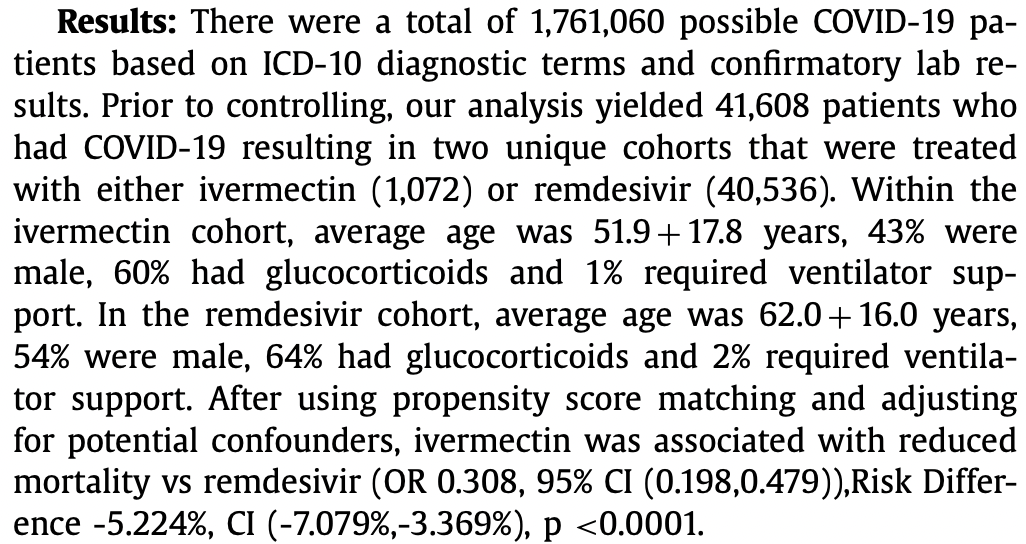
Treatment with Ivermectin Is Associated with Decreased Mortality in COVID-19 Patients: Analysis of a National Federated Database
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.12.096, Feb 2022
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PSM retrospective 41,608 patients in the USA, 1,072 treated with ivermectin and 40,536 treated with remdesivir, showing lower mortality with ivermectin treatment.
This study was presented at a conference (IMED 2021). Submissions were peer-reviewed. The treatment/control group sizes align with the estimated percentage of hospitals that used ivermectin vs. remdesivir. Hospitals in the USA receive financial incentives to use remdesivir.
Authors have self-censored the conference report of this result, not due to any error in the analysis, but because they believe ivermectin "has proven to be ineffective in clinical trials". This is incorrect, while some studies show no statistically significant effect, 65 studies show statistically significant positive results for one or more outcomes (38 prospective and 27 retrospective studies, including 28 RCTs)1-65.
The self-censorship and decision not to submit to a journal provide further evidence of a negative publication bias for ivermectin research.
This is the 80th of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
53 studies are RCTs, which show efficacy with p=0.000000087.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments66.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 69.2% lower, OR 0.31, p < 0.001, treatment 1,072, control 40,536, propensity score matching, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Chowdhury et al., A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients, Eurasian Journal of Medicine and Oncology, doi:10.14744/ejmo.2021.16263.
2.
Espitia-Hernandez et al., Effects of Ivermectin-azithromycin-cholecalciferol combined therapy on COVID-19 infected patients: A proof of concept study, Biomedical Research, 31:5, www.biomedres.info/biomedical-research/effects-of-ivermectinazithromycincholecalciferol-combined-therapy-on-covid19-infected-patients-a-proof-of-concept-study-14435.html.
3.
Mahmud et al., Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial, Journal of International Medical Research, doi:10.5061/dryad.qjq2bvqf6.
4.
Cadegiani et al., Early COVID-19 Therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in Outpatient Settings Significantly Improved COVID-19 outcomes compared to Known outcomes in untreated patients, New Microbes and New Infections, doi:10.1016/j.nmni.2021.100915.
5.
Ahmed et al., A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.11.191.
6.
