A COVID-19 Prophylaxis? Lower incidence associated with prophylactic administration of Ivermectin
et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106248, Nov 2020
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
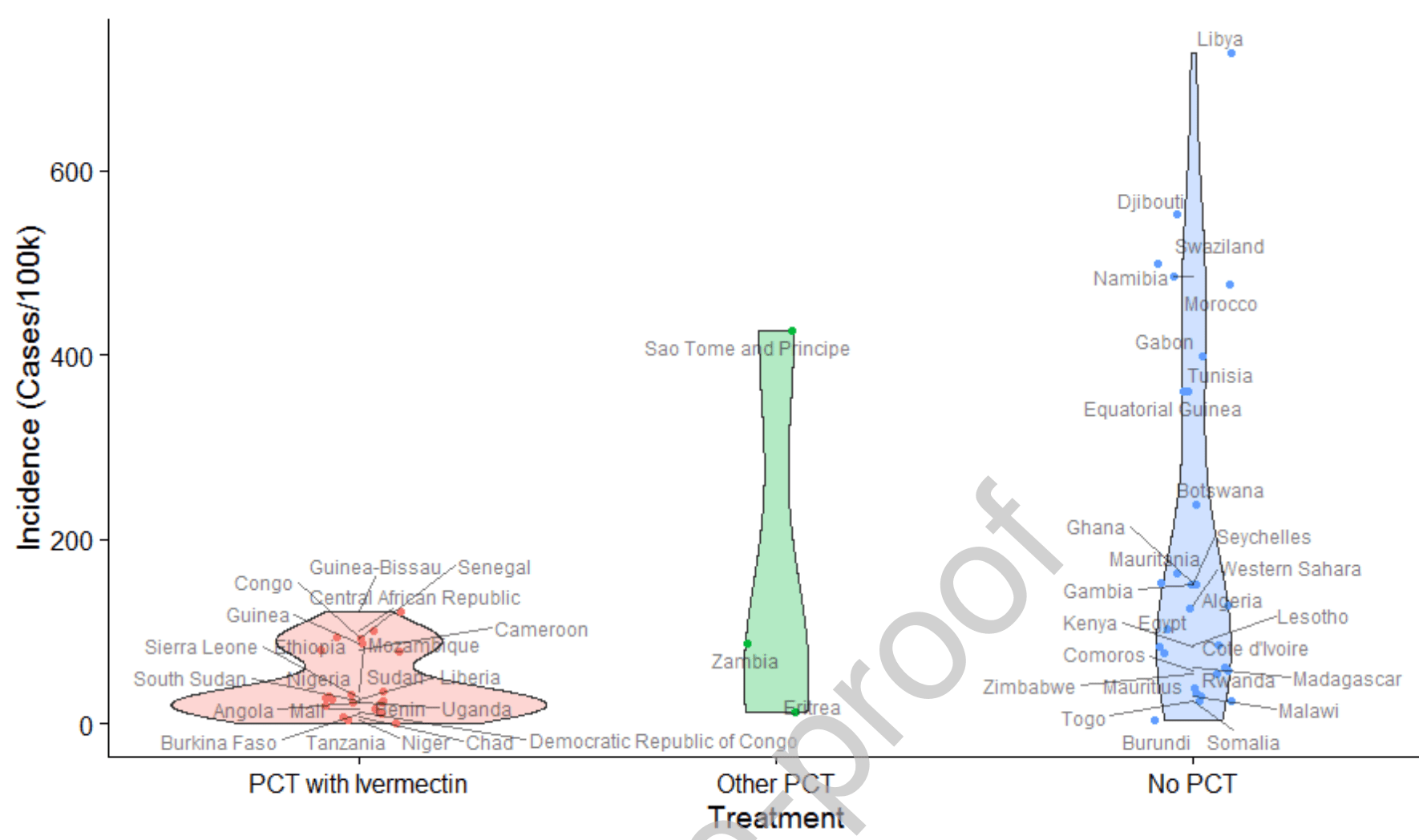
Analysis of COVID-19 cases vs. widespread prophylactic use of ivermectin for parasitic infections showing significantly lower incidence of COVID-19 cases.
This is the 22nd of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
53 studies are RCTs, which show efficacy with p=0.000000087.
This study is excluded in the after exclusion results of meta-analysis:
not a typical trial, analysis of African countries that used or did not use ivermectin prophylaxis for parasitic infections.
|
risk of case, 78.0% lower, RR 0.22, p < 0.02, African countries, PCTI vs. no PCT, relative cases per capita.
|
|
risk of case, 80.0% lower, RR 0.20, p < 0.001, worldwide, PCTI vs. no PCT, relative cases per capita.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hellwig et al., 28 Nov 2020, retrospective, ecological study, multiple countries, peer-reviewed, 2 authors, dosage 200μg/kg, dose varied, typically 150-200μg/kg.
