A quadruple blind, randomised controlled trial of gargling agents in reducing intraoral viral load among hospitalised COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial
et al., Trials, doi:10.1186/s13063-020-04634-2, GARGLES, NCT04341688, Jul 2022
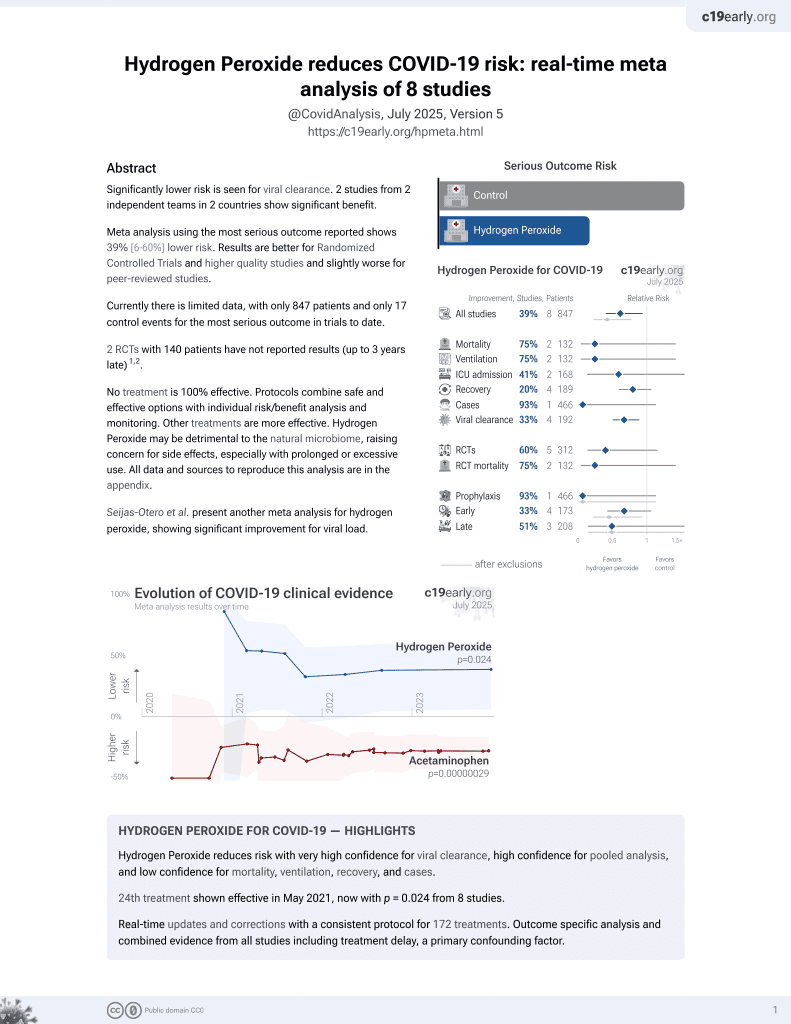
24th treatment shown to reduce risk in
May 2021, now with p = 0.024 from 8 studies.
Lower risk for viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Estimated 50 patient hydrogen peroxide early treatment RCT with results not reported over 3 years after estimated completion.
Study covers povidone-iodine and hydrogen peroxide.
Khan et al., 31 Jul 2022, Double Blind Randomized Controlled Trial, Pakistan, trial NCT04341688 (history) (GARGLES).
Contact: amjadkhan@lumhs.edu.pk.
Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19—Results From a Pilot Open-Label, Randomized Controlled Trial
Frontiers in Pharmacology, doi:10.3389/fphar.2022.898062
Background: Curcumin, quercetin, and vitamin D3 (cholecalciferol) are common natural ingredients of human nutrition and reportedly exhibit promising anti-inflammatory, immunomodulatory, broad-spectrum antiviral, and antioxidant activities. Objective: The present study aimed to investigate the possible therapeutic benefits of a single oral formulation containing supplements curcumin, quercetin, and cholecalciferol (combinedly referred to here as CQC) as an adjuvant therapy for early-stage of symptomatic coronavirus disease 2019 in a pilot open-label, randomized controlled trial conducted at
ETHICS STATEMENT The study was approved by the Institutional Review Board (IRB) of King Edward Medical University, Lahore, with approval No. 785/RC/KEMU. The patients/participants provided their written informed consent to participate in this study.
