Ivermectin A Potential Treatment In Covid-19, Related to Critical Illness
et al., Pakistan Journal of Medical and Health Sciences, doi:10.53350/pjmhs2216824, Aug 2022
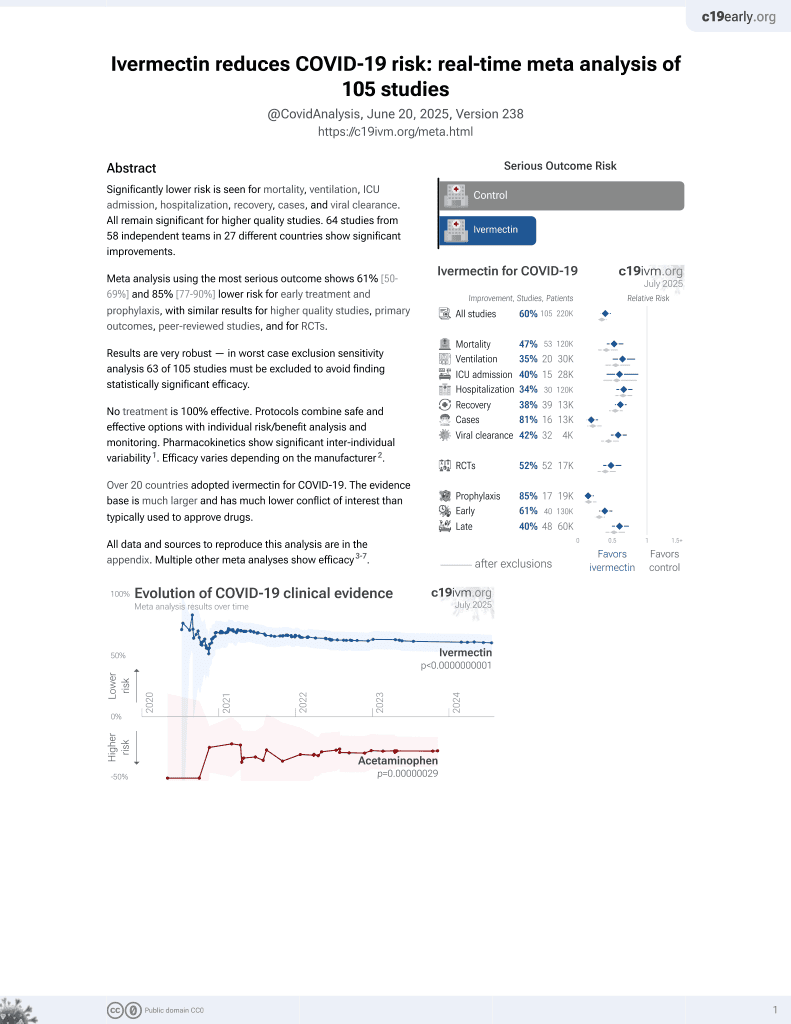
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective convenience sampling study of 210 hospitalized age-matched COVID-19 patients, showing faster viral clearance with ivermectin. Baseline information per group is not provided.
This is the 91st of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
53 studies are RCTs, which show efficacy with p=0.000000087.
This study is excluded in the after exclusion results of meta-analysis:
minimal baseline details provided.
|
risk of no viral clearance, 58.3% lower, RR 0.42, p < 0.001, treatment 35 of 105 (33.3%), control 84 of 105 (80.0%), NNT 2.1, mid-recovery, day 10.
|
|
risk of no viral clearance, 20.0% lower, RR 0.80, p < 0.001, treatment 84 of 105 (80.0%), control 105 of 105 (100.0%), NNT 5.0, day 7.
|
|
risk of no viral clearance, 98.6% lower, RR 0.01, p < 0.001, treatment 0 of 105 (0.0%), control 35 of 105 (33.3%), NNT 3.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Qadeer et al., 31 Aug 2022, prospective, Pakistan, peer-reviewed, median age 55.4, 6 authors, study period 1 November, 2020 - 30 May, 2021, dosage 12mg days 1-5.
Contact: darshan.kumar@duhs.edu.pk.
