Use of Intravenous Vitamin C in Critically Ill Patients With COVID-19 Infection
et al., Journal of Pharmacy Practice, doi:10.1177/08971900211015052, Jun 2021
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
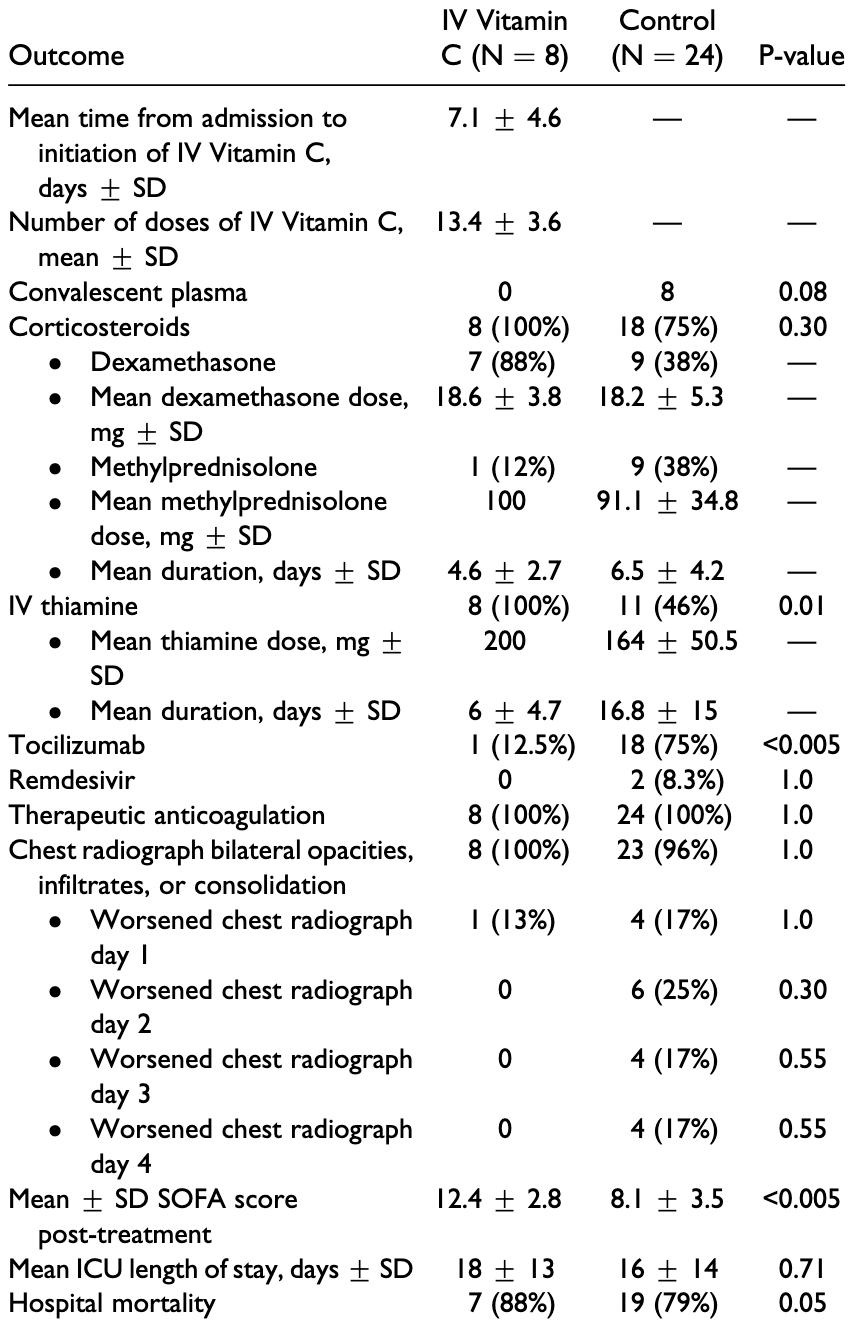
PSM retrospective 8 ICU patients treated with vitamin C and 24 matched controls, showing no significant difference. Authors note that "it is possible for the delayed timing of IV vitamin C to have blunted the beneficial effects as these patients may have already progressed to the late fibroproliferative phase or ARDS". IV vitamin C 1.5 grams every 6 hours.