AUTHOR CONTRIBUTIONS AK contributed to the study design, data interpretation, writing of the manuscript, and literature search; SI, SM, and SA contributed to the study design, data collection, data interpretation, and literature search; DP-F and MH critically reviewed and edited the manuscript; and SL helped in data analysis and revision of the manuscript. All authors approved the final manuscript.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abian, Ortega-Alarcon, Jimenez-Alesanco, Ceballos-Laita, Vega et al., Structural Stability of SARS-CoV-2 3CLpro and Identification of Quercetin as an Inhibitor by Experimental Screening, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2020.07.235
Aggarwal, Sung, Pharmacological Basis for the Role of Curcumin in Chronic Diseases: An Age-Old Spice with Modern Targets, Trends Pharmacol. Sci, doi:10.1016/j.tips.2008.11.002
Ahmadi, Salari, Sharifi, Reihani, Rostamiani et al., Oral Nano-Curcumin Formulation Efficacy in the Management of Mild to Moderate Outpatient Covid-19: A Randomized Triple-Blind Placebo-Controlled Clinical Trial, Food Sci. Nutr, doi:10.1002/fsn3.2226
Ali, Elevated Level of C-Reactive Protein May Be an Early Marker to Predict Risk for Severity of Covid-19, J. Med. Virol, doi:10.1002/jmv.26097
Andersen, Weber, Wood, Hughes, Murray et al., In Vitro Virucidal Activity of Selected Anthraquinones and Anthraquinone Derivatives, Antivir. Res, doi:10.1016/0166-3542(91)90024-l
Andres, Pevny, Ziegenhagen, Bakhiya, Schäfer et al., Safety Aspects of the Use of Quercetin as a Dietary Supplement, Mol. Nutr. Food Res, doi:10.1002/mnfr.201700447
Asadirad, Nashibi, Khodadadi, Ghadiri, Sadeghi et al., Antiinflammatory Potential of Nano-Curcumin as an Alternative Therapeutic Agent for the Treatment of Mild-To-Moderate Hospitalized Covid-19 Patients in a Placebo-Controlled Clinical Trial, Phytother. Res, doi:10.1002/ptr.7375
Babaei, Nassiri-Asl, Hosseinzadeh, Curcumin (A Constituent of Turmeric): New Treatment Option against Covid-19, Food Sci. Nutr, doi:10.1002/fsn3.1858
Bahun, Jukić, Oblak, Kranjc, Bajc et al., Inhibition of the SARS-CoV-2 3CLpro Main Protease by Plant Polyphenols, Food. Chem, doi:10.1016/j.foodchem.2021.131594
Bandyopadhyay, Farmer to Pharmacist: Curcumin as an Anti-invasive and Antimetastatic Agent for the Treatment of Cancer, Front. Chem, doi:10.3389/fchem.2014.00113
Basnet, Skalko-Basnet, Curcumin: An Anti-inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment, Molecules, doi:10.3390/molecules16064567
Biancatelli, Berrill, Catravas, Marik, Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of Sars-Cov-2 Related Disease (Covid-19), Front. Immunol, doi:10.3389/fimmu.2020.01451
Bormann, Alt, Schipper, Van De Sand, Le-Trilling et al., Turmeric Root and its Bioactive Ingredient Curcumin Effectively Neutralize Sars-Cov-2 In Vitro, Viruses, doi:10.3390/v13101914
Boroumand, Samarghandian, Hashemy, Immunomodulatory, Anti-inflammatory, and Antioxidant Effects of Curcumin, J. Herbmed. Pharmacol, doi:10.15171/jhp.2018.33
Bouillon, Marcocci, Carmeliet, Bikle, White et al., Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions, Endocr. Rev, doi:10.1210/er.2018-00126
Bowman, Applied Smoothing Techniques for Data Analysis : The Kernel Approach with S-Plus Illustrations
Bowman, Young, Graphical Comparison of Nonparametric Curves, Appl. Stat, doi:10.2307/2986225
Brockman-Schneider, Pickles, Gern, Effects of Vitamin D on Airway Epithelial Cell Morphology and Rhinovirus Replication, PLoS One, doi:10.1371/journal.pone.0086755
Chabot, Huntwork, Turmeric as a Possible Treatment for Covid-19-Induced Anosmia and Ageusia, Cureus, doi:10.7759/cureus.17829
Charoenngam, Shirvani, Holick, Vitamin D and its Potential Benefit for the Covid-19 Pandemic, Endocr. Pract, doi:10.1016/j.eprac.2021.03.006
