Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score Matched Study
et al., Infection & Chemotherapy, doi:10.3947/ic.2023.0087, Nov 2023
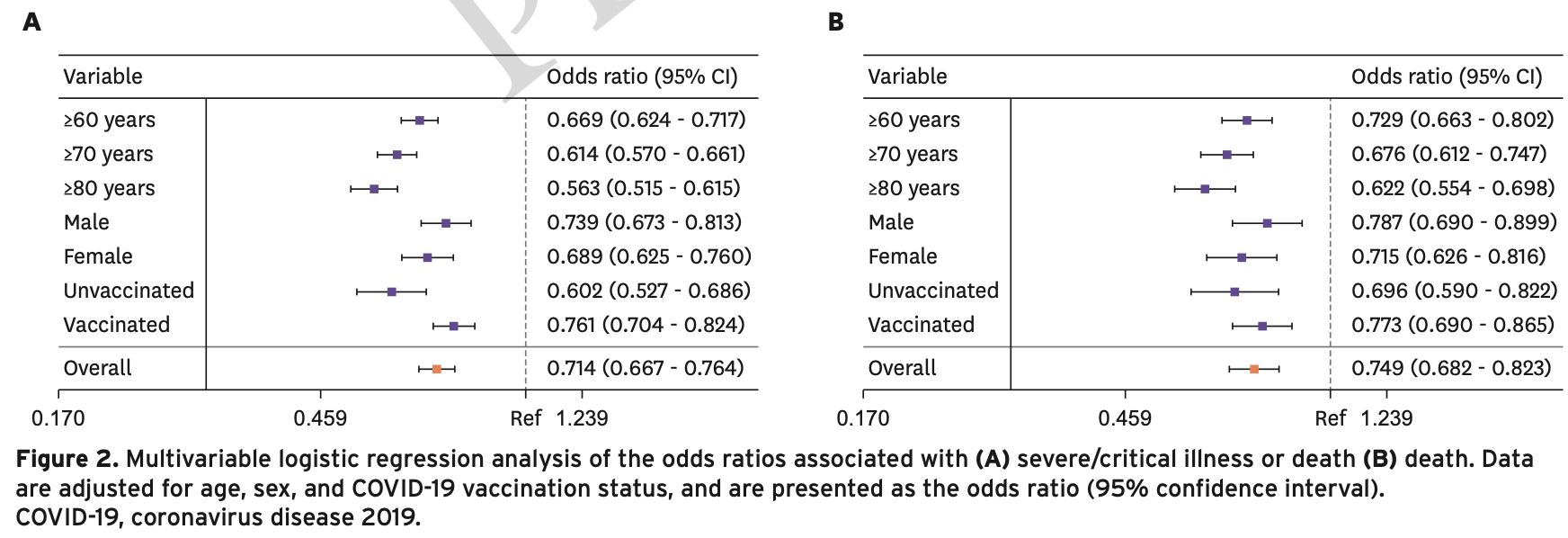
PSM retrospective 190,692 COVID-19 patients treated with molnupiravir and 762,768 matched controls, showing lower mortality and combined severe/critical illness and mortality with treatment.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending molnupiravir also recommended them, or
because the patient seeking out molnupiravir is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity4-18. Multiple analyses have identified variants potentially created by molnupiravir19-23. Studies show significantly increased risk of acute kidney injury24, cardiovascular toxocity25, and neurological symptoms24. Treatment may increase viral rebound26,27.
|
risk of death, 25.1% lower, OR 0.75, p < 0.001, RR approximated with OR.
|
|
risk of progression, 28.6% lower, OR 0.71, p < 0.001, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
5.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
6.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
7.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
8.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
9.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
10.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
11.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
12.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
13.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
14.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
15.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
16.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
17.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
18.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
19.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
20.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
21.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
22.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
24.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
25.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Park et al., 22 Nov 2023, retrospective, South Korea, peer-reviewed, 6 authors, study period 1 August, 2022 - 31 March, 2023.
Contact: erijykim@korea.kr.
Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score Matched Study
Infection & Chemotherapy, doi:10.3947/ic.2023.0087
Background: The MOVe-OUT (efficacy and safety of molnupiravir in non-hospitalized adult participants with ) trial reported that the administration of molnupiravir in unvaccinated patients with coronavirus disease 2019 (COVID-19) before the Omicron epidemic showed a preventive effect of 31% against hospitalization and death. However, studies on the preventive effect of molnupiravir against progression to severe disease and death in patients with COVID-19 during the Omicron epidemic are limited. This study aimed to evaluate the preventive effect of molnupiravir against severe/critical illness or death and death in Korean patients with COVID-19 who were vaccinated mostly during the Omicron epidemic.
