associated with lower of mortality among critically ill patients with COVID-19. Additional evidence is anticipated from relevant large randomized controlled trials which are currently underway.</jats:p></jats:sec><jats:sec><jats:title>Systematic Review Registration</jats:title><jats:p><jats:ext-link>https://www.crd.york.ac.uk/prospero/</jats:ext-link>, identifier: CRD42021276655.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2022.814587"
],
"author": [
{
"affiliation": [],
"family": "Gavrielatou",
"given": "Evdokia",
"sequence": "first"
},
{
"affiliation": [],
"family": "Xourgia",
"given": "Eleni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xixi",
"given": "Nikoleta A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mantelou",
"given": "Athina G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ischaki",
"given": "Eleni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanavou",
"given": "Aggeliki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zervakis",
"given": "Dimitris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Routsi",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kotanidou",
"given": "Anastasia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siempos",
"given": "Ilias I.",
"sequence": "additional"
}
],
"container-title": [
"Frontiers in Medicine"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
2,
11
]
],
"date-time": "2022-02-11T05:57:30Z",
"timestamp": 1644559050000
},
"deposited": {
"date-parts": [
[
2022,
2,
11
]
],
"date-time": "2022-02-11T05:57:35Z",
"timestamp": 1644559055000
},
"indexed": {
"date-parts": [
[
2022,
2,
11
]
],
"date-time": "2022-02-11T06:11:49Z",
"timestamp": 1644559909075
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2296-858X"
}
],
"issued": {
"date-parts": [
[
2022,
2,
11
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
11
]
],
"date-time": "2022-02-11T00:00:00Z",
"timestamp": 1644537600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.814587/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
2,
11
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
11
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/j.chest.2016.11.036",
"article-title": "Hydrocortisone, Vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study",
"author": "Marik",
"doi-asserted-by": "publisher",
"first-page": "1229",
"journal-title": "Chest.",
"key": "B1",
"volume": "151",
"year": "2017"
},
{
"DOI": "10.1001/jama.2019.11825",
"article-title": "Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the cITRIS-ALI randomized clinical trial",
"author": "Fowler",
"doi-asserted-by": "publisher",
"first-page": "1261",
"journal-title": "JAMA.",
"key": "B2",
"volume": "322",
"year": "2019"
},
{
"DOI": "10.1001/jama.2019.22176",
"article-title": "Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial",
"author": "Fujii",
"doi-asserted-by": "publisher",
"first-page": "423",
"journal-title": "JAMA.",
"key": "B3",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.24505",
"article-title": "Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial",
"author": "Sevransky",
"doi-asserted-by": "publisher",
"first-page": "742",
"journal-title": "JAMA.",
"key": "B4",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/j.chest.2020.02.049",
"article-title": "Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: the ORANGES trial",
"author": "Iglesias",
"doi-asserted-by": "publisher",
"first-page": "164",
"journal-title": "Chest.",
"key": "B5",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.02.065",
"article-title": "Combined treatment with hydrocortisone, vitamin c, and thiamine for sepsis and septic shock: a randomized controlled trial",
"author": "Chang",
"doi-asserted-by": "publisher",
"first-page": "174",
"journal-title": "Chest.",
"key": "B6",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1097/CCM.0000000000005263",
"article-title": "Effect of IV high-dose vitamin c on mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials",
"author": "Sato",
"doi-asserted-by": "publisher",
"first-page": "2121",
"journal-title": "Crit Care Med.",
"key": "B7",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0000000000003718",
"article-title": "Understanding vitamin c in critical illness: a focus on dose, route, and disease",
"author": "Hooper",
"doi-asserted-by": "publisher",
"first-page": "867",
"journal-title": "Crit Care Med.",
"key": "B8",
"volume": "47",
"year": "2019"
},
{
"DOI": "10.1201/9780429442025-7",
"article-title": "Vitamin C in pneumonia and sepsis",
"author": "Carr",
"doi-asserted-by": "crossref",
"key": "B9",
"volume-title": "Vitamin C: New Biochemical and Functional Insights [Internet]",
"year": "2020"
},
{
"DOI": "10.3390/nu13041172",
"article-title": "Vitamin C in the treatment of COVID-19",
"author": "Milani",
"doi-asserted-by": "publisher",
"first-page": "1172",
"journal-title": "Nutrients.",
"key": "B10",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/nu12113286",
"article-title": "The emerging role of vitamin c in the prevention and treatment of COVID-19",
"author": "Carr",
"doi-asserted-by": "publisher",
"first-page": "E3286",
"journal-title": "Nutrients.",
"key": "B11",
"volume": "12",
"year": "2020"
},
{
"key": "B12",
"unstructured": "COVID-19 Treatment Guidelines2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med.",
"key": "B13",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2020.614152",
"article-title": "Effect of Early vs. Delayed or no intubation on clinical outcomes of patients with COVID-19: an observational study",
"author": "Siempos",
"doi-asserted-by": "publisher",
"first-page": "614152",
"journal-title": "Front Med.",
"key": "B14",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2018.09.031",
"article-title": "Rapidly improving ARDS in therapeutic randomized controlled trials",
"author": "Schenck",
"doi-asserted-by": "publisher",
"first-page": "474",