Chen, Jiang, Wu, Fang, Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes, Mediat. Inflamm, doi:10.1155/2016/9340637
Chen, Li, Luo, Liu, Xu et al., Binding Interaction of Quercetin-3-Beta-Galactoside and its Synthetic Derivatives with Sars-Cov 3cl(Pro): Structure-Activity Relationship Studies Reveal Salient Pharmacophore Features, Bioorg. Med. Chem, doi:10.1016/j.bmc.2006.09.014
Chen, Zhang, Ge, Du, Deb et al., Vitamin D Receptor Inhibits Nuclear Factor κB Activation by Interacting with IκB Kinase β Protein, J. Biol. Chem, doi:10.1074/jbc.M113.467670
Cheng, Hsu, Lin, Hsu, Ho et al., Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-malignant Lesions, Anticancer Res
Chiang, Chiang, Liu, Lin, In Vitro Antiviral Activities of Caesalpinia Pulcherrima and its Related Flavonoids, J. Antimicrob. Chemother, doi:10.1093/jac/dkg291
Cleveland, Robust Locally Weighted Regression and Smoothing Scatterplots, J. Am. Stat. Assoc
Crp, mg/dl) a 34
Di Pierro, Derosa, Maffioli, Bertuccioli, Togni et al., Possible Therapeutic Effects of Adjuvant Quercetin Supplementation against Early-Stage Covid-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study, Int. J. Gen. Med, doi:10.2147/IJGM.S318720
Di Pierro, Iqtadar, Khan, Ullah Mumtaz, Masud Chaudhry et al., Potential Clinical Benefits of Quercetin in the Early Stage of Covid-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial, Int. J. Gen. Med, doi:10.2147/IJGM.S318949
Ferritin, median (IQR, Hematology Hemoglobin
Giordano, Tommonaro, Curcumin and Cancer, Nutrients, doi:10.3390/nu11102376
Glinsky, Tripartite Combination of Candidate Pandemic Mitigation Agents: Vitamin D, Quercetin, and Estradiol Manifest Properties of Medicinal Agents for Targeted Mitigation of the Covid-19 Pandemic Defined by Genomics-Guided Tracing of Sars-Cov-2 Targets in Human Cells, Biomedicines, doi:10.3390/biomedicines8050129
Gombart, Borregaard, Koeffler, Human Cathelicidin Antimicrobial Peptide (Camp) Gene Is a Direct Target of the Vitamin D Receptor and Is Strongly Up-Regulated in Myeloid Cells by 1,25-Dihydroxyvitamin D3, FASEB J, doi:10.1096/fj.04-3284com
Gonzalez, Fontanes, Raychaudhuri, Loo, Loo et al., The Heat Shock Protein Inhibitor Quercetin Attenuates Hepatitis C Virus Production, Hepatology, doi:10.1002/hep.23232
Grant, Lahore, Mcdonnell, Baggerly, French et al., Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and Covid-19 Infections and Deaths, Nutrients, doi:10.3390/nu12040988
Greiller, Suri, Jolliffe, Kebadze, Hirsman et al., Vitamin D Attenuates Rhinovirus-Induced Expression of Intercellular Adhesion Molecule-1 (Icam-1) and Platelet-Activating Factor Receptor (Pafr) in Respiratory Epithelial Cells, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2018.11.013
Gupta, Patchva, Aggarwal, Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials, AAPS J, doi:10.1208/s12248-012-9432-8
Harwood, Danielewska-Nikiel, Borzelleca, Flamm, Williams et al., A Critical Review of the Data Related to the Safety of Quercetin and Lack of Evidence of In Vivo Toxicity, Including Lack of Genotoxic/Carcinogenic Properties, Food Chem. Toxicol, doi:10.1016/j.fct.2007.05.015
Hassaniazad, Eftekhar, Inchehsablagh, Kamali, Tousi et al., A Triple-Blind, Placebo-Controlled, Randomized Clinical Trial to Evaluate the Effect of Curcumin-Containing Nanomicelles on Cellular Immune Responses Subtypes and Clinical Outcome in Covid-19 Patients, Phytother. Res, doi:10.1002/ptr.7294
Ilie, Stefanescu, Smith, The Role of Vitamin D in the Prevention of Coronavirus Disease 2019 Infection and Mortality, Aging Clin. Exp. Res, doi:10.1007/s40520-020-01570-8