A COVID-19 prophylaxis? Lower incidence associated with prophylactic administration of ivermectin
International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106248
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre -including this research content -immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020. 106248 .
References
Al-Tawfiq, Al-Homoud, Memish, Remdesivir as a possible therapeutic option for the COVID-19, Travel Med Infect Dis, doi:10.1016/j.tmaid.2020.101615
Allcott, Boxell, Conway, Gentzkow, Thaler et al., Polarization and public health: partisan differences in social distancing during the coronavirus pandemic, National Bureau of Economic Research, doi:10.3386/w26946
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Cohen, Vaccine designers take first shots at COVID-19, Science, doi:10.1126/science.368.6486.14
Colson, Rolain, Lagier, Brouqui, Raoult, Chloroquine and hydroxychloroquine as available weapons to fight COVID-19, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105932
Fink, Porras, Pharmacokinetics of ivermectin in animals and humans, doi:10.1007/978-1-4612-3626-9_7
Greene, Taylor, Cupp, Murphy, White et al., Comparison of ivermectin and diethylcarbamazine in the treatment of onchocerciasis, N Engl J Med, doi:10.1056/NEJM198507183130301
Heidary, Gharebaghi, a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot, doi:10.1038/s41429-020-0336-z
Munster, Koopmans, Van Doremalen, Van Riel, De, A novel coronavirus emerging in China-key questions for impact assessment, N Engl J Med, doi:10.1056/NEJMp2000929
Muñoz, Ballester, Antonijoan, Gich, Rodríguez et al., Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers, PLoS Negl Trop Dis, doi:10.1371/journal.pntd.0006020
Rajter, Sherman, Fatteh, Vogel, Sacks et al., ICON (Ivermectin in COvid Nineteen) Study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID-19, doi:10.2139/ssrn.3631261
Richard-Lenoble, Chandenier, Gaxotte, Ivermectin and filariasis, Fundam Clin Pharmacol, doi:10.1046/j.1472-8206.2003.00170.x
Scheim, Ivermectin for COVID-19 treatment: clinical response at quasithreshold doses via hypothesized alleviation of CD147-mediated vascular occlusion, doi:10.2139/ssrn.3636557
Schmith, Zhou, Lohmer, The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther, doi:10.1002/cpt.1889
Singer, The COVID-19 pandemic: growth patterns, power law scaling, and saturation, Phys Biol, doi:10.1088/1478-3975/ab9bf5
Van Norman Gadrugs, Devices, and the FDA: Part 1: an overview of approval processes for drugs, JACC Basic Transl Sci, doi:10.1016/j.jacbts.2016.03.002
Wang, Horby, Hayden, Gao, A novel coronavirus outbreak of global health concern, Lancet, doi:10.1016/S0140-6736(20)30185-9
Zhou, Hou, Shen, Huang, Martin et al., Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2, Cell Discov, doi:10.1038/s41421-020-0153-3
DOI record:
{
"DOI": "10.1016/j.ijantimicag.2020.106248",
"ISSN": [
"0924-8579"
],
"URL": "http://dx.doi.org/10.1016/j.ijantimicag.2020.106248",
"alternative-id": [
"S0924857920304684"
],
"article-number": "106248",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A COVID-19 prophylaxis? Lower incidence associated with prophylactic administration of ivermectin"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Antimicrobial Agents"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijantimicag.2020.106248"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier Ltd and International Society of Antimicrobial Chemotherapy. All rights reserved."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8841-7545",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hellwig",
"given": "Martin D.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Maia",
"given": "Anabela",
"sequence": "additional"
}
],
"container-title": "International Journal of Antimicrobial Agents",
"container-title-short": "International Journal of Antimicrobial Agents",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
11,
28
]
],
"date-time": "2020-11-28T16:15:37Z",
"timestamp": 1606580137000
},
"deposited": {
"date-parts": [
[
2021,
3,
8
]
],
"date-time": "2021-03-08T09:41:34Z",
"timestamp": 1615196494000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T16:36:50Z",
"timestamp": 1711643810150
},
"is-referenced-by-count": 40,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0924857920304684?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0924857920304684?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "106248",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30185-9",
"article-title": "A novel coronavirus outbreak of global health concern",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "470",
"journal-title": "Lancet",
"key": "10.1016/j.ijantimicag.2020.106248_bib0001",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2000929",
"article-title": "A novel coronavirus emerging in China—key questions for impact assessment",
"author": "Munster",
"doi-asserted-by": "crossref",
"first-page": "692",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijantimicag.2020.106248_bib0002",
"volume": "382",
"year": "2020"
},
{