Chaccour et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial, eClinicalMedicine, doi:10.1016/j.eclinm.2020.100720.
7.
Ghauri et al., Ivermectin Use Associated with Reduced Duration of Covid-19 Febrile Illness in a Community Setting, International Journal of Clinical Studies & Medical Case Reports, doi:10.46998/IJCMCR.2021.13.000320.
8.
Babalola et al., Ivermectin shows clinical benefits in mild to moderate COVID19: A randomised controlled double-blind, dose-response study in Lagos, QJM: An International Journal of Medicine, doi:10.1093/qjmed/hcab035.
9.
Bukhari et al., Efficacy of Ivermectin in COVID-19 Patients with Mild to Moderate Disease, medRxiv, doi:10.1101/2021.02.02.21250840.
10.
Biber et al., The effect of ivermectin on the viral load and culture viability in early treatment of non-hospitalized patients with mild COVID-19 – A double-blind, randomized placebo-controlled trial, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.07.003.
11.
Elalfy et al., Effect of a combination of Nitazoxanide, Ribavirin and Ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-1, Journal of Medical Virology, doi:10.1002/jmv.26880.
12.
Chahla et al., Randomized trials - Ivermectin repurposing for COVID-19 treatment of outpatients with mild disease in primary health care centers, Research, Society and Development, doi:10.33448/rsd-v11i8.30844.
13.
Mourya et al., Comparative Analytical Study of Two Different Drug Regimens in Treatment of Covid 19 Positive Patients in Index Medical College Hospital and Research Center, Indore, India, International Journal of Health and Clinical Research, 4:6, ijhcr.com/index.php/ijhcr/article/view/1263.
14.
Merino et al., Ivermectin and the odds of hospitalization due to COVID-19: evidence from a quasi-experimental analysis based on a public intervention in Mexico City, Preprint, web.archive.org/web/20211218011849/https://files.osf.io/v1/resources/r93g4/providers/osfstorage/609085945533b4031ee1c789?action=download&direct&version=1.
15.
Faisal et al., Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19, The Professional Medical Journal, doi:10.29309/TPMJ/2021.28.05.5867.
16.
Aref et al., Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19, International Journal of Nanomedicine, doi:10.2147/IJN.S313093.
17.
Mayer et al., Safety and Efficacy of a MEURI Program for the Use of High Dose Ivermectin in COVID-19 Patients, Frontiers in Public Health, doi:10.3389/fpubh.2022.813378.
18.
Borody et al., Combination Therapy For COVID-19 Based on Ivermectin in an Australian Population, TrialSite News, c19early.org/pdf/borody.pdf.
19.
Abbas et al., The Effect of Ivermectin on Reducing Viral Symptoms in Patients with Mild COVID-19, Indian Journal of Pharmaceutical Sciences, doi:10.36468/pharmaceutical-sciences.spl.416.
20.
de Jesús Ascencio-Montiel et al., A Multimodal Strategy to Reduce the Risk of Hospitalization/death in Ambulatory Patients with COVID-19, Archives of Medical Research, doi:10.1016/j.arcmed.2022.01.002.
21.
de la Rocha et al., Ivermectin compared with placebo in the clinical course in Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial, BMC Infectious Diseases, doi:10.1186/s12879-022-07890-6.
22.
Siripongboonsitti et al., A Randomized Trial to Assess the Acceleration of Viral Clearance by the Combination Favipiravir/Ivermectin/Niclosamide in Mild-to-Moderate COVID-19 Adult Patients (FINCOV), Journal of Infection and Public Health, doi:10.1016/j.jiph.2024.03.030.
23.
Wijewickrema et al., Efficacy and safety of oral ivermectin in the treatment of mild to moderate Covid-19 patients: a multi-centre double-blind randomized controlled clinical trial, BMC Infectious Diseases, doi:10.1186/s12879-024-09563-y.
24.
Gorial et al., Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management (Pilot Trial), medRxiv, doi:10.1101/2020.07.07.20145979.
25.