Ivermectin A Potential Treatment In Covid-19, Related to Critical Illness
Pakistan Journal of Medical and Health Sciences, doi:10.53350/pjmhs2216824
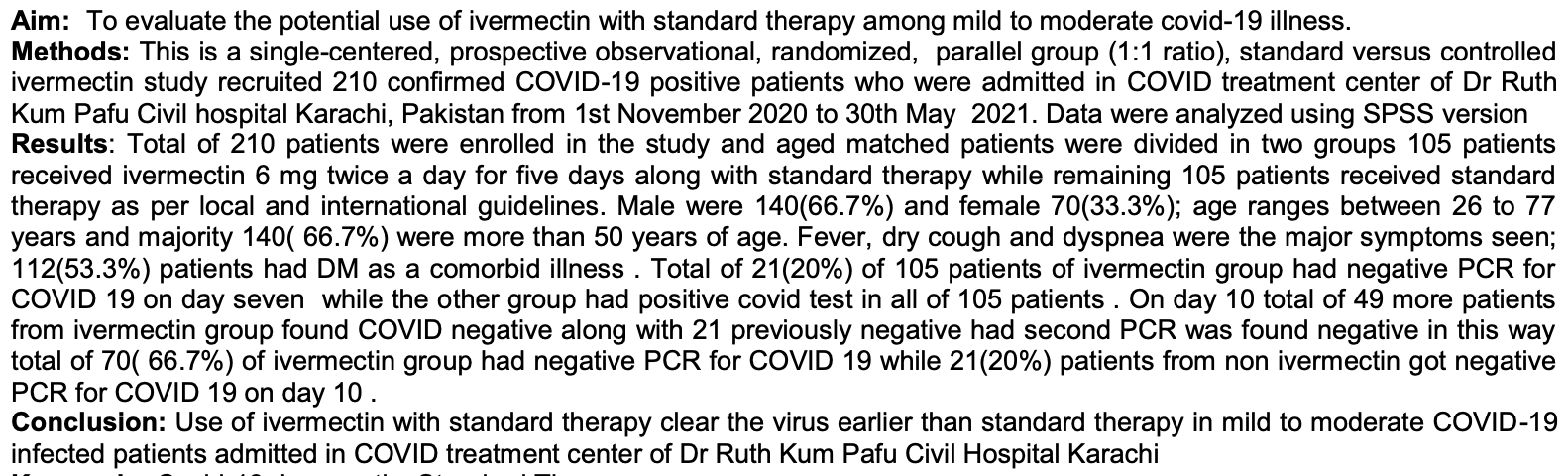
Aim: To evaluate the potential use of ivermectin with standard therapy among mild to moderate covid-19 illness. Methods: This is a single-centered, prospective observational, randomized, parallel group (1:1 ratio), standard versus controlled ivermectin study recruited 210 confirmed COVID-19 positive patients who were admitted in COVID treatment center of Dr Ruth Kum Pafu Civil hospital Karachi, Pakistan from 1st November 2020 to 30th May 2021. Data were analyzed using SPSS version Results: Total of 210 patients were enrolled in the study and aged matched patients were divided in two groups 105 patients received ivermectin 6 mg twice a day for five days along with standard therapy while remaining 105 patients received standard therapy as per local and international guidelines. Male were 140(66.7%) and female 70(33.3%); age ranges between 26 to 77 years and majority 140( 66.7%) were more than 50 years of age. Fever, dry cough and dyspnea were the major symptoms seen; 112(53.3%) patients had DM as a comorbid illness . Total of 21(20%) of 105 patients of ivermectin group had negative PCR for COVID 19 on day seven while the other group had positive covid test in all of 105 patients . On day 10 total of 49 more patients from ivermectin group found COVID negative along with 21 previously negative had second PCR was found negative in this way total of 70( 66.7%) of ivermectin group had negative PCR for COVID 19 while 21(20%) patients from non ivermectin got negative PCR for COVID 19 on day 10 . Conclusion: Use of ivermectin with standard therapy clear the virus earlier than standard therapy in mild to moderate COVID-19 infected patients admitted in COVID treatment center of Dr Ruth Kum Pafu Civil Hospital Karachi
References
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.191
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Formiga, Leblanc, De Souza Rebouças, Farias, De Oliveira et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J Control Release, doi:10.1016/j.jconrel.2020.10.009
Hellwig, A COVID-19 prophylaxis? Lower incidence associated with prophylactic administration of ivermectin, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106248
Jans, Wagstaff, Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?, Cells, doi:10.3390/cells9092100
Jans, Wagstaff, The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2020.10.042
Kaur, Shekhar, Sharma, Sarma, Prakash et al., Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes, Pharmacol Rep, doi:10.1007/s43440-020-00195-y
Khan, Khan, Debnath, Ivermectin Treatment May Improve the Prognosis of Patients With COVID-19, Arch Bronconeumol (Engl Ed), doi:10.1016/j.arbres.2020.08.007
Kinobe, Owens, A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin's possible mode of action against SARS-CoV-2, Fundam Clin Pharmacol, doi:10.1111/fcp.12644
Kory, Meduri, Varon, Iglesias, Marik, Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Am J Ther, doi:10.1097/MJT.0000000000001377
Majumder, Minko, Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19, AAPS J, doi:10.1208/s12248-020-00532-2
Marian, Current state of vaccine development and targeted therapies for COVID-19: impact of basic science discoveries, Cardiovasc Pathol, doi:10.1016/j.carpath.2020.107278
Martin, Jans, Antivirals that target the host IMPα/β1-virus interface, Biochem Soc Trans, doi:10.1042/BST20200568