This is the 28th of 74 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000068.
21 studies are RCTs, which show efficacy with p=0.0012.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients; very late stage, ICU patients.
|
risk of death, 10.5% higher, RR 1.11, p = 1.00, treatment 7 of 8 (87.5%), control 19 of 24 (79.2%), PSM.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Li et al., 8 Jun 2021, retrospective, propensity score matching, USA, peer-reviewed, 6 authors.
Use of Intravenous Vitamin C in Critically Ill Patients With COVID-19 Infection
Journal of Pharmacy Practice, doi:10.1177/08971900211015052
Introduction: The pathophysiology for Coronavirus Disease 2019 (COVID-19) infection is characterized by cytokine oxidative stress and endothelial dysfunction. Intravenous (IV) vitamin C has been utilized as adjuvant therapy in critically ill patients with sepsis for its protective effects against reactive oxygen species and immunomodulatory effects. The primary objective of this study was to evaluate the effects of IV vitamin C in critically ill patients with COVID-19 infection. Methods: Retrospective observational cohort study with propensity score matching of intensive care unit (ICU) patients who received 1.5 grams IV vitamin C every 6 hours for up to 4 days for COVID-19 infection. The primary study outcome was in-hospital mortality. Secondary outcomes included vasopressor requirements in norepinephrine equivalents, ICU length of stay, and change in Sequential Organ Failure Assessment (SOFA) score. Results: Eight patients received IV vitamin C and were matched to 24 patients. Patients in the IV vitamin C group had higher rates of hospital mortality [7 (88%) vs. 19 (79%), P ¼ 0.049]. There was no difference in the daily vasopressor requirement in the treatment group or between the 2 groups. The mean SOFA scores post-treatment was higher in the IV vitamin C group (12.4 + 2.8 vs. 8.1 + 3.5, P < 0.005). There was no difference in ICU length of stay between the treatment and control groups. Conclusion: Adjunctive IV vitamin C for the management of COVID-19 infection in critically ill patients may not decrease the incidence of mortality, vasopressor requirements, SOFA scores, or ventilator settings.
ORCID iD Matthew Li, PharmD, BCPS, BCCCP https://orcid.org/0000-0003-1320-7134
References
Annane, Adrenal insufficiency in sepsis, Curr Pharm Des, doi:10.2174/138161208784980626
Ascor, None
Bharara, Grossman, Grinnan, Intravenous vitamin C administered as adjunctive therapy for recurrent acute respiratory distress syndrome, Case Rep Crit Care, doi:10.1155/2016/8560871
Boretti, Banik, Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome, Pharma-Nutrition, doi:10.1016/j.phanu.2020.100190
Carr, Maggini, Vitamin C and immune function, Nutrients, doi:10.3390/nu9111211
Carr, Wohlrab, Young, Stability of intravenous vitamin C solutions: a technical report, Crit Care Resusc
Castelli, Cimini, Ferri, Cytokine storm in COVID-19: "When you come out of the storm, you won't be the same person who walked in, Front Immunol, doi:10.3389/fimmu.2020.02132
Corrao, Use of Ascorbic Acid in Patients with COVID 19
Cummings, Baldwin, Abrams, Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study, Lancet, doi:10.1016/S0140-6736(20)31189-2
Delgado-Roche, Mesta, Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection, Arch Med Res, doi:10.1016/j.arcmed.2020.04.019
Fowler, Iii, Truwit, Hite, Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial, JAMA, doi:10.1001/jama.2019.11825
Fujii, Luethi, Young, Effect of vitamin C, hydrocortisone, and thiamine versus hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the vitamins randomized clinical trial, JAMA, doi:10.1001/jama.2019.22176