Khan et al., Ivermectin treatment may improve the prognosis of patients with COVID-19, Archivos de Bronconeumología, doi:10.1016/j.arbres.2020.08.007.
26.
Soto-Becerra et al., Real-World Effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: Results of a target trial emulation using observational data from a nationwide Healthcare System in Peru, medRxiv, doi:10.1101/2020.10.06.20208066.
27.
Rajter et al., Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID-19 (ICON study), Chest, doi:10.1016/j.chest.2020.10.009.
28.
Hashim et al., Controlled randomized clinical trial on using Ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq, Iraqi Journal of Medical Science, 19:1, www.iraqijms.net/upload/pdf/iraqijms60db8b76d3b1e.pdf.
29.
Spoorthi et al., Utility of Ivermectin and Doxycycline combination for the treatment of SARSCoV-2, IAIM, 2020, 7:10, 177-182, iaimjournal.com/wp-content/uploads/2020/10/iaim_2020_0710_23.pdf.
30.
Budhiraja et al., Clinical Profile of First 1000 COVID-19 Cases Admitted at Tertiary Care Hospitals and the Correlates of their Mortality: An Indian Experience, medRxiv, doi:10.1101/2020.11.16.20232223.
31.
Okumuş et al., Evaluation of the Effectiveness and Safety of Adding Ivermectin to Treatment in Severe COVID-19 Patients, BMC Infectious Diseases, doi:10.1186/s12879-021-06104-9.
32.
Shahbaznejad et al., Effects of Ivermectin in Patients With COVID-19: A Multicenter, Double-blind, Randomized, Controlled Clinical Trial, Clinical Therapeutics, doi:10.1016/j.clinthera.2021.04.007.
33.
Lima-Morales et al., Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.02.014.
34.
Ahsan et al., Clinical Variants, Characteristics, and Outcomes Among COVID-19 Patients: A Case Series Analysis at a Tertiary Care Hospital in Karachi, Pakistan, Cureus, doi:10.7759/cureus.14761.
35.
Rezk et al., miRNA-223-3p, miRNA- 2909 and Cytokines Expression in COVID-19 Patients Treated with Ivermectin, Zagazig University Medical Journal, doi:10.21608/zumj.2021.92746.2329.
36.
Lim et al., Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial, JAMA, doi:10.1001/jamainternmed.2022.0189.
37.
Ozer et al., Effectiveness and Safety of Ivermectin in COVID-19 Patients: A Prospective Study at A Safety-Net Hospital, Journal of Medical Virology, doi:10.1002/jmv.27469.
38.
Shimizu et al., Ivermectin administration is associated with lower gastrointestinal complications and greater ventilator-free days in ventilated patients with COVID-19: A propensity score analysis, Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2021.12.024.
39.
Thairu et al., A Comparison of Ivermectin and Non Ivermectin Based Regimen for COVID-19 in Abuja: Effects on Virus Clearance, Days-to-discharge and Mortality, Journal of Pharmaceutical Research International, doi:10.9734/jpri/2022/v34i44A36328.
40.
Efimenko et al., Treatment with Ivermectin Is Associated with Decreased Mortality in COVID-19 Patients: Analysis of a National Federated Database, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.12.096.
41.
Naggie et al., Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.18590.
42.
Rezai et al., Non-effectiveness of Ivermectin on Inpatients and Outpatients With COVID-19; Results of Two Randomized, Double-Blinded, Placebo-Controlled Clinical Trials, Frontiers in Medicine, doi:10.3389/fmed.2022.919708.
43.
Qadeer et al., Ivermectin A Potential Treatment In Covid-19, Related to Critical Illness, Pakistan Journal of Medical and Health Sciences, doi:10.53350/pjmhs2216824.
44.
Aref (B) et al., Possible Role of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Recovery of Post-COVID-19 Anosmia, Infection and Drug Resistance, doi:10.2147/IDR.S381715.
45.
Osati et al., Clinical manifestations and mortality among hospitalized COVID-19 patients in Tanzania, 2021-2022., medRxiv, doi:10.1101/2023.07.13.23292643.
