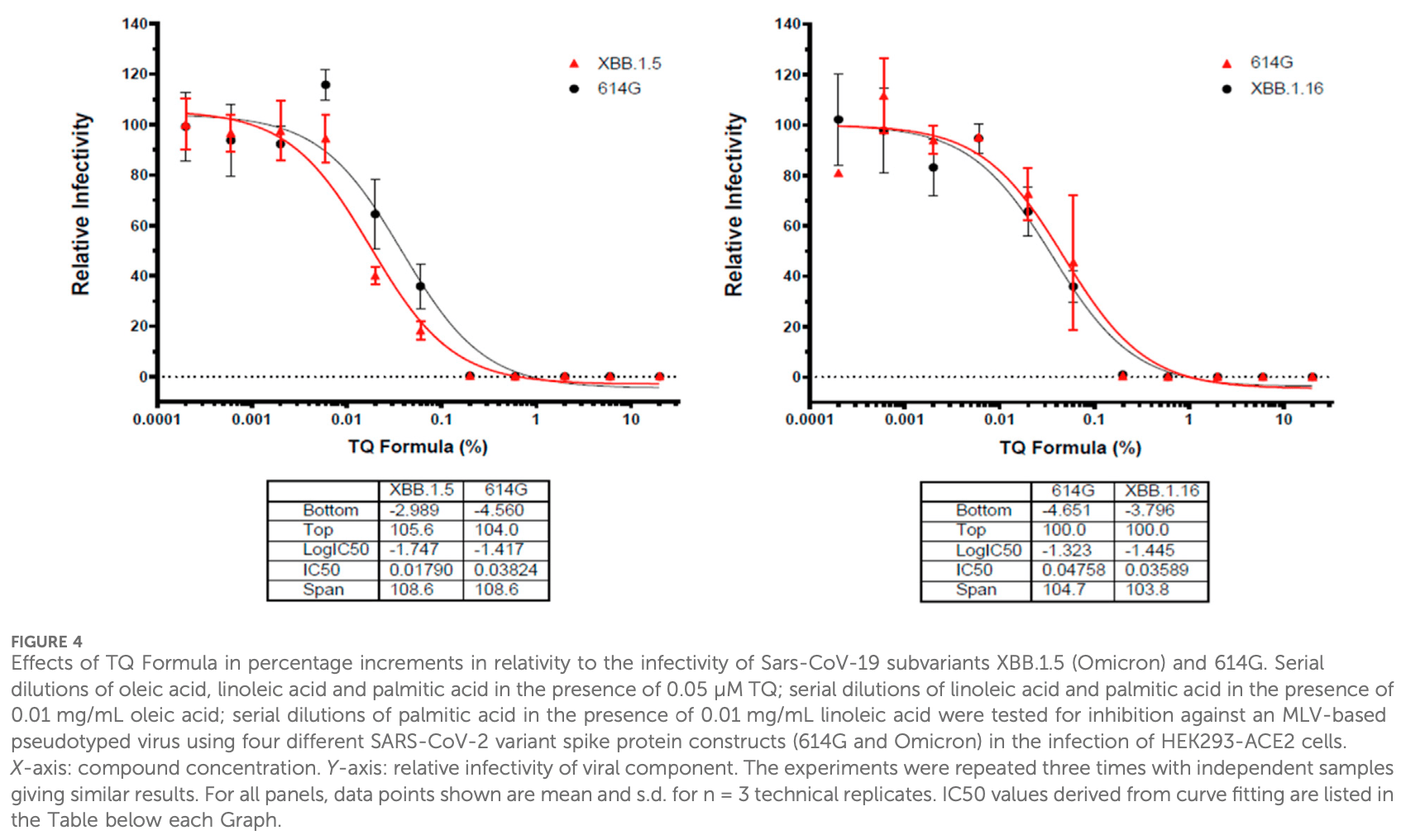
Individual ingredients of NP-101 (Thymoquinone formula) inhibit SARS-CoV-2 pseudovirus infection
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2024.1291212, Feb 2024
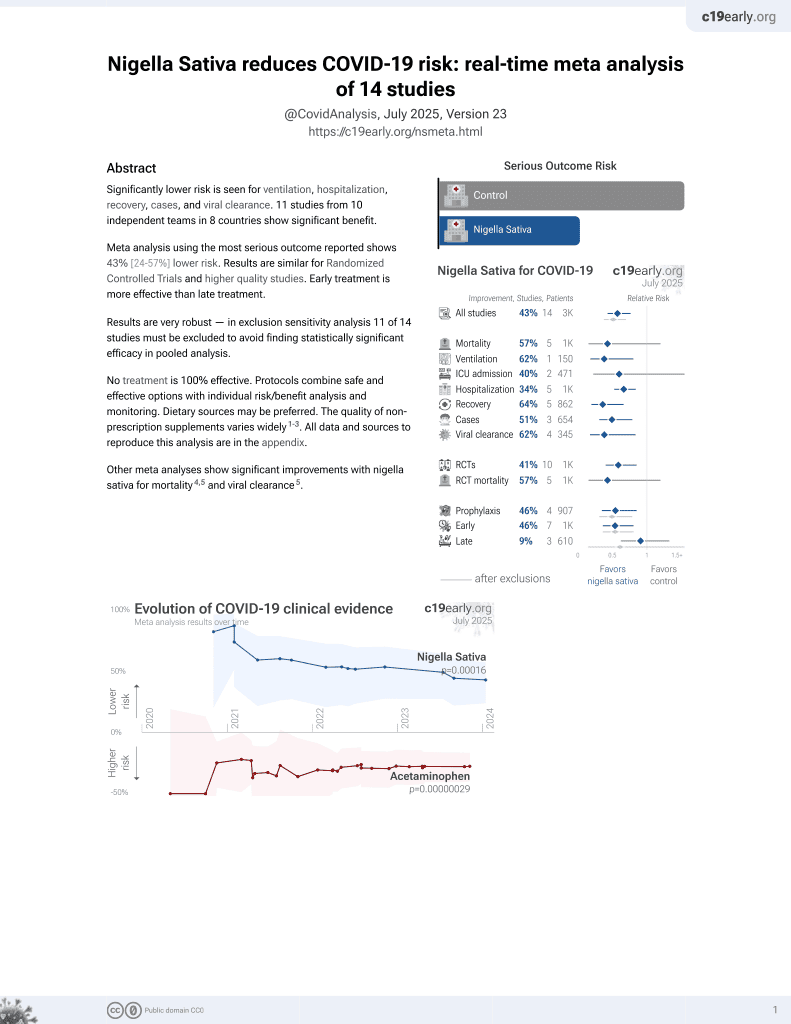
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing that components of thymoquinone formulation NP-101, including thymoquinone, oleic acid, linoleic acid, and palmitic acid, showed dose-dependent inhibition of SARS-CoV-2 variants in a pseudovirus model. Combinations of TQ and fatty acids also inhibited variants, with palmitic acid showing increased potency. A large randomized trial is planned to validate the potential of TQF for COVID-19 treatment. The results suggest thymoquinone and fatty acids in NP-101 could provide therapeutic benefits, likely by blocking viral entry via the ACE2 receptor.
22 preclinical studies support the efficacy of nigella sativa for COVID-19:
1.
Rahman et al., In Silico Screening of Potential Drug Candidate against Chain a of Coronavirus Binding Protein from Major Nigella Bioactive Compounds, Asian Journal of Advanced Research and Reports, doi:10.9734/ajarr/2024/v18i7697.
2.
Zafar Nayak Snehasis, S., Molecular Docking to Discover Potential Bio-Extract Substitutes for Hydroxychloroquine against COVID-19 and Malaria, International Journal of Science and Research (IJSR), doi:10.21275/SR24323192940.
3.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
4.
Ali et al., Computational Prediction of Nigella sativa Compounds as Potential Drug Agents for Targeting Spike Protein of SARS-CoV-2, Pakistan BioMedical Journal, doi:10.54393/pbmj.v6i3.853.
5.
Miraz et al., Nigelladine A among Selected Compounds from Nigella sativa Exhibits Propitious Interaction with Omicron Variant of SARS-CoV-2: An In Silico Study, International Journal of Clinical Practice, doi:10.1155/2023/9917306.
6.