Gavriilaki, Anyfanti, Gavriilaki, Endothelial dysfunction in COVID-19: lessons learned from coronaviruses, Curr Hypertens Rep, doi:10.1007/s11906-020-01078-6
Gupta, Wang, Hayek, Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19, JAMA Intern Med, doi:10.1001/jamainternmed.2020.6252
Hermine, Tharaux, Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial, JAMA Intern Med, doi:10.1001/jamain-ternmed.2020.6820
Hiedra, Lo, Elbashabsheh, The use of IV vitamin C for patients with COVID-19: a case series, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2020.1794819
Hoang, Shaw, Fang, Possible application of highdose vitamin C in the prevention and therapy of coronavirus infection, J Glob Antimicrob Resist, doi:10.1016/j.jgar.2020.09.025
Horby, Pessoa-Amorim, Peto, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomized, controlled, open-label, platform trial, medRxiv, doi:10.1101/2021.02.11.21249258
Kouakanou, Xu, Peters, Vitamin C promotes the proliferation and effector functions of human gd T cells, Cell Mol Immunol, doi:10.1038/s41423-019-0247-8
Lamontagne, Lessening Organ Dysfunction with Vitamin C-COVID-19 (LOVIT-COVID)
Liu, Zhu, Zhang, Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicenter randomized controlled trial, BMJ Open, doi:10.1136/bmjopen-2020-039519
Marik, Khangoora, Rivera, Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock, Chest, doi:10.1016/j.chest.2016.11.036
Moskowitz, Huang, Hou, Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial, JAMA, doi:10.1001/jama.2020.11946
Ragab, Salah Eldin, Taeimah, The COVID-19 cytokine storm; what we know so far, Front Immunol, doi:10.3389/fimmu.2020.01446
Russell, Walley, Singer, Vasopressin versus norepinephrine infusion in patients with septic shock, N Engl J Med, doi:10.1056/NEJMoa067373
Stone, Frigault, Boyd, Efficacy of tocilizumab in patients hospitalized with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2028836
Tsai, Diawara, Nahass, Impact of tocilizumab administration on mortality in severe COVID-19, Sci Re, doi:10.1038/s41598-020-76187-y
Wajanaponsan, Reade, Milbrandt, Steroids in late ARDS?, Crit Care, doi:10.1186/cc5954
Wilson, Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium, Biofactors, doi:10.1002/biof.7
Woolum, Abner, Kelly, Effect of thiamine administration on lactate clearance and mortality in patients with septic shock, Crit Care Med
Ye, Wang, Mao, The pathogenesis and treatment of the "Cytokine Storm" in COVID-19, J Infect, doi:10.1016/j.jinf.2020.03.037
DOI record:
{
"DOI": "10.1177/08971900211015052",
"ISSN": [
"0897-1900",
"1531-1937"
],
"URL": "http://dx.doi.org/10.1177/08971900211015052",
"abstract": "<jats:sec><jats:title>Introduction</jats:title><jats:p> The pathophysiology for Coronavirus Disease 2019 (COVID-19) infection is characterized by cytokine oxidative stress and endothelial dysfunction. Intravenous (IV) vitamin C has been utilized as adjuvant therapy in critically ill patients with sepsis for its protective effects against reactive oxygen species and immunomodulatory effects. The primary objective of this study was to evaluate the effects of IV vitamin C in critically ill patients with COVID-19 infection. </jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p> Retrospective observational cohort study with propensity score matching of intensive care unit (ICU) patients who received 1.5 grams IV vitamin C every 6 hours for up to 4 days for COVID-19 infection. The primary study outcome was in-hospital mortality. Secondary outcomes included vasopressor requirements in norepinephrine equivalents, ICU length of stay, and change in Sequential Organ Failure Assessment (SOFA) score. </jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p> Eight