46.
Hayward et al., Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes, Journal of Infection, doi:10.1016/j.jinf.2024.106130.
47.
Varnaseri et al., Ivermectin as a Potential Addition to the Limited Anti-COVID-19 Arsenal: A Double-Blinded Clinical Trial, Jundishapur Journal of Health Sciences, doi:10.5812/jjhs-146703.
48.
Shouman et al., Use of Ivermectin as a Potential Chemoprophylaxis for COVID-19 in Egypt: A Randomised Clinical Trial, Journal of Clinical and Diagnostic Research, doi:10.7860/JCDR/2021/46795.14529.
49.
Carvallo et al., Usefulness of Topical Ivermectin and Carrageenan to Prevent Contagion of Covid 19 (IVERCAR), NCT04425850, clinicaltrials.gov/ct2/show/results/NCT04425850.
50.
Behera et al., Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study, PLOS ONE, doi:10.1371/journal.pone.0247163.
51.
Carvallo (B) et al., Study of the Efficacy and Safety of Topical Ivermectin + Iota-Carrageenan in the Prophylaxis against COVID-19 in Health Personnel, Journal of Biomedical Research and Clinical Investigation, doi:10.31546/2633-8653.1007.
52.
Hellwig et al., A COVID-19 Prophylaxis? Lower incidence associated with prophylactic administration of Ivermectin, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106248.
53.
Bernigaud et al., Ivermectin benefit: from scabies to COVID-19, an example of serendipity, Annals of Dermatology and Venereology, doi:10.1016/j.annder.2020.09.231.
54.
Alam et al., Ivermectin as Pre-exposure Prophylaxis for COVID-19 among Healthcare Providers in a Selected Tertiary Hospital in Dhaka – An Observational Study, European Journal of Medical and Health Sciences, doi:10.24018/ejmed.2020.2.6.599.
55.
IVERCOR PREP, Ivermectina en agentes de salud e IVERCOR COVID19, Preliminary Results, web.archive.org/web/20210226215453/https://twitter.com/Covid19Crusher/status/1365420061859717124.
56.
Chahla (B) et al., Intensive Treatment With Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers From Tucuman, Argentina, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001433.
57.
Behera (B) et al., Prophylactic Role of Ivermectin in Severe Acute Respiratory Syndrome Coronavirus 2 Infection Among Healthcare Workers, Cureus, doi:10.7759/cureus.16897.
58.
Tanioka et al., Why COVID-19 is not so spread in Africa: How does Ivermectin affect it?, medRxiv, doi:10.1101/2021.03.26.21254377.
59.
Seet et al., Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.04.035.
60.
Morgenstern et al., Ivermectin as a SARS-CoV-2 Pre-Exposure Prophylaxis Method in Healthcare Workers: A Propensity Score-Matched Retrospective Cohort Study, Cureus, doi:10.7759/cureus.17455.
61.
Mondal et al., Prevalence of COVID-19 Infection and Identification of Risk Factors among Asymptomatic Healthcare Workers: A Serosurvey Involving Multiple Hospitals in West Bengal, Journal of the Indian Medical Association, 119:5, onlinejima.com/read_journals.php?article=683.
62.
Samajdar et al., Ivermectin and Hydroxychloroquine for Chemo-Prophylaxis of COVID-19: A Questionnaire Survey of Perception and Prescribing Practice of Physicians vis-a-vis Outcomes, Journal of the Association of Physicians India, 69:11, www.researchgate.net/publication/356294136_Ivermectin_and_Hydroxychloroquine_for_Chemo-Prophylaxis_of_COVID-19_A_Questionnaire_Survey_of_Perception_and_Prescribing_Practice_of_Physicians_vis-a-vis_Outcomes.
63.
Kerr et al., Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching, Cureus, doi:10.7759/cureus.21272.
64.
Desort-Henin et al., The SAIVE Trial, Post-Exposure use of ivermectin in Covid-19 prevention: Efficacy and Safety Results, ECCMID 2023 (results released 1/5/2023), www.medincell.com/wp-content/uploads/2024/03/Poster-SAIVE-April2023-OK3.pdf.