Jena, Kanungo, Nayak, Chainy, Dandapat, Catechin and Curcumin Interact with S Protein of Sars-Cov2 and Ace2 of Human Cell Membrane: Insights from Computational Studies, Sci. Rep, doi:10.1038/s41598-021-81462-7
Jennings, Parks, Curcumin as an Antiviral Agent, Viruses, doi:10.3390/v12111242
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib Plus Remdesivir for Hospitalized Adults with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2031994
Kamel, Abdelseed, Albalawi, Aslsalameen, Almutairi et al., Evaluation of the Effect of Zinc, Quercetin, Bromelain and Vitamin C on Covid-19 Patients, doi:10.1101/2020.12.22.20245993
Karimi, Ghodsi, Kooshki, Karimi, Asghariazar et al., Therapeutic Effects of Curcumin on Sepsis and Mechanisms of Action: A Systematic Review of Preclinical Studies, Phytother. Res, doi:10.1002/ptr.6467
Kim, Jeon, Ko, Flavanone Glycosides from Citrus Junos and Their Anti-influenza Virus Activity, Planta Med, doi:10.1055/s-2001-16484
Kunnumakkara, Bordoloi, Padmavathi, Monisha, Roy et al., Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases, Br. J. Pharmacol, doi:10.1111/bph.13621
Lakhanpal, Rai, Quercetin: A Versatile Flavonoid, Internet J. Med. Update, doi:10.4314/ijmu.v2i2.39851
Ldh, None, U/l) c
Lee, Yu, Trimpert, Benthani, Mairhofer et al., Virus-Induced Senescence Is a Driver and Therapeutic Target in Covid-19, Nature, doi:10.1038/s41586-021-03995-1
Leyva-López, Gutierrez-Grijalva, Ambriz-Perez, Heredia, Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases, Int. J. Mol. Sci, doi:10.3390/ijms17060921
Lopes, Da Costa, Genova Ribeiro, Da Silva, Lima et al., Quercetin Pentaacetate Inhibits In Vitro Human Respiratory Syncytial Virus Adhesion, Virus Res, doi:10.1016/j.virusres.2019.197805
Majeed, Nagabhushanam, Shah, Mundkur, A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of a Nutritional Supplement (ImmuActiveTM) for COVID-19
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data, BMJ, doi:10.1136/bmj.i6583
Marín-Palma, Tabares-Guevara, Zapata-Cardona, Flórez-Álvarez, Yepes et al., Curcumin Inhibits In Vitro Sars-Cov-2 Infection in Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900
Mazumder, Raghavan, Weinstein, Kohn, Pommier, Inhibition of Human Immunodeficiency Virus Type-1 Integrase by Curcumin, Biochem. Pharmacol, doi:10.1016/0006-2952(95)98514-a
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics with Covid-19 Test Results, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.19722
Meng, Xiong, He, Lin, Hao et al., Ct Imaging and Clinical Course of Asymptomatic Cases with Covid-19 Pneumonia at Admission in Wuhan, China, J. Infect, doi:10.1016/j.jinf.2020.04.004
Mercola, Grant, Wagner, Evidence Regarding Vitamin D and Risk of Covid-19 and its Severity, Nutrients, doi:10.3390/nu12113361
Merzon, Tworowski, Gorohovski, Vinker, Golan Cohen et al., Low Plasma 25(Oh) Vitamin D Level Is Associated with Increased Risk of Covid-19 Infection: An Israeli Population-Based Study, FEBS J, doi:10.1111/febs.15495
Mohammadi-Sartang, Mazloom, Sherafatmanesh, Ghorbani, Firoozi, Effects of Supplementation with Quercetin on Plasma C-Reactive Protein Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Eur. J. Clin. Nutr, doi:10.1038/ejcn.2017.55
Nabavi, Daglia, Moghaddam, Habtemariam, Nabavi, Curcumin and Liver Disease: From Chemistry to Medicine, Compr. Rev. Food Sci. Food Saf, doi:10.1111/1541-4337.12047
Nair, Kandaswami, Mahajan, Chadha, Chawda et al., The Flavonoid, Quercetin, Differentially Regulates Th-1 (Ifngamma) and Th-2 (Il4) Cytokine Gene Expression by Normal Peripheral Blood Mononuclear Cells, Biochim. Biophys. Acta, doi:10.1016/s0167-4889(02)00328-2
Ono, Nakane, Mechanisms of Inhibition of Various Cellular DNA and Rna Polymerases by Several Flavonoids, J. Biochem, doi:10.1093/oxfordjournals.jbchem.a123251