Materials and Methods: This study used large-scale retrospective cohort data to select patients with COVID-19 who were either treated or not treated with molnupiravir, between August 2022 and March 2023, at a ratio of 1 : 4 using the propensity score matching method. In total, 762,768 patients comprised the non-administered group, and 190,692 patients comprised the molnupiravir-administered group. The preventive effect of molnupiravir against severe/ critical illness or death and death was analyzed using logistic regression analysis. Results: The preventive effect of molnupiravir against severe/critical illness or death and death, represented by the odds ratio (OR) and 95% confidence interval (CI), in the molnupiravir-administered and non-administered group was (OR: 0.714; CI: 0.667 -0.764) and (OR: 0.749; CI: 0.682 -0.823), respectively. As age increased, the preventive effect against severe/critical illness or death and death increased. The preventive effect against severe/critical illness or death at ≥60 years was (OR: 0.
Conflict of interest No conflict of interest.
Author Contributions
References
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Bajema, Berry, Streja, Rajeevan, Li et al., Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: Target trial emulation studies with one-month and six-month outcomes, Ann Intern Med, doi:10.7326/M22-3565
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Williams-8/10 icjournal
Bestetti, Furlan-Daniel, Silva, Pharmacological Treatment of Patients with Mild to Moderate COVID-19: a comprehensive review, Int J Environ Res Public Health, doi:10.3390/ijerph18137212
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an openlabel, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Diaz, Brown, Du, Pedley, Assaid et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Fiolet, Kherabi, Macdonald, Ghosn, Peiffer-Smadja, Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.10.005
Gandhi, Lynch, Rio, Mild or moderate Covid-19, N Engl J Med, doi:10.1056/NEJMcp2009249
Guan, Liang, Zhao, Liang, Chen et al., China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis, Eur Respir J, doi:10.1183/13993003.00547-2020
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hoertel, Boulware, Sánchez-Rico, Burgun, Limosin, Prevalence of contraindications to nirmatrelvir-ritonavir among hospitalized patients with COVID-19 at risk for progression to severe disease, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.42140
Ito, Piantham, Nishiura, Relative instantaneous reproduction number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark, J Med Virol, doi:10.1002/jmv.27560
Kim, Yoo, Bae, Kim, Lee, Effectiveness of paxlovid, an oral antiviral drug, against the Omicron BA.5 variant in Korea: severe progression and death between July and November 2022, J Korean Med Sci, doi:10.3346/jkms.2023.38.e211
Lee, Hsieh, Ko, Molnupiravir-A novel oral anti-SARS-CoV-2 agent, Antibiotics, doi:10.3390/antibiotics10111294
Lv, Lv, Clinical characteristics and analysis of risk factors for disease progression of COVID-19: a retrospective Cohort Study, Int J Biol Sci, doi:10.7150/ijbs.50654
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of molnupiravir in highrisk patients: a propensity score matched analysis, Clin Infect Dis, doi:10.1093/cid/ciac781
Painter, Holman, Bush, Almazedi, Malik et al., Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob Agents Chemother, doi:10.1128/AAC.02428-20
Paraskevis, Gkova, Mellou, Gerolymatos, Psalida et al., Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in high-risk patients, J Infect Dis
Park, Park, Lee, Yu, Song et al., The effectiveness of Paxlovid treatment in long-term care facilities in South Korea during the outbreak of the Omicron variant of SARS-CoV-2, Osong Public Health Res Perspect, doi:10.24171/j.phrp.2022.0262
Rosenbaum, Db, The central role of the propensity score in observational studies for causal effects, Biometrika
Ross, Bortolussi-Courval, Hanula, Lee, Wilson et al., Drug interactions with nirmatrelvirritonavir in older adults using multiple medications, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.20184
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant, doi:10.1016/j.healun.2020.03.012
Soy, Keser, Atagündüz, Pathogenesis and treatment of cytokine storm in COVID-19, Turk J Biol, doi:10.3906/biy-2105-37
Suzuki, Shibata, Minemura, Nikaido, Tanino et al., Real-world clinical outcomes of treatment with molnupiravir for patients with mild-to-moderate coronavirus disease 2019 during the Omicron variant pandemic, Clin Exp Med, doi:10.1007/s10238-022-00949-3
