A Comparison of Ivermectin and Non Ivermectin Based Regimen for COVID-19 in Abuja: Effects on Virus Clearance, Days-to-discharge and Mortality
et al., Journal of Pharmaceutical Research International, doi:10.9734/jpri/2022/v34i44A36328, Feb 2022 (preprint)
Ivermectin for COVID-19
4th treatment shown to reduce risk in
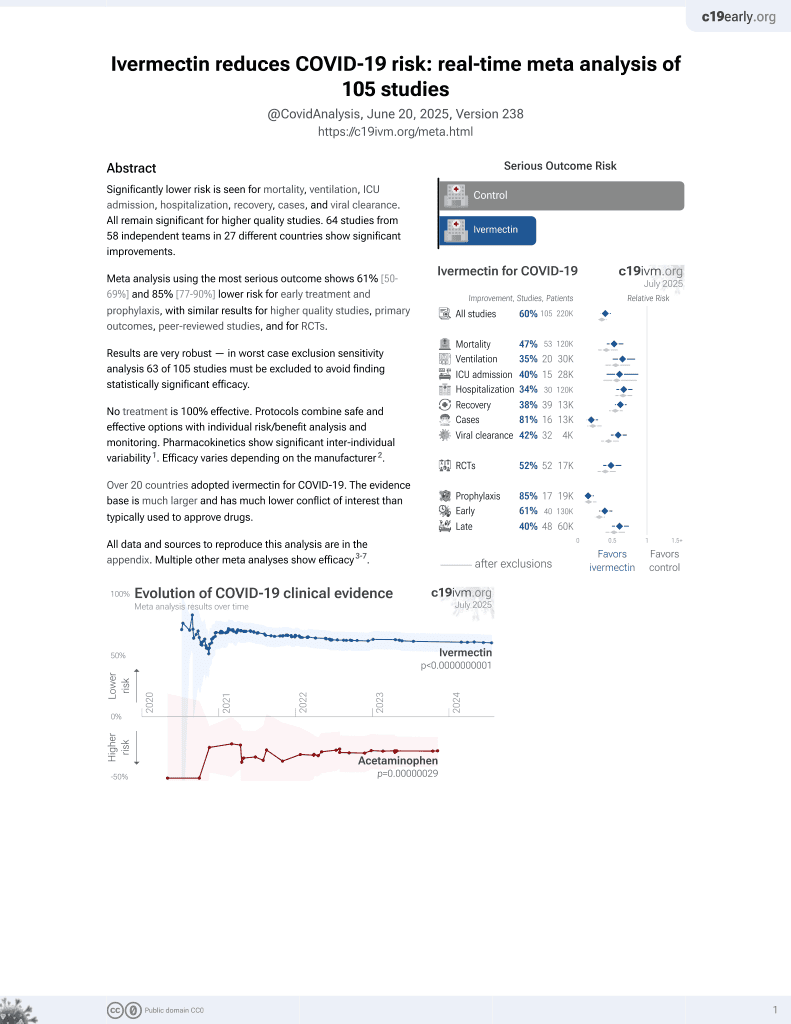
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PSM retrospective 87 patients in Nigeria, 61 treated with ivermectin, showing lower mortality, faster recovery, and faster viral clearance with ivermectin treatment. All patients received zinc and vitamin C. A synergistic effect was seen for viral clearance when ivermectin and remdesivir were combined, as predicted by in vitro research1. Subject to confounding by time, with ivermectin patients from April-June 2021, and non-ivermectin patients from September-November 2021.
This is the 79th of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
53 studies are RCTs, which show efficacy with p=0.000000087.
This study is excluded in the after exclusion results of meta-analysis:
significant confounding by time possible due to separation of groups in different time periods.
|
risk of death, 87.9% lower, RR 0.12, p = 0.12, treatment 0 of 21 (0.0%), control 4 of 26 (15.4%), NNT 6.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), propensity score matching.
|
|
risk of death, 93.0% lower, RR 0.07, p = 0.007, treatment 0 of 61 (0.0%), control 4 of 26 (15.4%), NNT 6.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), all patients.