"journal-title": "Chest.",
"key": "B15",
"volume": "155",
"year": "2019"
},
{
"DOI": "10.1136/bmj.b2700",
"article-title": "The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration",
"author": "Liberati",
"doi-asserted-by": "publisher",
"first-page": "b2700",
"journal-title": "BMJ.",
"key": "B16",
"volume": "339",
"year": "2009"
},
{
"DOI": "10.1186/s13054-021-03540-6",
"article-title": "Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies",
"author": "Papoutsi",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Crit Care.",
"key": "B17",
"volume": "25",
"year": "2021"
},
{
"key": "B18",
"unstructured": "Contributed by the CLARITY Group at McMaster University2021"
},
{
"DOI": "10.1136/bmj.l4898",
"article-title": "RoB 2: a revised tool for assessing risk of bias in randomised trials",
"author": "Sterne",
"doi-asserted-by": "publisher",
"first-page": "l4898",
"journal-title": "BMJ.",
"key": "B19",
"volume": "366",
"year": "2019"
},
{
"DOI": "10.1186/s12874-017-0442-1",
"article-title": "When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts",
"author": "Jakobsen",
"doi-asserted-by": "publisher",
"first-page": "162",
"journal-title": "BMC Med Res Methodol.",
"key": "B20",
"volume": "17",
"year": "2017"
},
{
"key": "B21",
"year": "2020"
},
{
"key": "B22",
"unstructured": "HigginsJ\n ThomasJ\n ChandlerJ\n CumpstonM\n LiM\n PageM\n Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021)2021"
},
{
"DOI": "10.1038/s41598-021-96703-y",
"article-title": "Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: a propensity score matched study",
"author": "Al Sulaiman",
"doi-asserted-by": "publisher",
"first-page": "17648",
"journal-title": "Sci Rep.",
"key": "B23",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.18632/aging.202557",
"article-title": "The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "7020",
"journal-title": "Aging.",
"key": "B24",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1186/s40001-021-00490-1",
"article-title": "Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial",
"author": "JamaliMoghadamSiahkali",
"doi-asserted-by": "publisher",
"first-page": "20",
"journal-title": "Eur J Med Res.",
"key": "B25",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/j.jclinane.2020.110005",
"article-title": "Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia",
"author": "Krishnan",
"doi-asserted-by": "publisher",
"first-page": "110005",
"journal-title": "J Clin Anesth.",
"key": "B26",
"volume": "67",
"year": "2020"
},
{
"DOI": "10.7759/cureus.11779",
"article-title": "The role of vitamin c as adjuvant therapy in COVID-19",
"author": "Kumari",
"doi-asserted-by": "publisher",
"first-page": "e11779",
"journal-title": "Cureus",
"key": "B27",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1177/08971900211015052",
"article-title": "Use of intravenous vitamin c in critically ill patients with covid-19 infection",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "8971900211015052",
"journal-title": "J Pharm Pract.",
"key": "B28",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1186/s13613-020-00792-3",
"article-title": "Pilot trial of high-dose vitamin C in critically ill COVID-19 patients",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "5",
"journal-title": "Ann Intensive Care.",
"key": "B29",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1515/med-2021-0361",
"article-title": "No significant benefit of moderate-dose vitamin C on severe COVID-19 cases",
"author": "Zheng",
"doi-asserted-by": "publisher",
"first-page": "1403",
"journal-title": "Open Med Wars Pol.",
"key": "B30",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.22037/jcma.v6i2.32182",
"article-title": "Efficacy of High dose vitamin C, melatonin and zinc in iranian patients with acute respiratory syndrome due to coronavirus infection: a pilot randomized trial",
"author": "Darban",
"doi-asserted-by": "publisher",
"first-page": "164",
"journal-title": "J Cell Mol Anesth.",
"key": "B31",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1186/s13063-021-05795-4",
"article-title": "The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial",
"author": "Beigmohammadi",
"doi-asserted-by": "publisher",
"first-page": "802",
"journal-title": "Trials.",
"key": "B32",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2021.102324",
"article-title": "Vitamin C and COVID-19 treatment: a systematic review and meta-analysis of randomized controlled trials",
"author": "Rawat",
"doi-asserted-by": "publisher",
"first-page": "102324",
"journal-title": "Diabetes Metab Syndr.",
"key": "B33",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.3390/life11111166",
"article-title": "Vitamin C intervention for critical COVID-19: a pragmatic review of the current level of evidence",
"author": "Holford",
"doi-asserted-by": "publisher",
"first-page": "1166",
"journal-title": "Life.",
"key": "B34",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1007/s00134-021-06389-z",
"article-title": "Natural history, trajectory, and management of mechanically ventilated COVID-19 patients in the United Kingdom",
"author": "Patel",
"doi-asserted-by": "publisher",
"first-page": "549",
"journal-title": "Intensive Care Med.",
"key": "B35",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0000000000004396",
"article-title": "Harm of IV high-dose vitamin C therapy in adult patients: a scoping review",
"author": "Yanase",
"doi-asserted-by": "publisher",
"first-page": "e620",
"journal-title": "Crit Care Med.",
"key": "B36",
"volume": "48",
"year": "2020"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"score": 1,
"short-container-title": [
"Front. Med."
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Effect of Vitamin C on Clinical Outcomes of Critically Ill Patients With COVID-19: An Observational Study and Subsequent Meta-Analysis"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "9"
}