"key": "10.1016/j.ijantimicag.2020.106248_bib0003",
"series-title": "COVID-19 coronavirus pandemic",
"year": "2020"
},
{
"article-title": "Polarization and public health: partisan differences in social distancing during the coronavirus pandemic. Working Paper 26946",
"author": "Allcott",
"journal-title": "National Bureau of Economic Research",
"key": "10.1016/j.ijantimicag.2020.106248_bib0004",
"year": "2020"
},
{
"DOI": "10.1088/1478-3975/ab9bf5",
"article-title": "The COVID-19 pandemic: growth patterns, power law scaling, and saturation",
"author": "Singer",
"doi-asserted-by": "crossref",
"journal-title": "Phys Biol",
"key": "10.1016/j.ijantimicag.2020.106248_bib0005",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1126/science.368.6486.14",
"article-title": "Vaccine designers take first shots at COVID-19",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Science",
"key": "10.1016/j.ijantimicag.2020.106248_bib0006",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/j.jacbts.2016.03.002",
"article-title": "Drugs, devices, and the FDA: Part 1: an overview of approval processes for drugs",
"doi-asserted-by": "crossref",
"first-page": "170",
"journal-title": "JACC Basic Transl Sci",
"key": "10.1016/j.ijantimicag.2020.106248_bib0007",
"volume": "1",
"year": "2016"
},
{
"DOI": "10.1038/s41421-020-0153-3",
"article-title": "Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Cell Discov",
"key": "10.1016/j.ijantimicag.2020.106248_bib0008",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105932",
"article-title": "Chloroquine and hydroxychloroquine as available weapons to fight COVID-19",
"author": "Colson",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.ijantimicag.2020.106248_bib0009",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101615",
"article-title": "Remdesivir as a possible therapeutic option for the COVID-19",
"author": "Al-Tawfiq",
"doi-asserted-by": "crossref",
"journal-title": "Travel Med Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106248_bib0010",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ijantimicag.2020.106248_bib0011",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1046/j.1472-8206.2003.00170.x",
"article-title": "Ivermectin and filariasis",
"author": "Richard-Lenoble",
"doi-asserted-by": "crossref",
"first-page": "199",
"journal-title": "Fundam Clin Pharmacol",
"key": "10.1016/j.ijantimicag.2020.106248_bib0012",
"volume": "17",
"year": "2003"
},
{
"DOI": "10.1056/NEJM198507183130301",
"article-title": "Comparison of ivermectin and diethylcarbamazine in the treatment of onchocerciasis",
"author": "Greene",
"doi-asserted-by": "crossref",
"first-page": "133",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijantimicag.2020.106248_bib0013",
"volume": "313",
"year": "1985"
},
{
"DOI": "10.2139/ssrn.3636557",
"article-title": "Ivermectin for COVID-19 treatment: clinical response at quasi-threshold doses via hypothesized alleviation of CD147-mediated vascular occlusion",
"author": "Scheim",
"doi-asserted-by": "crossref",
"journal-title": "SSRN",
"key": "10.1016/j.ijantimicag.2020.106248_bib0014",
"year": "2020"
},
{
"DOI": "10.2139/ssrn.3631261",
"article-title": "ICON (Ivermectin in COvid Nineteen) Study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID-19",
"author": "Rajter",
"doi-asserted-by": "crossref",
"journal-title": "SSRN",
"key": "10.1016/j.ijantimicag.2020.106248_bib0015",
"year": "2020"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"article-title": "a systematic review from antiviral effects to COVID-19 complementary regimen",
"author": "Heidary",
"doi-asserted-by": "crossref",
"first-page": "593",
"journal-title": "J Antibiot (Tokyo)",
"key": "10.1016/j.ijantimicag.2020.106248_bib0016",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1371/journal.pntd.0006020",
"article-title": "Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers",
"author": "Muñoz",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Negl Trop Dis",
"key": "10.1016/j.ijantimicag.2020.106248_bib0017",
"volume": "12",
"year": "2018"
},
{
"key": "10.1016/j.ijantimicag.2020.106248_bib0018",
"series-title": "PCT Databank",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1889",
"article-title": "The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19",
"author": "Schmith",
"doi-asserted-by": "crossref",
"first-page": "762",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.ijantimicag.2020.106248_bib0019",
"volume": "108",
"year": "2020"
},
{
"article-title": "Pharmacokinetics of ivermectin in animals and humans",
"author": "Fink",
"first-page": "113",
"key": "10.1016/j.ijantimicag.2020.106248_bib0020",
"series-title": "Ivermectin and abamectin",
"year": "1989"
},
{
"key": "10.1016/j.ijantimicag.2020.106248_bib0021",
"series-title": "COVID-19 and ivermectin intended for animals",
"year": "2020"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0924857920304684"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "A COVID-19 prophylaxis? Lower incidence associated with prophylactic administration of ivermectin",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "57"
}
