Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial
et al., Complementary Therapies in Medicine, doi:10.1016/j.ctim.2021.102769, NCT04401202, Aug 2021
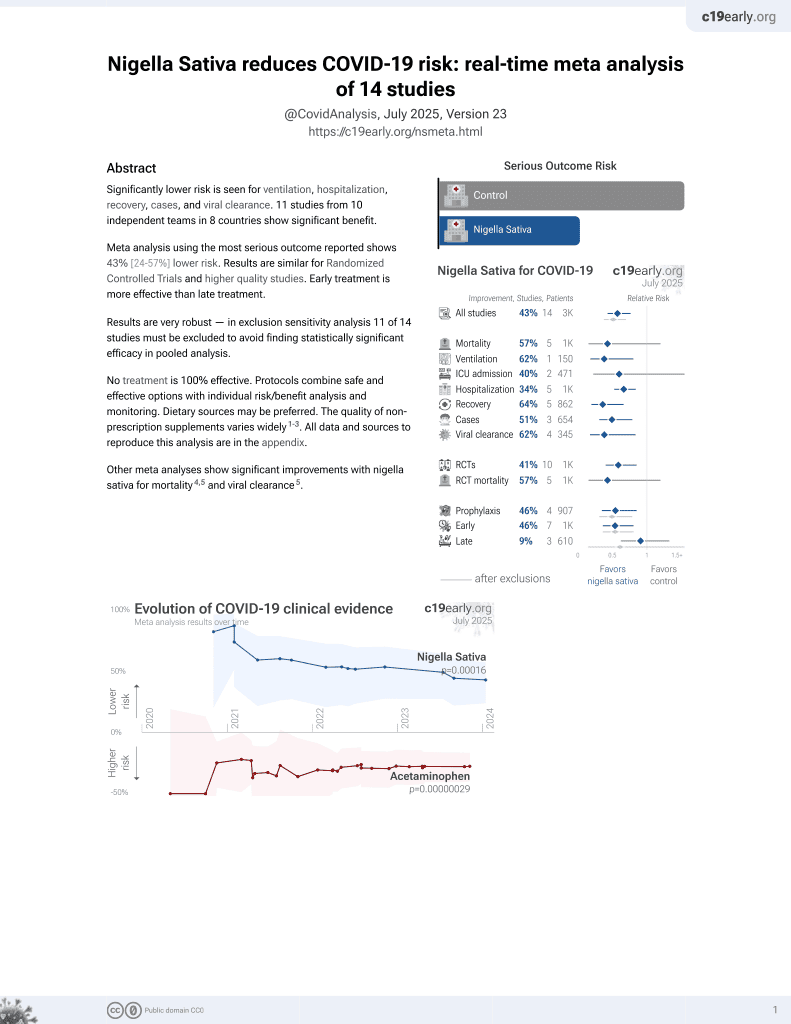
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
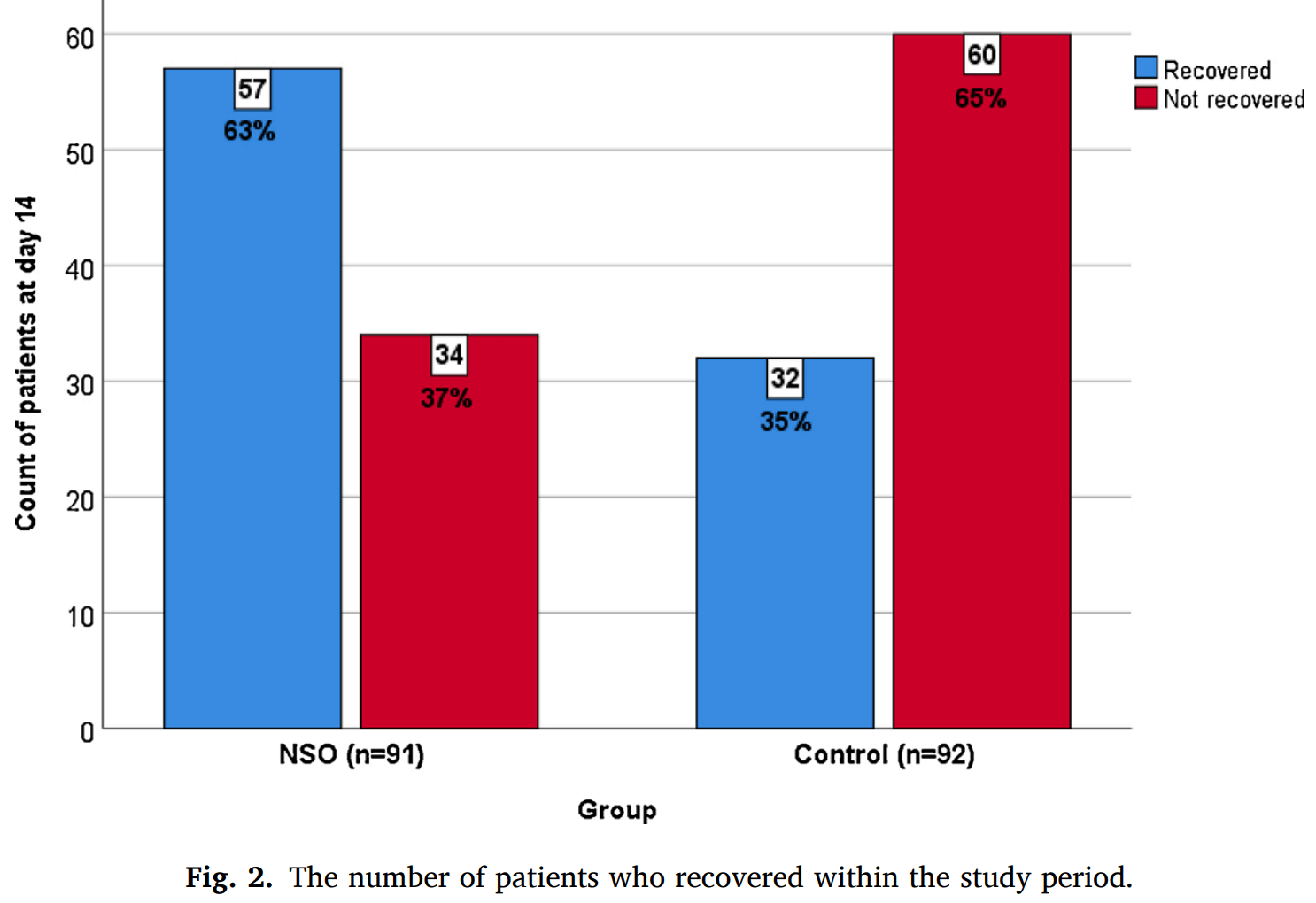
RCT 183 mild COVID-19 outpatients in Saudi Arabia, 91 treated with Nigella Sativa, showing lower hospitalization and faster recovery with treatment. 500mg Nigella Sativa oil (MARNYS Cuminmar) twice daily for 10 days. NCT04401202 (history).
|
risk of hospitalization, 74.7% lower, RR 0.25, p = 0.37, treatment 1 of 91 (1.1%), control 4 of 92 (4.3%), NNT 31.
|
|
risk of no recovery, 42.7% lower, RR 0.57, p < 0.001, treatment 34 of 91 (37.4%), control 60 of 92 (65.2%), NNT 3.6.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Koshak et al., 15 Aug 2021, Randomized Controlled Trial, Saudi Arabia, peer-reviewed, 10 authors, study period 1 May, 2020 - 30 September, 2020, trial NCT04401202 (history).
Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial
Complementary Therapies in Medicine, doi:10.1016/j.ctim.2021.102769
Background: Effective treatment for Coronavirus Disease-2019 (COVID-19) is under intensive research. Nigella sativa oil (NSO) is a herbal medicine with antiviral and immunomodulatory activities, and has been recommended for the treatment of COVID-19. This study aimed to evaluate the efficacy of NSO treatment in patients with COVID-19. Methods: All adult patients with mild COVID-19 symptoms presented to King
Declaration of Competing Interest The authors report no declarations of interest.
Appendix A. Supplementary data Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ctim.2021.102769.