Sherwani et al., Pharmacological Profile of Nigella sativa Seeds in Combating COVID-19 through In-Vitro and Molecular Docking Studies, Processes, doi:10.3390/pr10071346.
7.
Khan et al., Inhibitory effect of thymoquinone from Nigella sativa against SARS-CoV-2 main protease. An in-silico study, Brazilian Journal of Biology, doi:10.1590/1519-6984.25066.
8.
Esharkawy et al., In vitro Potential Antiviral SARS-CoV-19- Activity of Natural Product Thymohydroquinone and Dithymoquinone from Nigela sativia, Bioorganic Chemistry, doi:10.1016/j.bioorg.2021.105587.
9.
Banerjee et al., Nigellidine (Nigella sativa, black-cumin seed) docking to SARS CoV-2 nsp3 and host inflammatory proteins may inhibit viral replication/transcription, Natural Product Research, doi:10.1080/14786419.2021.2018430.
10.
Rizvi et al., Identifying the Most Potent Dual-Targeting Compound(s) against 3CLprotease and NSP15exonuclease of SARS-CoV-2 from Nigella sativa: Virtual Screening via Physicochemical Properties, Docking and Dynamic Simulation Analysis, Processes, doi:10.3390/pr9101814.
11.
Mir et al., Identification of SARS-CoV-2 RNA-dependent RNA polymerase inhibitors from the major phytochemicals of Nigella sativa: An in silico approach, Saudi Journal of Biological Sciences, doi:10.1016/j.sjbs.2021.09.002.
12.
Hardianto et al., Exploring the Potency of Nigella sativa Seed in Inhibiting SARS-CoV-2 Main Protease Using Molecular Docking and Molecular Dynamics Simulations, Indonesian Journal of Chemistry, doi:10.22146/ijc.65951.
13.
Maiti et al., Active-site Molecular docking of Nigellidine to nucleocapsid/Nsp2/Nsp3/MPro of COVID-19 and to human IL1R and TNFR1/2 may stop viral-growth/cytokine-flood, and the drug source Nigella sativa (black cumin) seeds show potent antioxidant role in experimental rats, Research Square, doi:10.21203/rs.3.rs-26464/v1.
14.
Duru et al., In silico identification of compounds from Nigella sativa seed oil as potential inhibitors of SARS-CoV-2 targets, Bulletin of the National Research Centre, doi:10.1186/s42269-021-00517-x.
15.
Bouchentouf et al., Identification of Compounds from Nigella Sativa as New Potential Inhibitors of 2019 Novel Coronasvirus (Covid-19): Molecular Docking Study, ChemRxiv, doi:10.26434/chemrxiv.12055716.v1.
16.
Ali (B) et al., In vitro inhibitory effect of Nigella sativa L. extracts on SARS-COV-2 spike protein-ACE2 interaction, Current Therapeutic Research, doi:10.1016/j.curtheres.2024.100759.
17.
Bostancıklıoğlu et al., Nigella sativa, Anthemis hyaline and Citrus sinensis extracts reduce SARS-CoV-2 replication by fluctuating Rho GTPase, PI3K-AKT, and MAPK/ERK pathways in HeLa-CEACAM1a cells, Gene, doi:10.1016/j.gene.2024.148366.
Maen et al., 6 Feb 2024, peer-reviewed, 15 authors.
Contact: akaseb@mdanderson.org, mabdelrahim@houstonmethodist.org.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Individual ingredients of NP-101 (Thymoquinone formula) inhibit SARS-CoV-2 pseudovirus infection
Frontiers in Pharmacology, doi:10.3389/fphar.2024.1291212
Thymoquinone TQ, an active ingredient of Nigella Sativa, has been shown to inhibit COVID-19 symptoms in clinical trials. Thymoquinone Formulation (TQF or NP-101) is developed as a novel enteric-coated medication derivative from Nigella Sativa. TQF consists of TQ with a favorable concentration and fatty acids, including palmitic, oleic, and linoleic acids. In this study, we aimed to investigate the roles of individual ingredients of TQF on infection of SARS-CoV-2 variants in-vitro, by utilizing Murine Leukemia Virus (MLV) based pseudovirus particles. We demonstrated that NP-101, TQ, and other individual ingredients, including oleic, linoleic, and palmitic acids inhibited SARS-CoV-2 infection in the MLV-based pseudovirus model. A large, randomized phase 2 study of NP-101 is planned in outpatient COVID-19 patients.