Padmanaban, Rangarajan, Curcumin as an Adjunct Drug for Infectious Diseases, Trends Pharmacol. Sci, doi:10.1016/j.tips.2015.09.007
Patel, Acharya, Ray, Agrawal, Raghuwanshi et al., Cellular and Molecular Mechanisms of Curcumin in Prevention and Treatment of Disease, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2018.1552244
Pawar, Mastud, Pawar, Pawar, Bhoite et al., Oral Curcumin with Piperine as Adjuvant Therapy for the Treatment of Covid-19: A Randomized Clinical Trial, Front. Pharmacol, doi:10.3389/fphar.2021.669362
Perrone, Ardito, Giannatempo, Dioguardi, Troiano et al., Biological and Therapeutic Activities, and Anticancer Properties of Curcumin, Exp. Ther. Med, doi:10.3892/etm.2015.2749
Praditya, Kirchhoff, Brüning, Rachmawati, Steinmann et al., Anti-Infective Properties of the Golden Spice Curcumin, Front. Microbiol, doi:10.3389/fmicb.2019.00912
Prietl, Treiber, Pieber, Amrein, Vitamin D and Immune Function, Nutrients, doi:10.3390/nu5072502
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short Term, High-Dose Vitamin D Supplementation for Covid-19 Disease: A Randomised, Placebo-Controlled, Study (Shade Study), Postgrad. Med. J, doi:10.1136/postgradmedj-2020-139065
Richart, Li, Mizushina, Chang, Chung et al., Synergic Effect of Curcumin and its Structural Analogue (Monoacetylcurcumin) on Anti-influenza Virus Infection, J. Food Drug Anal, doi:10.1016/j.jfda.2017.12.006
Rizzuti, Grande, Conforti, Jimenez-Alesanco, Ceballos-Laita et al., Rutin Is a Low Micromolar Inhibitor of Sars-Cov-2 Main Protease 3clpro: Implications for Drug Design of Quercetin Analogs, Biomedicines, doi:10.3390/biomedicines9040375
Robaszkiewicz, Balcerczyk, Bartosz, Antioxidative and Prooxidative Effects of Quercetin on A549 Cells, Cell Biol. Int, doi:10.1016/j.cellbi.2007.04.009
Saber-Moghaddam, Salari, Hejazi, Amini, Taherzadeh et al., Oral Nano-curcumin Formulation Efficacy in Management of Mild to Moderate Hospitalized Coronavirus Disease -19 Patients: An Open Label Nonrandomized Clinical Trial, Phytotherapy Res, doi:10.1002/ptr.7004
Sabico, Enani, Sheshah, Aljohani, Aldisi et al., Effects of a 2-Week 5000 Iu versus 1000 Iu Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial, Nutrients, doi:10.3390/nu13072170
Sadeghi-Haddad-Zavareh, Bayani, Shokri, Ebrahimpour, Babazadeh et al., C-reactive Protein as a Prognostic Indicator in Covid-19 Patients, Interdiscip. Perspect. Infect. Dis, doi:10.1155/2021/5557582
Salehi, Machin, Monzote, Sharifi-Rad, Ezzat et al., Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health, ACS Omega, doi:10.1021/acsomega.0c01818
Semple, Pyke, Reynolds, Flower, In Vitro Antiviral Activity of the Anthraquinone Chrysophanic Acid against Poliovirus, Antivir. Res, doi:10.1016/s0166-3542(01)00125-5
Sharma, Prateekshasingh, Singh, Singh, Rao et al., Nanocurcumin Potently Inhibits SARS-CoV-2 Spike Protein-Induced Cytokine Storm by Deactivation of MAPK/NF-κB Signaling in Epithelial Cells, ACS Appl. Bio. Mat, doi:10.1021/acsabm.1c00874
Shohan, Nashibi, Mahmoudian-Sani, Abolnezhadian, Ghafourian et al., The Therapeutic Efficacy of Quercetin in Combination with Antiviral Drugs in Hospitalized Covid-19 Patients: A Randomized Controlled Trial, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2021.174615
Sánchez-Zuno, González-Estevez, Matuz-Flores, Macedo-Ojeda, Hernández-Bello et al., Vitamin D Levels in Covid-19 Outpatients from Western Mexico: Clinical Correlation and Effect of its Supplementation, J. Clin. Med, doi:10.3390/jcm10112378
Tahmasebi, El-Esawi, Mahmoud, Timoshin, Valizadeh et al., Immunomodulatory Effects of Nanocurcumin on Th17 Cell Responses in Mild and Severe Covid-19 Patients, J. Cell. Physiol, doi:10.1002/jcp.30233