patients received IV vitamin C and were matched to 24 patients. Patients in the IV vitamin C group had higher rates of hospital mortality [7 (88%) vs. 19 (79%), P = 0.049]. There was no difference in the daily vasopressor requirement in the treatment group or between the 2 groups. The mean SOFA scores post-treatment was higher in the IV vitamin C group (12.4 ± 2.8 vs. 8.1 ± 3.5, P < 0.005). There was no difference in ICU length of stay between the treatment and control groups. </jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p> Adjunctive IV vitamin C for the management of COVID-19 infection in critically ill patients may not decrease the incidence of mortality, vasopressor requirements, SOFA scores, or ventilator settings. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/08971900211015052"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1320-7134",
"affiliation": [
{
"name": "New York City Health + Hospitals/Queens, Jamaica, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Li",
"given": "Matthew",
"sequence": "first"
},
{
"affiliation": [
{
"name": "New York City Health + Hospitals/Queens, Jamaica, NY, USA"
},
{
"name": "Icahn School of Medicine at Mount Sinai, NY, USA"
}
],
"family": "Ching",
"given": "Tsung Han",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Independent Researcher"
}
],
"family": "Hipple",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "New York City Health + Hospitals/Queens, Jamaica, NY, USA"
},
{
"name": "Icahn School of Medicine at Mount Sinai, NY, USA"
}
],
"family": "Lopez",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "New York City Health + Hospitals/Queens, Jamaica, NY, USA"
},
{
"name": "Icahn School of Medicine at Mount Sinai, NY, USA"
}
],
"family": "Sahibzada",
"given": "Asad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "New York City Health + Hospitals/Queens, Jamaica, NY, USA"
},
{
"name": "Icahn School of Medicine at Mount Sinai, NY, USA"
}
],
"family": "Rahman",
"given": "Habibur",
"sequence": "additional"
}
],
"container-title": "Journal of Pharmacy Practice",
"container-title-short": "Journal of Pharmacy Practice",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
6,
8
]
],
"date-time": "2021-06-08T09:02:03Z",
"timestamp": 1623142923000
},
"deposited": {
"date-parts": [
[
2023,
2,
2
]
],
"date-time": "2023-02-02T02:50:22Z",
"timestamp": 1675306222000
},
"indexed": {
"date-parts": [
[
2023,
9,
19
]
],
"date-time": "2023-09-19T18:14:51Z",
"timestamp": 1695147291985
},
"is-referenced-by-count": 19,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
6,
8
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://journals.sagepub.com/page/policies/text-and-data-mining-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
8
]
],
"date-time": "2021-06-08T00:00:00Z",
"timestamp": 1623110400000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/08971900211015052",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/08971900211015052",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/08971900211015052",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "60-66",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2021,
6,
8
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
8
]
]
},
"published-print": {
"date-parts": [
[
2023,
2
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1016/j.arcmed.2020.04.019",
"doi-asserted-by": "publisher",
"key": "bibr1-08971900211015052"
},
{
"DOI": "10.1007/s11906-020-01078-6",
"doi-asserted-by": "publisher",
"key": "bibr2-08971900211015052"
},
{
"DOI": "10.3389/fimmu.2020.01446",
"doi-asserted-by": "publisher",
"key": "bibr3-08971900211015052"
},
{
"DOI": "10.1097/CCM.0000000000003311",
"doi-asserted-by": "publisher",