Author contributions AbM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing-original draft, Writing-review and editing. BG: Conceptualization, Investigation, Software, Writing-original draft, Writing-review and editing. YM: Conceptualization, Data
Conflict of interest Author JL was employed by Codex BioSolutions Inc. Authors MoK, OK, and MG were employed by Novatek Pharmaceuticals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their
Supplementary material The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1291212/ full#supplementary-material
References
Abdelrahim, Esmail, Al Saadi, Zsigmond, Al Najjar et al., Thymoquinone's antiviral effects: it is time to be proven in the covid-19 pandemic era and its Omicron variant surge, Front. Pharmacol, doi:10.3389/fphar.2022.848676
Ashraf, Ashraf, Ashraf, Imran, Kalsoom et al., Therapeutic efficacy of Honey and Nigella sativa against COVID-19: a multicenter randomized controlled clinical trial (HNS-COVID-PK), medRxiv, doi:10.1101/2020.10.30.20217364
Barakat, El Wakeel, Hagag, Effects of Nigella sativa on outcome of hepatitis C in Egypt, World J. Gastroenterol, doi:10.3748/wjg.v19.i16.2529
Bencheqroun, Ahmed, Kocak, Villa, Barrera et al., A randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety and efficacy of ThymoQuinone formula (TQF) for treating outpatient SARS-CoV-2, Pathogens, doi:10.3390/pathogens11050551
Chaudhry, Lavandero, Xie, Sabharwal, Zheng et al., Manipulation of ACE2 expression in COVID-19, Open Heart, doi:10.1136/openhrt-2020-001424
Chen, Xu, Pradhan, Gorshkov, Petersen et al., Identifying SARS-CoV-2 entry inhibitors through drug repurposing screens of SARS-S and MERS-S pseudotyped particles, ACS Pharmacol. Transl. Sci, doi:10.1101/2020.07.10.197988
Chen, Zhang, Li, Li, Liu et al., The impact of ACE2 polymorphisms on COVID-19 disease: susceptibility, severity, and therapy, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2021.753721
Das, Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review, J. Adv. Res, doi:10.1016/j.jare.2018.01.001
Goc, Niedzwiecki, Rath, Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry, Sci. Rep, doi:10.1038/s41598-021-84850-1
Gu, Chen, Yang, He, Fan et al., Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy, Science, doi:10.1126/science.abc4730
Kaseb, Mohamed, Malek, Raad, Ii et al., The impact of angiotensin-converting enzyme 2 (ACE2) expression on the incidence and severity of COVID-19 infection, Pathogens, doi:10.3390/pathogens10030379
Kirola, Genetic emergence of B.1.617.2 in COVID-19, New Microbes New Infect, doi:10.1016/j.nmni.2021.100929
Kohn, Gitelman, Inbar, Interaction of polyunsaturated fatty acids with animal cells and enveloped viruses, Antimicrob. Agents Chemother, doi:10.1128/AAC.18.6.962
Kohn, Gitelman, Inbar, Unsaturated free fatty acids inactivate animal enveloped viruses, Arch. Virol, doi:10.1007/bf01320626
Koshak, Koshak, Mobeireek, Badawi, Wali et al., Nigella sativa for the treatment of COVID-19: an open-label randomized controlled clinical trial, Complement. Ther. Med, doi:10.1016/j.ctim.2021.102769
Koshak, Koshak, Mobeireek, Badawi, Wali et al., Nigella sativa for the treatment of COVID-19: an open-label randomized controlled clinical trial, Complementary Ther. Med, doi:10.1016/j.ctim.2021.102769
Liu, Wei, Kappler, Marrack, Zhang, SARS-CoV-2 variants of concern and variants of interest receptor binding domain mutations and virus infectivity, Front. Immunol, doi:10.3389/fimmu.2022.825256
Millet, Whittaker, Murine leukemia virus (MLV)-based Coronavirus spike-pseudotyped particle production and infection, Bio Protoc, doi:10.21769/BioProtoc.2035
Mostofa, Hossain, Basak, Sayeed, Thymoquinone as a potential adjuvant therapy for cancer treatment: evidence from preclinical studies, Front. Pharmacol, doi:10.3389/fphar.2017.00295
Ni, Yang, Yang, Bao, Li et al., Role of angiotensinconverting enzyme 2 (ACE2) in COVID-19, Crit. Care, doi:10.1186/s13054-020-03120-0
Obukhov, Stevens, Prasad, Li Calzi, Boulton et al., SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes, Diabetes, doi:10.2337/dbi20-0019
Onifade, Jewell, Adedeji, Nigella sativa concoction induced sustained seroreversion in HIV patient, Afr. J. traditional, complementary, Altern. Med, doi:10.4314/ajtcam.v10i5.18