Tahmasebi, Saeed, Temirgalieva, Yumashev, El-Esawi et al., Nanocurcumin Improves Treg Cell Responses in Patients with Mild and Severe Sars-Cov2, Life Sci, doi:10.1016/j.lfs.2021.119437
Tamer, Mesçi, Role of Vitamin D in the Immune System, Tjem, doi:10.4274/Tjem.1938
Tőzsér, Benkő, Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1βProduction, Mediat. Inflamm, doi:10.1155/2016/5460302
Uchide, Toyoda, Antioxidant Therapy as a Potential Approach to Severe Influenza-Associated Complications, Molecules, doi:10.3390/molecules16032032
Wang, C-reactive Protein Levels in the Early Stage of Covid-19, Med. Mal. Infect, doi:10.1016/j.medmal.2020.03.007
Wen, Kuo, Jan, Liang, Wang et al., Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus, J. Med. Chem, doi:10.1021/jm070295s
Wu, Li, Li, He, Jiang et al., Quercetin as an Antiviral Agent Inhibits Influenza a Virus (Iav) Entry, Viruses, doi:10.3390/v8010006
Yang, Wang, Long, Li, Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine, Oxid. Med. Cell. Longev, doi:10.1155/2020/8825387
Yi, Li, Yuan, Qu, Chen et al., Small Molecules Blocking the Entry of Severe Acute Respiratory Syndrome Coronavirus into Host Cells, J. Virol, doi:10.1128/JVI.78.20.11334-11339.2004
Young, Bowman, Non-Parametric Analysis of Covariance, Biometrics, doi:10.2307/2532993
Zahedipour, Hosseini, Sathyapalan, Majeed, Jamialahmadi et al., Potential Effects of Curcumin in the Treatment of Covid-19 Infection, Phytother. Res, doi:10.1002/ptr.6738
Önal, Arslan, Üçüncü Ergun, Topuz, Yilmaz Semerci et al., Treatment of Covid-19 Patients with Quercetin: A Prospective, Single Center, Randomized, Controlled Trial, Turk. J. Biol, doi:10.3906/biy-2104-16
DOI record:
{
"DOI": "10.1186/s13063-020-04634-2",
"ISSN": [
"1745-6215"
],
"URL": "http://dx.doi.org/10.1186/s13063-020-04634-2",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n<jats:title>Objectives</jats:title>\n<jats:p>1- To compare the effectiveness of 1% Hydrogen peroxide, 0.2% Povidone-Iodine, 2% hypertonic saline and a novel solution Neem extract (<jats:italic>Azardirachta indica</jats:italic>) in reducing intra-oral viral load in COVID-19 positive patients.</jats:p>\n<jats:p>2- To determine the salivary cytokine profiles of IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ and IL- 17 among COVID-19 patients subjected to 1% Hydrogen peroxide, 0.2% Povidone-Iodine, 2% hypertonic saline or Neem extract (<jats:italic>Azardirachta indica)</jats:italic> based gargles.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Trial design</jats:title>\n<jats:p>This will be a parallel group, quadruple blind-randomised controlled pilot trial with an add on laboratory based study.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Participants</jats:title>\n<jats:p>A non-probability, purposive sampling technique will be followed to identify participants for this study.</jats:p>\n<jats:p>The clinical trial will be carried out at the Aga Khan University Hospital (AKUH), Karachi, Pakistan. The viral PCR tests will be done at main AKUH clinical laboratories whereas the immunological tests (cytokine analysis) will be done at the Juma research laboratory of AKUH.</jats:p>\n<jats:p>The inclusion criteria are laboratory-confirmed COVID-19 positive patients, male or female, in the age range of 18-65 years, with mild to moderate disease, already admitted to the AKUH. Subjects with low Glasgow coma score, with a history of radiotherapy or chemotherapy, who are more than 7 days past the onset of COVID- 19 symptoms, or intubated or edentulous patients will be excluded. Patients who are being treated with any form of oral or parenteral antiviral therapy will be excluded, as well as patients with known pre-existing chronic mucosal lesions such as lichen planus.