"key": "bibr4-08971900211015052"
},
{
"DOI": "10.2174/138161208784980626",
"doi-asserted-by": "publisher",
"key": "bibr5-08971900211015052"
},
{
"DOI": "10.3390/nu9111211",
"doi-asserted-by": "publisher",
"key": "bibr6-08971900211015052"
},
{
"DOI": "10.1016/j.chest.2016.11.036",
"doi-asserted-by": "publisher",
"key": "bibr7-08971900211015052"
},
{
"DOI": "10.1001/jama.2019.22176",
"doi-asserted-by": "publisher",
"key": "bibr8-08971900211015052"
},
{
"DOI": "10.1001/jama.2019.11825",
"doi-asserted-by": "publisher",
"key": "bibr9-08971900211015052"
},
{
"DOI": "10.1001/jama.2020.11946",
"doi-asserted-by": "publisher",
"key": "bibr10-08971900211015052"
},
{
"DOI": "10.1016/S0140-6736(20)31189-2",
"doi-asserted-by": "publisher",
"key": "bibr11-08971900211015052"
},
{
"DOI": "10.1080/14787210.2020.1794819",
"doi-asserted-by": "publisher",
"key": "bibr12-08971900211015052"
},
{
"DOI": "10.1136/bmjopen-2020-039519",
"doi-asserted-by": "publisher",
"key": "bibr13-08971900211015052"
},
{
"DOI": "10.1056/NEJMoa067373",
"doi-asserted-by": "publisher",
"key": "bibr14-08971900211015052"
},
{
"author": "Bharara A",
"journal-title": "Case Rep Crit Care",
"key": "bibr15-08971900211015052",
"volume": "2016",
"year": "2016"
},
{
"DOI": "10.1016/j.jgar.2020.09.025",
"doi-asserted-by": "publisher",
"key": "bibr16-08971900211015052"
},
{
"key": "bibr17-08971900211015052",
"unstructured": "Corrao S.Use of Ascorbic Acid in Patients with COVID 19. ClinicalTrials.gov. National Library of Medicine (US). Published 2020. Accessed April 29, 2021. https://clinicaltrials.gov/ct2/show/NCT04323514"
},
{
"key": "bibr18-08971900211015052",
"unstructured": "Lamontagne F. Lessening Organ Dysfunction with Vitamin C—COVID-19 (LOVIT-COVID). ClinicalTrials.gov. National Library of Medicine (US). Published 2020. Accessed April 29, 2021. https://clinicaltrials.gov/ct2/show/NCT04401150"
},
{
"DOI": "10.3389/fimmu.2020.02132",
"doi-asserted-by": "publisher",
"key": "bibr19-08971900211015052"
},
{
"DOI": "10.1016/j.jinf.2020.03.037",
"doi-asserted-by": "publisher",
"key": "bibr20-08971900211015052"
},
{
"DOI": "10.1186/cc5954",
"doi-asserted-by": "publisher",
"key": "bibr21-08971900211015052"
},
{
"DOI": "10.1016/j.phanu.2020.100190",
"doi-asserted-by": "publisher",
"key": "bibr22-08971900211015052"
},
{
"DOI": "10.1002/biof.7",
"doi-asserted-by": "publisher",
"key": "bibr23-08971900211015052"
},
{
"DOI": "10.1038/s41423-019-0247-8",
"doi-asserted-by": "publisher",
"key": "bibr24-08971900211015052"
},
{
"DOI": "10.1001/jamainternmed.2020.6252",
"doi-asserted-by": "publisher",
"key": "bibr25-08971900211015052"
},
{
"DOI": "10.1001/jamainternmed.2020.6820",
"doi-asserted-by": "publisher",
"key": "bibr26-08971900211015052"
},
{
"author": "Tsai A",
"first-page": "19131",
"issue": "1",
"journal-title": "Sci Re",
"key": "bibr27-08971900211015052",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2028836",
"doi-asserted-by": "publisher",
"key": "bibr28-08971900211015052"
},
{
"author": "Horby PW",
"first-page": "605",
"issue": "10274",
"journal-title": "medRxiv",
"key": "bibr29-08971900211015052",
"volume": "397",
"year": "2021"
},
{
"key": "bibr30-08971900211015052",
"unstructured": "ASCOR [package insert]. Santa Ana, CA: McGuff Pharmaceuticals, Inc; 2017."
},
{
"author": "Carr A",
"first-page": "180",
"issue": "3",
"journal-title": "Crit Care Resusc",
"key": "bibr31-08971900211015052",
"volume": "20",
"year": "2018"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/08971900211015052"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)"
],
"subtitle": [],
"title": "Use of Intravenous Vitamin C in Critically Ill Patients With COVID-19 Infection",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "36"
}