Onifade, Jewell, Okesina, Seronegative conversion of an HIV positive subject treated with Nigella sativa and honey, Afr. J. Infect. Dis, doi:10.4314/ajid.v9i2.6
Pop, Sabin, Suciu, Vesa, Socaci et al., Nigella sativa's anti-inflammatory and antioxidative effects in experimental inflammation, Antioxidants, doi:10.3390/antiox9100921
Rahman, Potential benefits of combination of Nigella sativa and Zn supplements to treat COVID-19, J. Herb. Med, doi:10.1016/j.hermed.2020.100382
Starr, Greaney, Hilton, Ellis, Crawford et al., Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding, Cell, doi:10.1016/j.cell.2020.08.012
Villoutreix, Calvez, Marcelin, Khatib, In silico investigation of the new UK (B.1.1.7) and South African (501Y.V2) SARS-CoV-2 variants with a focus at the ACE2-spike RBD interface, doi:10.3390/ijms22041695
Xiao, Lu, Zhang, Johnson, Mckay et al., A trimeric human angiotensin-converting enzyme 2 as an anti-SARS-CoV-2 agent, Nat. Struct. Mol. Biol, doi:10.1038/s41594-020-00549-3
DOI record:
{
"DOI": "10.3389/fphar.2024.1291212",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2024.1291212",
"abstract": "<jats:p>Thymoquinone TQ, an active ingredient of Nigella Sativa, has been shown to inhibit COVID-19 symptoms in clinical trials. Thymoquinone Formulation (TQF or NP-101) is developed as a novel enteric-coated medication derivative from Nigella Sativa. TQF consists of TQ with a favorable concentration and fatty acids, including palmitic, oleic, and linoleic acids. In this study, we aimed to investigate the roles of individual ingredients of TQF on infection of SARS-CoV-2 variants <jats:italic>in-vitro</jats:italic>, by utilizing Murine Leukemia Virus (MLV) based pseudovirus particles. We demonstrated that NP-101, TQ, and other individual ingredients, including oleic, linoleic, and palmitic acids inhibited SARS-CoV-2 infection in the MLV-based pseudovirus model. A large, randomized phase 2 study of NP-101 is planned in outpatient COVID-19 patients.</jats:p>",
"alternative-id": [
"10.3389/fphar.2024.1291212"
],
"author": [
{
"affiliation": [],
"family": "Maen",
"given": "Abdelrahim",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gok Yavuz",
"given": "Betul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohamed",
"given": "Yehia I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Esmail",
"given": "Abdullah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Jianming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohamed",
"given": "Amr",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Azmi",
"given": "Asfar S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaseb",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kasseb",
"given": "Osama",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gocio",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kocak",
"given": "Mehmet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Selim",
"given": "Abdelhafez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Qing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaseb",
"given": "Ahmed O.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2024,
2,
6
]
],
"date-time": "2024-02-06T04:31:58Z",
"timestamp": 1707193918000
},
"deposited": {
"date-parts": [
[
2024,
2,
6
]
],
"date-time": "2024-02-06T04:32:02Z",
"timestamp": 1707193922000
},
"indexed": {
"date-parts": [
[
2024,
2,
7
]
],
"date-time": "2024-02-07T00:48:33Z",
"timestamp": 1707266913632
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2,
6
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
6
]
],
"date-time": "2024-02-06T00:00:00Z",
"timestamp": 1707177600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2024.1291212/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2024,
2,
6
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
6
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.3389/fphar.2022.848676",
"article-title": "Thymoquinone's antiviral effects: it is time to be proven in the covid-19 pandemic era and its Omicron variant surge",
"author": "Abdelrahim",
"doi-asserted-by": "publisher",
"first-page": "848676",
"journal-title": "Front. Pharmacol.",
"key": "B1",
"volume": "13",
"year": "2022"
},
{
"key": "B2",