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Intervention and comparator</jats:title>\n<jats:p>Group A (n=10) patients on 10 ml gargle and nasal lavage using 0.2% Povidone-Iodine (Betadiene® by Aviro Health Inc./ Pyodine® by Brooks Pharma Inc.) for 20-30 seconds, thrice daily for 6 days. Group B (n=10) patients will be subjected to 10 ml gargle and nasal lavage using 1% Hydrogen peroxide (HP® by Karachi Chemicals Products Inc./ ActiveOxy® by Boumatic Inc.) for 20-30 seconds, thrice daily for 6 days. Group C will comprised of (n=10) subjects on 10ml gargle and nasal lavage using Neem extract solution (<jats:italic>Azardirachta indica</jats:italic>) formulated by Karachi University (chemistry department laboratories) for 20-30 seconds, thrice daily for 6 days. Group D (n=10) patients will use 2% hypertonic saline (Plabottle® by Otsuka Inc.) gargle and nasal lavage for a similar time period. Group E (n=10) will serve as positive controls. These will be given simple distilled water gargles and nasal lavage for 20-30 seconds, thrice daily for six days. For nasal lavage, a special douche syringe will be provided to each participant. Its use will be thoroughly explained by the data collection officer. After each use, the patient is asked not to eat, drink, or rinse their mouth for the next 30 minutes.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Main outcomes</jats:title>\n<jats:p>The primary outcome is the reduction in the intra-oral viral load confirmed with real time quantitative PCR.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Randomisation</jats:title>\n<jats:p>The assignment to the study group/ allocation will be done using the sealed envelope method under the supervision of Clinical Trial Unit (CTU) of Aga Khan University, Karachi, Pakistan. The patients will be randomised to their respective study group (1:1:1:1:1 allocation ratio) immediately after the eligibility assessment and consent administration is done.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Blinding (masking)</jats:title>\n<jats:p>The study will be quadruple-blinded. Patients, intervention provider, outcome assessor and the data collection officer will be blinded. The groups will be labelled as A, B, C, D or E. The codes of the intervention will be kept in lock & key at the CTU and will only be revealed at the end of study or if the study is terminated prematurely.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Numbers to be randomised (sample size)</jats:title>\n<jats:p>As there is no prior work on this research question, so no assumptions for the sample size calculation could be made. The present study will serve as a pilot trial. We intend to study <jats:bold>50</jats:bold> patients in five study groups with 10 patients in each study group. For details, please refer to Fig. 1 for details.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Trial Status</jats:title>\n<jats:p>Protocol version is 7.0, approved by the department and institutional ethics committees and clinical trial unit of the university hospital. Recruitment is planned to start as soon as the funding is sanctioned. The total duration of the study is expected to be 6 months i.e. August 2020-January 2021.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Trial registration</jats:title>\n<jats:p>This study protocol was registered at <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"http://www.clinicaltrials.gov\">www.clinicaltrials.gov</jats:ext-link> on 10 April 2020 <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04341688\">NCT04341688</jats:ext-link>.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Full protocol</jats:title>\n<jats:p>The full protocol is attached as an additional file, accessible from the Trials website (Additional file 1). In the interest in expediting dissemination of this material, the familiar formatting has been eliminated; this Letter serves as a summary of the key elements of the full protocol. The study protocol has been reported in accordance with the Standard Protocol Items: Recommendations for Clinical Interventional Trials (SPIRIT) guidelines (Additional file 2).</jats:p>\n\n</jats:sec>",