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant, N Engl J Med, doi:10.1056/NEJMc2119407
Takashita, Yamayoshi, Simon, Van Bakel, Sordillo et al., Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants, N Engl J Med, doi:10.1056/NEJMc2207519
Watson, Barnsley, Toor, Hogan, Winskill et al., Global impact of the first year of COVID-19 vaccination: a mathematical modelling study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00320-6
Wen, Chen, Tang, Wang, Zhou et al., Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis, Ann Med, doi:10.1080/07853890.2022.2034936
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00507-2
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Wu, Chen, Cai, Xia, Zhou et al., Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med, doi:10.1001/jamainternmed.2020.0994
Xie, Bowe, Al-Aly, Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records, BMJ, doi:10.1136/bmj-2022-072705
Yang, Zheng, Gou, Pu, Chen et al., Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis, Int J Infect Dis, doi:10.1016/j.ijid.2020.03.017
Ying-Hao, Yuan-Yuan, Dong, Qiu-Hua, Xue-Ran et al., Clinical characteristics and analysis of risk factors for disease progression of patients with SARS-CoV-2 omicron variant infection: a retrospective study of 25207 cases in a Fangcang hospital, Front Cell Infect Microbiol, doi:10.3389/fcimb.2022.1009894
DOI record:
{
"DOI": "10.3947/ic.2023.0087",
"ISSN": [
"2093-2340",
"2092-6448"
],
"URL": "http://dx.doi.org/10.3947/ic.2023.0087",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"value": "2023-09-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"value": "2023-11-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published online",
"name": "published_online",
"value": "2023-11-22"
},
{
"group": {
"label": "Copyright and Licensing",
"name": "Copyright_and_licensing"
},
"label": "Copyright",
"name": "copyright",
"value": "Copyright © 2023 by The Korean Society of Infectious Diseases, Korean Society for Antimicrobial Therapy, and The Korean Society for AIDS"
},
{
"explanation": {
"URL": "https://creativecommons.org/licenses/by-nc/4.0/"
},
"group": {
"label": "Copyright and Licensing",
"name": "Copyright_and_licensing"
},
"label": "License",
"name": "license",
"value": "This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8719-9387",
"affiliation": [
{
"name": "Patient Management Team, Central Disease Control Headquarters for COVID-19, Korea Disease Control and Prevention Agency, Cheongju, Korea."
}
],
"authenticated-orcid": false,
"family": "Park",
"given": "Hye Rim",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8153-6813",
"affiliation": [
{
"name": "Patient Management Team, Central Disease Control Headquarters for COVID-19, Korea Disease Control and Prevention Agency, Cheongju, Korea."
},
{
"name": "Division of Public Health Emergency Response Research, Bureau of Public Health Emergency Preparedness, Korea Disease Control and Prevention Agency, Cheongju, Korea."
}
],
"authenticated-orcid": false,
"family": "Yoo",
"given": "Min-Gyu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5840-6965",
"affiliation": [
{
"name": "Patient Management Team, Central Disease Control Headquarters for COVID-19, Korea Disease Control and Prevention Agency, Cheongju, Korea."
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Jong Mu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9031-4825",
"affiliation": [
{
"name": "Patient Management Team, Central Disease Control Headquarters for COVID-19, Korea Disease Control and Prevention Agency, Cheongju, Korea."
}
],
"authenticated-orcid": false,
"family": "Bae",
"given": "Soon Jong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3478-3704",
"affiliation": [
{
"name": "Patient Management Team, Central Disease Control Headquarters for COVID-19, Korea Disease Control and Prevention Agency, Cheongju, Korea."
},
{
"name": "Division of Emerging Infectious Disease, Bureau of Infectious Disease Risk Response, Korea Disease Control and Prevention Agency, Cheongju, Korea."
},
{
"name": "Division of Vaccine-Prevetable Diseases Control and National Immunization Program, Korea Disease Control and Prevention Agency, Cheongju, Korea."
}
],
"authenticated-orcid": false,
"family": "Lee",
"given": "Hyungmin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2732-1544",
"affiliation": [
{
"name": "Patient Management Team, Central Disease Control Headquarters for COVID-19, Korea Disease Control and Prevention Agency, Cheongju, Korea."
},
{
"name": "Division of Emerging Infectious Disease, Bureau of Infectious Disease Risk Response, Korea Disease Control and Prevention Agency, Cheongju, Korea."