Efimenko et al., 28 Feb 2022, retrospective, propensity score matching, USA, peer-reviewed, 6 authors, study period 1 January, 2020 - 11 July, 2021, dosage not specified, this trial compares with another treatment - results may be better when compared to placebo, self-censored, see notes.
Development of Duplex and Multiplex Reverse Transcription Loop Mediated Isothermal Amplification (RT-LAMP) Assays for Clinical Diagnosis of SARS-COV-2 in Sri Lanka
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.12.095
Purpose: Despite the rollout of several vaccines targeting SARS-CoV-2, attainment of near-universal vaccination is a challenging task, particularly for low-and middle-income nations such as Sri Lanka. Rapid, reliable diagnostics for the detection of the virus is of vital importance for the predominantly export-and tourismbased economy of the country. Herein, we report the development of a RT-LAMP assay as an alternative to the gold-standard RT-qPCR method for diagnostic laboratories in Sri Lanka in a cost-effective and highly reliable manner. Methods & Materials: About 313 nasopharyngeal and oropharyngeal samples from the community were collected and subjected to RNA purification and subjected to simultaneous RT-qPCR and RT-LAMP experiments by using previously published primers in a thermocycler. Duplex (containing N and E gene primers) and multiplex (containing N, E and ORF1ab gene primers) RT-LAMP assay results were compared with standard RT-qPCR results using an agreement attribute statistical test. The effect of guanidine hydrochloride was also analyzed. Results: The limit of detection for the duplex assay was found to be 10 copies μL-1 at a constant temperature of 63 °C, and 5 copies μL-1 for multiplex assays at 66.4 °C. Both types of RT-LAMP assay were specific only for the SARS-COV-2 virus, successfully distinguishing it from multiple other human viruses. Attribute agreement analysis between duplex-and multiplex RT-LAMP vs RT-qPCR yielded 93% and 96.5% scores, respectively. Moreover, both RT-LAMP assays showed 100% agreement with RT-qPCR when Ct was < 25 in positive samples and showed 100% (duplex) or 97.22% (multiplex) at 35 ≥ Ct ≥25. The discrepancy between agreements at higher Ct values was attributed to the higher sensitivity of the multiplex RT-LAMP assay. The addition of guanidine hydrochloride increased the sensitivity and decreased detection time significantly for both the duplex and multiplex assays. Conclusion: Overall, we have demonstrated a potentially rapidly deployable diagnostic test kit not only for widespread community use but particularly for high-risk locations such as ports of entry or manufacturing facilities to mitigate the effects of the SARS-CoV-2 virus in Sri Lanka.
DOI record:
{
"DOI": "10.1016/j.ijid.2021.12.096",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2021.12.096",
"alternative-id": [
"S1201971221009887"
],
"author": [
{
"affiliation": [],
"family": "Efimenko",
"given": "I.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Nackeeran",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jabori",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zamora",
"given": "J.A. Gonzalez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Danker",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "D.",
"sequence": "additional"
}
],
"container-title": [
"International Journal of Infectious Diseases"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
28
]
],
"date-time": "2022-02-28T23:44:22Z",
"timestamp": 1646091862000
},
"deposited": {
"date-parts": [
[
2022,
2,
28
]
],
"date-time": "2022-02-28T23:44:22Z",
"timestamp": 1646091862000
},
"indexed": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:14:48Z",
"timestamp": 1646093688074
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1201-9712"
}
],
"issued": {
"date-parts": [
[
2022,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:00:00Z",
"timestamp": 1646092800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
12
]
],
"date-time": "2021-12-12T00:00:00Z",
"timestamp": 1639267200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971221009887?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971221009887?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "S40",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"International Journal of Infectious Diseases"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": [
"Treatment with Ivermectin Is Associated with Decreased Mortality in COVID-19 Patients: Analysis of a National Federated Database"
],
"type": "journal-article",
"volume": "116"
}