"unstructured": "Therapeutic efficacy of Honey and Nigella sativa against COVID-19: a multi-center randomized controlled clinical trial (HNS-COVID-PK)\n AshrafS.\n AshrafS.\n AshrafM.\n ImranM. A.\n KalsoomL.\n SiddiquiU. N.\n 2020"
},
{
"DOI": "10.3748/wjg.v19.i16.2529",
"article-title": "Effects of Nigella sativa on outcome of hepatitis C in Egypt",
"author": "Barakat",
"doi-asserted-by": "publisher",
"first-page": "2529",
"journal-title": "World J. Gastroenterol.",
"key": "B3",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.3390/pathogens11050551",
"article-title": "A randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety and efficacy of ThymoQuinone formula (TQF) for treating outpatient SARS-CoV-2",
"author": "Bencheqroun",
"doi-asserted-by": "publisher",
"first-page": "551",
"journal-title": "Pathogens",
"key": "B4",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1136/openhrt-2020-001424",
"article-title": "Manipulation of ACE2 expression in COVID-19",
"author": "Chaudhry",
"doi-asserted-by": "publisher",
"first-page": "e001424",
"journal-title": "Open Heart",
"key": "B5",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.10.197988",
"article-title": "Identifying SARS-CoV-2 entry inhibitors through drug repurposing screens of SARS-S and MERS-S pseudotyped particles",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "1165",
"journal-title": "ACS Pharmacol. Transl. Sci.",
"key": "B6",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.3389/fcimb.2021.753721",
"article-title": "The impact of ACE2 polymorphisms on COVID-19 disease: susceptibility, severity, and therapy",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "753721",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "B7",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.jare.2018.01.001",
"article-title": "Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review",
"author": "Das",
"doi-asserted-by": "publisher",
"first-page": "57",
"journal-title": "J. Adv. Res.",
"key": "B8",
"volume": "11",
"year": "2018"
},
{
"key": "B9",
"unstructured": "U.S. Food and drug administration2022"
},
{
"DOI": "10.1038/s41598-021-84850-1",
"article-title": "Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry",
"author": "Goc",
"doi-asserted-by": "publisher",
"first-page": "5207",
"journal-title": "Sci. Rep.",
"key": "B10",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1126/science.abc4730",
"article-title": "Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy",
"author": "Gu",
"doi-asserted-by": "publisher",
"first-page": "1603",
"journal-title": "Science",
"key": "B11",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.3390/pathogens10030379",
"article-title": "The impact of angiotensin-converting enzyme 2 (ACE2) expression on the incidence and severity of COVID-19 infection",
"author": "Kaseb",
"doi-asserted-by": "publisher",
"first-page": "379",
"journal-title": "Pathogens",
"key": "B12",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.nmni.2021.100929",
"article-title": "Genetic emergence of B.1.617.2 in COVID-19",
"author": "Kirola",
"doi-asserted-by": "publisher",
"first-page": "100929",
"journal-title": "New Microbes New Infect.",
"key": "B13",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1007/bf01320626",
"article-title": "Unsaturated free fatty acids inactivate animal enveloped viruses",
"author": "Kohn",
"doi-asserted-by": "publisher",
"first-page": "301",
"journal-title": "Arch. Virol.",
"key": "B14",
"volume": "66",
"year": ""
},
{
"DOI": "10.1128/AAC.18.6.962",
"article-title": "Interaction of polyunsaturated fatty acids with animal cells and enveloped viruses",
"author": "Kohn",
"doi-asserted-by": "publisher",
"first-page": "962",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "B15",
"volume": "18",
"year": ""
},
{
"DOI": "10.1016/j.ctim.2021.102769",
"article-title": "Nigella sativa for the treatment of COVID-19: an open-label randomized controlled clinical trial",
"author": "Koshak",
"doi-asserted-by": "publisher",
"first-page": "102769",
"journal-title": "Complementary Ther. Med.",
"key": "B16",
"volume": "61",
"year": ""
},
{
"DOI": "10.1016/j.ctim.2021.102769",
"article-title": "Nigella sativa for the treatment of COVID-19: an open-label randomized controlled clinical trial",