},
{
"name": "Division of Clinical Research, Center for Emerging Virus Research, Natinal Institute of Infectious Disease, Korea Disease Control and Prevention Agency, Cheongju, Korea."
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Jungyeon",
"sequence": "additional"
}
],
"container-title": "Infection & Chemotherapy",
"container-title-short": "Infect Chemother",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"icjournal.org"
]
},
"created": {
"date-parts": [
[
2023,
11,
23
]
],
"date-time": "2023-11-23T01:52:36Z",
"timestamp": 1700704356000
},
"deposited": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T04:34:30Z",
"timestamp": 1701146070000
},
"indexed": {
"date-parts": [
[
2023,
11,
29
]
],
"date-time": "2023-11-29T00:23:14Z",
"timestamp": 1701217394293
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
}
],
"link": [
{
"URL": "https://icjournal.org/pdf/10.3947/ic.2023.0087",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://icjournal.org/DOIx.php?id=10.3947/ic.2023.0087",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://icjournal.org/DOIx.php?id=10.3947/ic.2023.0087",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "18617",
"original-title": [],
"prefix": "10.3947",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"publisher": "XMLink",
"reference": [
{
"key": "10.3947/ic.2023.0087_ref1",
"unstructured": "World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard. Accessed 24 May 2023. Available at: https://covid19.who.int"
},
{
"key": "10.3947/ic.2023.0087_ref2",
"unstructured": "Korea Disease Control and Prevention Agency (KDCA). Coronavirus disease 19 (COVID-19). Accessed 27 May 2023. Available at: https://ncov.kdca.go.kr"
},
{
"DOI": "10.1183/13993003.00547-2020",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "2000547",
"journal-title": "Eur Respir J",
"key": "10.3947/ic.2023.0087_ref3",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.03.017",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Int J Infect Dis",
"key": "10.3947/ic.2023.0087_ref4",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "934",
"journal-title": "JAMA Intern Med",
"key": "10.3947/ic.2023.0087_ref5",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1002/jmv.27560",
"author": "Ito",
"doi-asserted-by": "crossref",
"first-page": "2265",
"journal-title": "J Med Virol",
"key": "10.3947/ic.2023.0087_ref6",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.3390/ijerph18137212",
"author": "Bestetti",
"doi-asserted-by": "crossref",
"first-page": "7212",
"journal-title": "Int J Environ Res Public Health",
"key": "10.3947/ic.2023.0087_ref7",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"author": "Siddiqi",
"doi-asserted-by": "crossref",
"first-page": "405",
"journal-title": "J Heart Lung Transplant",
"key": "10.3947/ic.2023.0087_ref8",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1056/NEJMcp2009249",
"author": "Gandhi",
"doi-asserted-by": "crossref",
"first-page": "1757",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2023.0087_ref9",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.7150/ijbs.50654",
"author": "Lv",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Int J Biol Sci",
"key": "10.3947/ic.2023.0087_ref10",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.3389/fcimb.2022.1009894",
"author": "Ying-Hao",
"doi-asserted-by": "crossref",
"first-page": "1009894",
"journal-title": "Front Cell Infect Microbiol",
"key": "10.3947/ic.2023.0087_ref11",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3906/biy-2105-37",
"author": "Soy",
"doi-asserted-by": "crossref",
"first-page": "372",
"journal-title": "Turk J Biol",
"key": "10.3947/ic.2023.0087_ref12",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"author": "Wen",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Ann Med",
"key": "10.3947/ic.2023.0087_ref13",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2023.0087_ref14",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2023.0087_ref15",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3390/antibiotics10111294",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "1294",
"journal-title": "Antibiotics (Basel)",
"key": "10.3947/ic.2023.0087_ref16",
"volume": "10",
"year": "2021"
},
{
"author": "Painter",
"first-page": "e02428",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.3947/ic.2023.0087_ref17",
"volume": "65",
"year": "2021"
},
{
"key": "10.3947/ic.2023.0087_ref18",
"unstructured": "US Food and Drug Administration (FDA). Fact sheet for healthcare providers: Emergency use authorization for Lagevrio™ (molnupiravir) capsules. Accessed 1 February 2023. Available at: https://www.fda.gov/media/155054/download"
},
{
"key": "10.3947/ic.2023.0087_ref19",