|
|
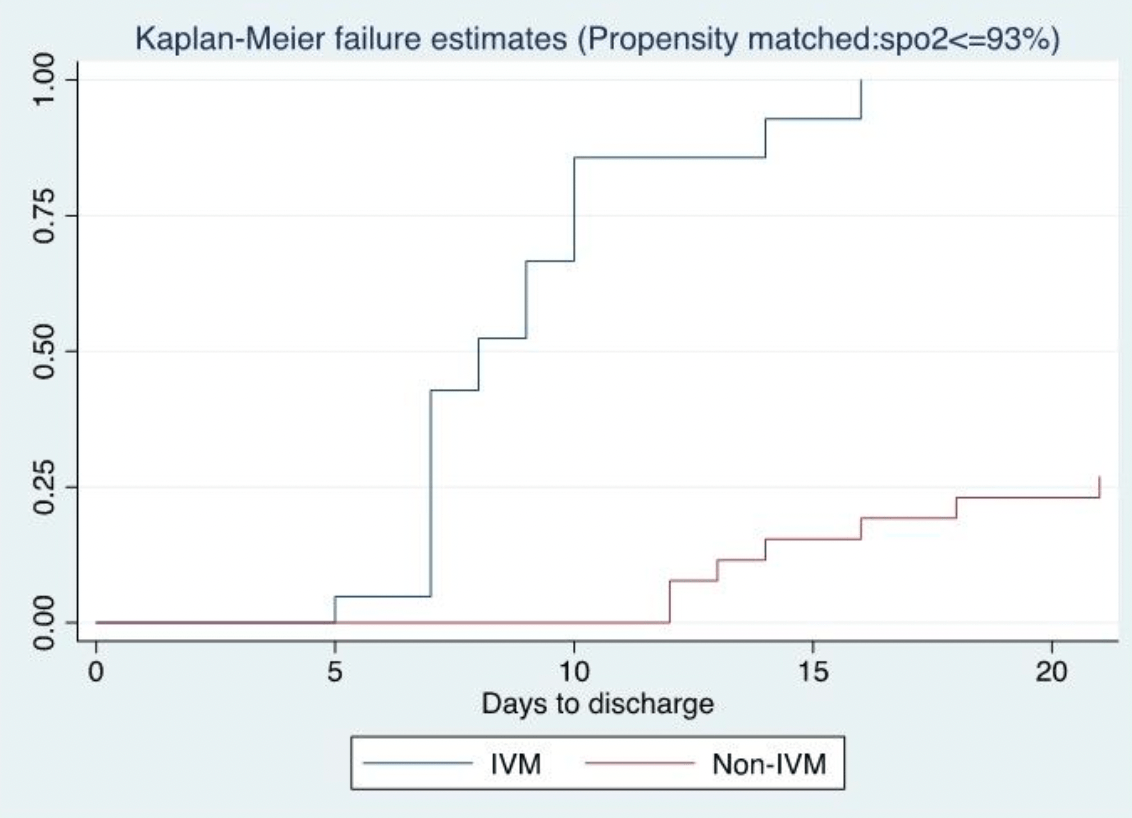
time to discharge, 54.6% lower, relative time 0.45, p < 0.001, treatment 61, control 26, propensity score matching.
|
|
risk of no viral clearance, 94.8% lower, RR 0.05, p = 0.001, treatment 0 of 21 (0.0%), control 10 of 26 (38.5%), NNT 2.6, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), propensity score matching, day 21.
|
|
risk of no viral clearance, 95.2% lower, RR 0.05, p < 0.001, treatment 1 of 21 (4.8%), control 26 of 26 (100.0%), NNT 1.1, propensity score matching, day 14.
|
|
risk of no viral clearance, 28.6% lower, RR 0.71, p = 0.005, treatment 15 of 21 (71.4%), control 26 of 26 (100.0%), NNT 3.5, propensity score matching, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Thairu et al., 25 Feb 2022, retrospective, Nigeria, peer-reviewed, mean age 41.7, 6 authors, study period April 2021 - November 2021, dosage 200μg/kg days 1-5.
Contact: bablo57@gmail.com.
A Comparison of Ivermectin and Non Ivermectin Based Regimen for COVID-19 in Abuja: Effects on Virus Clearance, Days-to-discharge and Mortality
Journal of Pharmaceutical Research International, doi:10.9734/jpri/2022/v34i44a36328
Aim: To compare outcomes from ivermectin (IVM) -and non-ivermectin (NIVM)-based treatments for COVID-19 in Abuja, Nigeria. Methods: Sixty-one consecutive virology-proven cases were recruited and managed with IVMbased regimes. A subsequent cohort of 26 patients was treated with NIVM due to physician preference, with varying combinations of lopinavir/ritonavir (Alluvia), remdesivir, azithromycin, and enoxapramin. All patients received zinc sulfate, vitamin C and supportive therapy. Propensity matching was carried out as indicated, and Repeat Measures Analysis of Variance (RMANOVA) allowing for time*treatment interaction was carried out for time dependent variables, deriving Likelihood Ratio (LR) and P values.