Mohamadian, Chiti, Shoghli, Biglari, Parsamanesh et al., COVID-19: Virology, biology and novel laboratory diagnosis, J Gene Med, doi:10.1002/jgm.3303
Navarro, Camprubí, Requena-Méndez, Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother, doi:10.1093/jac/dkz524
Padhy, Mohanty, Das, Meher, Therapeutic potential of ivermectin as add on treatment in COVID 19: A systematic review and meta-analysis, J Pharm Pharm Sci, doi:10.18433/jpps31457
Phelan, Katz, Lo, The novel coronavirus originating in Wuhan, China: challenges for global health governance, Jama
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study, Chest, doi:10.1016/j.chest.2020.10.009
Taiub, Chowdhury, Shahbaz, Karim, Islam et al., A randomized trial of ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID19 patients n
Vallejos, Zoni, Bangher, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19): a structured summary of a study protocol for a randomized controlled trial, Cochrane Database Syst Rev, doi:10.1186/s13063-020-04813-1
Vora, Arora, Behera, Tripathy, White paper on Ivermectin as a potential therapy for COVID-19, Indian J Tuberc, doi:10.1016/j.ijtb.2020.07.031
Wu, Ho, Huang, Jin, Li et al., SARS-CoV-2 is an appropriate name for the new coronavirus, The Lancet
DOI record:
{
"DOI": "10.53350/pjmhs2216824",
"URL": "http://dx.doi.org/10.53350/pjmhs2216824",
"abstract": "<jats:p>Aim: To evaluate the potential use of ivermectin with standard therapy among mild to moderate covid-19 illness. Methods: This is a single-centered, prospective observational, randomized, parallel group (1:1 ratio), standard versus controlled ivermectin study recruited 210 confirmed COVID-19 positive patients who were admitted in COVID treatment center of Dr Ruth Kum Pafu Civil hospital Karachi, Pakistan from 1st November 2020 to 30th May 2021. Data were analyzed using SPSS version Results: Total of 210 patients were enrolled in the study and aged matched patients were divided in two groups 105 patients received ivermectin 6 mg twice a day for five days along with standard therapy while remaining 105 patients received standard therapy as per local and international guidelines.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Qadeer",
"given": "Rashid",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kashif",
"given": "Syed Muhmmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Darshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehmmood",
"given": "Madiha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lal",
"given": "Jawahar",
"sequence": "additional"
},
{
"affiliation": [],
"family": ".",
"given": "Faizan",
"sequence": "additional"
}
],
"container-title": "Pakistan Journal of Medical and Health Sciences",
"container-title-short": "PJMHS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T09:18:30Z",
"timestamp": 1662455910000
},
"deposited": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T09:18:43Z",
"timestamp": 1662455923000
},
"indexed": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T09:41:49Z",
"timestamp": 1662457309628
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2022,
8,
31
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2022,
8,
31
]
]
}
},
"member": "31137",
"original-title": [],
"page": "24-26",
"prefix": "10.53350",
"published": {
"date-parts": [
[
2022,
8,
31
]
]
},
"published-print": {
"date-parts": [
[
2022,
8,
31
]
]
},
"publisher": "Lahore Medical and Dental College",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://pjmhsonline.com/index.php/pjmhs/article/view/2153"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Ivermectin A Potential Treatment In Covid-19, Related to Critical Illness",
"type": "journal-article",
"volume": "16"
}