"unstructured": "Korea Disease Control and Prevention Agency (KDCA). Guide to use of coronavirus infectious diseases-19 treatment in South Korea (edition 11). Accessed 1 September 2023. Available at: https://ncov.kdca.go.kr/extendedCareBoardList.jsp?brdId=8&brdGubun=81"
},
{
"DOI": "10.1056/NEJMc2119407",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2023.0087_ref20",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2207519",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "468",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2023.0087_ref21",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"author": "Arbel",
"doi-asserted-by": "crossref",
"first-page": "790",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2023.0087_ref22",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Lancet",
"key": "10.3947/ic.2023.0087_ref23",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac781",
"author": "Najjar-Debbiny",
"doi-asserted-by": "crossref",
"first-page": "453",
"journal-title": "Clin Infect Dis",
"key": "10.3947/ic.2023.0087_ref24",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1681",
"journal-title": "Lancet Infect Dis",
"key": "10.3947/ic.2023.0087_ref25",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1213",
"journal-title": "Lancet",
"key": "10.3947/ic.2023.0087_ref26",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.7326/M22-3565",
"author": "Bajema",
"doi-asserted-by": "crossref",
"first-page": "807",
"journal-title": "Ann Intern Med",
"key": "10.3947/ic.2023.0087_ref27",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1136/bmj-2022-072705",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "e072705",
"journal-title": "BMJ",
"key": "10.3947/ic.2023.0087_ref28",
"volume": "380",
"year": "2023"
},
{
"DOI": "10.1007/s10238-022-00949-3",
"author": "Suzuki",
"doi-asserted-by": "crossref",
"first-page": "2715",
"journal-title": "Clin Exp Med",
"key": "10.3947/ic.2023.0087_ref29",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.24171/j.phrp.2022.0262",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "443",
"journal-title": "Osong Public Health Res Perspect",
"key": "10.3947/ic.2023.0087_ref30",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1093/biomet/70.1.41",
"author": "Rosenbaum",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Biometrika",
"key": "10.3947/ic.2023.0087_ref31",
"volume": "70",
"year": "1983"
},
{
"key": "10.3947/ic.2023.0087_ref32",
"unstructured": "Korea Disease Control and Prevention Agency (KDCA). Coronavirus disease 2019 response and management guidelines for local governments in South Korea (edition 13-1). Accessed 22 August 2023. Available at: https://ncov.kdca.go.kr/duBoardList.do?brdId=2&brdGubun=28"
},
{
"DOI": "10.1016/j.cmi.2021.10.005",
"author": "Fiolet",
"doi-asserted-by": "crossref",
"first-page": "202",
"journal-title": "Clin Microbiol Infect",
"key": "10.3947/ic.2023.0087_ref33",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00320-6",
"author": "Watson",
"doi-asserted-by": "crossref",
"first-page": "1293",
"journal-title": "Lancet Infect Dis",
"key": "10.3947/ic.2023.0087_ref34",
"volume": "22",
"year": "2022"
},
{
"key": "10.3947/ic.2023.0087_ref35",
"unstructured": "Our world in Data. Share of people who received at least one dose of COVID-19 vaccine. Accessed 1 February 2023. Available at: https://ourworldindata.org/grapher/share-people-vaccinated-covid"
},
{
"DOI": "10.1001/jamanetworkopen.2022.42140",
"author": "Hoertel",
"doi-asserted-by": "crossref",
"first-page": "e2242140",
"journal-title": "JAMA Netw Open",
"key": "10.3947/ic.2023.0087_ref36",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.20184",
"author": "Ross",
"doi-asserted-by": "crossref",
"first-page": "e2220184",
"journal-title": "JAMA Netw Open",
"key": "10.3947/ic.2023.0087_ref37",
"volume": "5",
"year": "2022"
},
{
"key": "10.3947/ic.2023.0087_ref38",
"unstructured": "Korea Disease Control and Prevention Agency (KDCA). Press reference data. Accessed 29 December 2022. Available at: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=721541&cg_code=&act=view&nPage=16"
},
{
"DOI": "10.3346/jkms.2023.38.e211",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "e211",
"journal-title": "J Korean Med Sci",
"key": "10.3947/ic.2023.0087_ref39",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1093/infdis/jiad324",
"author": "Paraskevis",
"doi-asserted-by": "crossref",
"first-page": "jiad324",
"journal-title": "J Infect Dis",
"key": "10.3947/ic.2023.0087_ref40",
"year": "2023"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://icjournal.org/DOIx.php?id=10.3947/ic.2023.0087"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases"
],
"subtitle": [],
"title": "Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score Matched Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3947/crossmark_policy",
"volume": "55"
}