Original Research Article Main Outcome Measures: Change in cycle threshold (viral load) over time, positivity status by day 5, improvement in clinical status using myalgia scores, days to discharge (DTD), change in SpO2 and death. Results: IVM was associated with a greater and faster reduction in viral clearance (LR=64.2 p< 0.0001 for the N gene): 31% and 95% were negative by days 5 and 14, respectively, versus 0% on NIVM. The mean DTD on IVM was 8.8 days versus 19.4 days, p< 0.0001. IVM proved significantly superior for Myalgia scores, LR= 23.45, P=0.0007. The mortality rate was 0/61 (0%) in IVM but 4/26 (15.3%) in NIVM. Three of the 4 deaths were in females, and 2 had been vaccinated, one fully. The SP02% increased significantly more on IVM (p < 0.0001 RMANOVA) than the NIVM group. C-reactive protein and D-dimer levels dropped significantly more sharply during IVM (P= 0.0068, 0.063), suggesting anti-inflammatory and antifibrinolytic activity. Conclusions: The IVM-based regimen caused earlier discharge from treatment and reduced mortality, in addition to clinical and laboratory improvements. Vaccination did not protect some patients from SARS-CoV-2 breakthrough infection and mortality.
ETHICS APPROVAL AND CONSENT The Project was approved by the University of Abja Teaching Hospital Human Research Ethics Committee. The Approval number was UATH/HREC/PR/2020/015/10. Consent to participate was obtained from each individual patient using a standard consent form in which the project was explained.
COMPETING INTERESTS Authors have declared that no competing interests exist.
References
Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of Ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Arshad, Pertinez, Box, Prioritization of Anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther, doi:10.1002/cpt.1909
Babalola, Bode, Ajayi, Alakaloko, Akase et al., A Randomized Controlled Trial of Ivermectin Monotherapy versus Hydroxychloroquine, Ivermectin, and Azithromycin Combination Therapy in COVID-19 Patients in Nigeria, J Infect Dis Epidemiol, doi:.org/10.23937/2474-3658/1510233
Bobrowski, Chen, Eastman, Synergistic and Antagonistic Drug Combinations against SARS-CoV-2, Mol Ther, doi:10.1016/j.ymthe.2020.12.016
Bryant, Lawrie, Dowswell, Fordham, Mitchell, Ivermectin for prevention and treatment of COVID-19 infection: A systematic review, metaanalysis, and trial sequential analysis to inform clinical guidelines, Am J Ther
Budhiraja, Soni, Jha, Clinical Profile of First 1000 COVID-19 Cases Admitted at Tertiary Care Hospitals and the Correlates of their Mortality: An Indian Experience, doi:10.1101/2020.11.16.20232223v1
Buonfrate, Chesini, Martini, High-dose Ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2021.106516
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research
Chahla, Ruiz, Mena, Cluster Randomised Trials -Ivermectin Repurposing For COVID-19 Treatment of Outpatients With Mild Disease In Primary Health Care Centers, doi:10.21203/rs.3.rs-495945/v1
Chandrima, Evaluation of Ivermectin as a Potential Treatment for Mild to Moderate COVID-19: A Double-Blind Randomized Placebo Controlled Trial in Eastern India, Journal of Pharmacy and Pharmaceutical Sciences, doi:10.18433/jpps32105
Dinicolantonio, Barroso, Mccarty, Ivermectin may be a clinically useful antiinflammatory agent for late-stage COVID-19 [published correction appears, Open Heart
Elalfy, Besheer, El-Mesery, Effect of a combination of nitazoxanide, ribavirin, and Ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19, J Med Virol, doi:10.1002/jmv.26880
Hay, Arnott, Ivermectin and coagulation: an in vitro study, Ann Trop Med Parasitol
Kory, Gianfranco, Varon, Iglesias, Marik, Review of the emerging evidence demonstrating the efficacy of Ivermectin in the prophylaxis and treatment of COVID-19, Am J Ther
Lehrer, Rheinstein, Ivermectin Docks to the SARS-CoV-2 Spike Receptorbinding Domain Attached to ACE2, Vivo, doi:10.21873/invivo.12134
Mahmud, Rahman, Alam, Ivermectin in combination with doxycycline for treating COVID-19 symptoms: A randomized trial, Journal of International Medical Research, doi:10.1177/03000605211013550
Maiada, Hashem Clinicaltrials, None
Maragakis, Kelen, Breakthrough infections: Coronavirus after vaccination
Matsuyama, Kubli, Yoshinaga, An aberrant STAT pathway is central to COVID-19, Cell Death Differ, doi:10.1038/s41418-020-00633-7
Mohan, Tiwari, Suri, Singledose oral Ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial, J Infect Chemother, doi:10.1016/j.jiac.2021.08.021
Omura, Crump, Ivermectin: panacea for resource-poor communities?, Trends Parasitol, doi:10.1016/j.pt.2014.07.005
Rajter, Sherman, Fatteh, Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID-19 (ICON study), doi:10.1016/j.chest.2020.10.009
Ravi, Ranjini, Pattadar
Richards, Mcneeley, Bryan, Ivermectin and prothrombin time, The Lancet
Seth, Mas, Conod, Mueller, Siems et al., Long-Lasting WNT-TCF response blocking and epigenetic modifying activities of withanolide f in human cancer cells, PLoS One
Stone, Ndarukwa, Scheim, Rapid increase od SpO2 on room air for 34 severe COVID-19 patients after Ivermectin-based combination treatement, doi:10.21203/rs.3.rs-1048271/v1
Thakur, Bhola, Thakur, Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe, Infection, doi:10.1007/s15010-021-01734-2
Wagstaff, Ivermectin Global Summit
Whitworth, Hay, Mcnicholas, Morgan, Maude et al., Coagulation abnormalities and Ivermectin, Ann Trop Med Parasitol
Yan, Ci, Chen, Anti-Inflammatory effects of Ivermectin in mouse model of allergic asthma, Inflamm Res
Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral Ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antivir Res
Zhang, Song, Ci, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPSinduced survival in mice, Inflamm Res, doi:10.1007/s00011-008-8007-8
DOI record:
{
"DOI": "10.9734/jpri/2022/v34i44a36328",
"ISSN": [
"2456-9119"
],
"URL": "http://dx.doi.org/10.9734/jpri/2022/v34i44A36328",
"abstract": "<jats:p>Aim: To compare outcomes from ivermectin (IVM) - and non-ivermectin (NIVM)-based treatments for COVID-19 in Abuja, Nigeria.