"author": "Koshak",
"doi-asserted-by": "publisher",
"first-page": "102769",
"journal-title": "Complement. Ther. Med.",
"key": "B17",
"volume": "61",
"year": ""
},
{
"DOI": "10.3389/fimmu.2022.825256",
"article-title": "SARS-CoV-2 variants of concern and variants of interest receptor binding domain mutations and virus infectivity",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "825256",
"journal-title": "Front. Immunol.",
"key": "B18",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.21769/BioProtoc.2035",
"article-title": "Murine leukemia virus (MLV)-based Coronavirus spike-pseudotyped particle production and infection",
"author": "Millet",
"doi-asserted-by": "publisher",
"first-page": "e2035",
"journal-title": "Bio Protoc.",
"key": "B19",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.3389/fphar.2017.00295",
"article-title": "Thymoquinone as a potential adjuvant therapy for cancer treatment: evidence from preclinical studies",
"author": "Mostofa",
"doi-asserted-by": "publisher",
"first-page": "295",
"journal-title": "Front. Pharmacol.",
"key": "B20",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1186/s13054-020-03120-0",
"article-title": "Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19",
"author": "Ni",
"doi-asserted-by": "publisher",
"first-page": "422",
"journal-title": "Crit. Care",
"key": "B21",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.2337/dbi20-0019",
"article-title": "SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes",
"author": "Obukhov",
"doi-asserted-by": "publisher",
"first-page": "1875",
"journal-title": "Diabetes",
"key": "B22",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.4314/ajtcam.v10i5.18",
"article-title": "Nigella sativa concoction induced sustained seroreversion in HIV patient",
"author": "Onifade",
"doi-asserted-by": "publisher",
"first-page": "332",
"journal-title": "Afr. J. traditional, complementary, Altern. Med.",
"key": "B23",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.4314/ajid.v9i2.6",
"article-title": "Seronegative conversion of an HIV positive subject treated with Nigella sativa and honey",
"author": "Onifade",
"doi-asserted-by": "publisher",
"first-page": "47",
"journal-title": "Afr. J. Infect. Dis.",
"key": "B24",
"volume": "9",
"year": "2015"
},
{
"DOI": "10.3390/antiox9100921",
"article-title": "Nigella sativa’s anti-inflammatory and antioxidative effects in experimental inflammation",
"author": "Pop",
"doi-asserted-by": "publisher",
"first-page": "921",
"journal-title": "Antioxidants",
"key": "B25",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.hermed.2020.100382",
"article-title": "Potential benefits of combination of Nigella sativa and Zn supplements to treat COVID-19",
"author": "Rahman",
"doi-asserted-by": "publisher",
"first-page": "100382",
"journal-title": "J. Herb. Med.",
"key": "B26",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.08.012",
"article-title": "Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding",
"author": "Starr",
"doi-asserted-by": "publisher",
"first-page": "1295",
"journal-title": "Cell",
"key": "B27",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.3390/ijms22041695",
"article-title": "In silico investigation of the new UK (B.1.1.7) and South African (501Y.V2) SARS-CoV-2 variants with a focus at the ACE2-spike RBD interface",
"author": "Villoutreix",
"doi-asserted-by": "publisher",
"first-page": "1695",
"journal-title": "Int. J. Mol. Sci.",
"key": "B28",
"volume": "22",
"year": "2021"
},
{
"key": "B29",
"unstructured": "WHO Coronavirus (COVID-19) dashboard: world health organization2022"
},
{
"DOI": "10.1038/s41594-020-00549-3",
"article-title": "A trimeric human angiotensin-converting enzyme 2 as an anti-SARS-CoV-2 agent",
"author": "Xiao",
"doi-asserted-by": "publisher",
"first-page": "202",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "B30",
"volume": "28",
"year": "2021"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2024.1291212/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Individual ingredients of NP-101 (Thymoquinone formula) inhibit SARS-CoV-2 pseudovirus infection",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "15"
}