"alternative-id": [
"4634"
],
"article-number": "785",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "20 July 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "26 July 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "14 September 2020"
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The study protocol has been approved by the ethics review committee of the Aga Khan University, Karachi Pakistan. Approved on 16<sup>th</sup> July 2020. Reference # 2020-4926-11376. The authors certify that this trial has received ethical approval from the appropriate ethical committee as described above. Informed consent will be obtained in Urdu language (or in English for those who are well versed with the latter), where the harms and benefits of the gargles and nasal lavage will be explained along with the method of their use in a simple language. In addition to the clinical trial, this consent will also entail the use of biological specimens for the laboratory testing and analysis. The details are stated in the Urdu and English consent forms. The signed consent forms will be retained for the record purpose."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5650-6268",
"affiliation": [],
"authenticated-orcid": false,
"family": "Khan",
"given": "Farhan Raza",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-6934-6945",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kazmi",
"given": "Syed Murtaza Raza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iqbal",
"given": "Najeeha Talat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iqbal",
"given": "Junaid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Syed Tariq",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3911-1632",
"affiliation": [],
"authenticated-orcid": false,
"family": "Abbas",
"given": "Syed Akbar",
"sequence": "additional"
}
],
"container-title": "Trials",
"container-title-short": "Trials",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
14
]
],
"date-time": "2020-09-14T10:02:59Z",
"timestamp": 1600077779000
},
"deposited": {
"date-parts": [
[
2021,
9,
14
]
],
"date-time": "2021-09-14T00:02:06Z",
"timestamp": 1631577726000
},
"indexed": {
"date-parts": [
[
2023,
8,
19
]
],
"date-time": "2023-08-19T01:34:27Z",
"timestamp": 1692408867704
},
"is-referenced-by-count": 13,
"issue": "1",
"issued": {
"date-parts": [
[
2020,
9,
14
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2020,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
9,
14
]
],
"date-time": "2020-09-14T00:00:00Z",
"timestamp": 1600041600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
9,
14
]
],
"date-time": "2020-09-14T00:00:00Z",
"timestamp": 1600041600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13063-020-04634-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13063-020-04634-2/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13063-020-04634-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2020,
9,
14
]
]
},
"published-online": {
"date-parts": [
[
2020,
9,
14
]
]
},
"published-print": {
"date-parts": [
[
2020,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-020-04634-2"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": "A quadruple blind, randomised controlled trial of gargling agents in reducing intraoral viral load among hospitalised COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "21"
}
khan6