\nMethods: Sixty-one consecutive virology-proven cases were recruited and managed with IVM-based regimes. A subsequent cohort of 26 patients was treated with NIVM due to physician preference, with varying combinations of lopinavir/ritonavir (Alluvia), remdesivir, azithromycin, and enoxapramin. All patients received zinc sulfate, vitamin C and supportive therapy. Propensity matching was carried out as indicated, and Repeat Measures Analysis of Variance (RMANOVA) allowing for time*treatment interaction was carried out for time dependent variables, deriving Likelihood Ratio (LR) and P values.
\nMain Outcome Measures: Change in cycle threshold (viral load) over time, positivity status by day 5, improvement in clinical status using myalgia scores, days to discharge (DTD), change in SpO2 and death.
\nResults: IVM was associated with a greater and faster reduction in viral clearance (LR=64.2 p< 0.0001 for the N gene): 31% and 95% were negative by days 5 and 14, respectively, versus 0% on NIVM. The mean DTD on IVM was 8.8 days versus 19.4 days, p< 0.0001. IVM proved significantly superior for Myalgia scores, LR= 23.45, P=0.0007. The mortality rate was 0/61 (0%) in IVM but 4/26 (15.3%) in NIVM. Three of the 4 deaths were in females, and 2 had been vaccinated, one fully. The SP02% increased significantly more on IVM (p < 0.0001 RMANOVA) than the NIVM group. C-reactive protein and D-dimer levels dropped significantly more sharply during IVM (P= 0.0068, 0.063), suggesting anti-inflammatory and antifibrinolytic activity.
\nConclusions: The IVM-based regimen caused earlier discharge from treatment and reduced mortality, in addition to clinical and laboratory improvements. Vaccination did not protect some patients from SARS-CoV-2 breakthrough infection and mortality.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Thairu",
"given": "Y.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Babalola",
"given": "O. E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ajayi",
"given": "A. A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ndanusa",
"given": "Y.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ogedengbe",
"given": "J. O.",
"sequence": "first"
},
{
"affiliation": [],
"family": "O.",
"given": "Omede",
"sequence": "first"
}
],
"container-title": "Journal of Pharmaceutical Research International",
"container-title-short": "JPRI",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
11
]
],
"date-time": "2022-07-11T04:33:25Z",
"timestamp": 1657514005000
},
"deposited": {
"date-parts": [
[
2022,
7,
11
]
],
"date-time": "2022-07-11T04:33:25Z",
"timestamp": 1657514005000
},
"indexed": {
"date-parts": [
[
2022,
9,
1
]
],
"date-time": "2022-09-01T04:55:38Z",
"timestamp": 1662008138842
},
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2022,
7,
8
]
]
},
"link": [
{
"URL": "https://journaljpri.com/index.php/JPRI/article/download/36328/68696",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journaljpri.com/index.php/JPRI/article/download/36328/68697",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journaljpri.com/index.php/JPRI/article/download/36328/68696",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4694",
"original-title": [],
"page": "1-19",
"prefix": "10.9734",
"published": {
"date-parts": [
[
2022,
7,
8
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
8
]
]
},
"publisher": "Sciencedomain International",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journaljpri.com/index.php/JPRI/article/view/36328"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "A Comparison of Ivermectin and Non Ivermectin Based Regimen for COVID-19 in Abuja: Effects on Virus Clearance, Days-to-discharge and Mortality",
"type": "journal-article"
}