References
Ahmad, Husain, Mujeeb, A review on therapeutic potential of Nigella sativa: a miracle herb, Asian Pac J Trop Biomed, doi:10.1016/S2221-1691(13)60075-1
Ashraf, Ashraf, Ashraf, Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): a multi-center placebo-controlled randomized clinical trial, medRxiv, doi:10.1101/2020.10.30.20217364
Babaei, Mirzababaei, Nassiri-Asl, Hosseinzadeh, Review of registered clinical trials for the treatment of COVID-19, Drug Dev Res, doi:10.1002/ddr.21762
Barakat, Shoman, Dina, Alfarouk, Antiviral activity and mode of action of Dianthus caryophyllus L. and Lupinus termes L. seed extracts against in vitro herpes simplex and hepatitis A viruses infection, J Microbiol Antimicrob
Barakat, Wakeel, Hagag, Effects of Nigella sativa on outcome of hepatitis C in Egypt, World J Gastroenterol, doi:10.3748/wjg.v19.i16.2529
Boskabady, Keyhanmanesh, Khamneh, Ebrahimi, The effect of Nigella sativa extract on tracheal responsiveness and lung inflammation in ovalbuminsensitized guinea pigs, Clinics, doi:10.1590/S1807-59322011000500027
Dorra, El-Berrawy, Sallam, Evaluation of antiviral and antioxidant activity of selected herbal extracts, J High Inst Public Heal, doi:10.21608/jhiph.2019.29464
Drugs, Diseases, What are complications of patients with coronavirus disease 2019 (COVID-19)?
Food, Coronavirus (COVID-19) update: FDA reiterates importance of close patient supervision for "off-label" use of antimalarial drugs to mitigate known risks, including heart rhythm problems
Food, FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems
Gholamnezhad, Boskabady, Hosseini, Effect of Nigella sativa on immune response in treadmill exercised rat, BMC Complement Altern Med, doi:10.1186/1472-6882-14-437
Gilani, Aziz, Khurram, Chaudhary, Iqbal, Bronchodilator, spasmolytic and calcium antagonist activities of Nigella sativa seeds (Kalonji): a traditional herbal product with multiple medicinal uses, J Pak Med Assoc
Haq, Abdullatif, Lobo, Khabar, Sheth et al., Nigella sativa: effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity, Immunopharmacology, doi:10.1016/0162-3109(95)00016-M
Islam, Hossain, Sarker, Revisiting pharmacological potentials of Nigella sativa seed: a promising option for COVID-19 prevention and cure, Phyther Res, doi:10.31219/osf.io/56pq9
Khazdair, Ghafari, Sadeghi, Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19, Pharm Biol, doi:10.1080/13880209.2021.1931353
Koshak, Fiebich, Koshak, Heinrich, Comparative anti-inflammatory/ immunomodulatory effect of different extracts of medicinal plant Nigella sativa
Koshak, Koshak, Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: a mini review of in silico studies, Curr Ther Res -Clin Exp, doi:10.1016/j.curtheres.2020.100602
Koshak, Wei, Koshak, Nigella sativa supplementation improves asthma control and biomarkers: a randomized, double-blind, placebo-controlled trial, Phyther Res, doi:10.1002/ptr.5761
Lin, Hsu, Lin, Antiviral natural products and herbal medicines, J Tradit Complement Med, doi:10.4103/2225-4110.124335
Mady, Arafa, Hussein, Aly, Madbouly, Nigella sativa oil as an immunostimulant adjuvant in H5 based DNA vaccine of H5N1 avian influenza virus, Glob Vet, doi:10.5829/idosi.gv.2013.10.6.73101
Majdalawieh, Fayyad, Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review, Int Immunopharmacol, doi:10.1016/j.intimp.2015.06.023
Mfea, Li, Mehmood, Waqas, Li et al., Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: a hope to decelerate the COVID-19 pandemic, Phytomedicine, doi:10.1016/j.phymed.2020.153277
Mitjà, Corbacho-Monné, Ubals, Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial, Clin Infect Dis, doi:10.1093/cid/ciaa1009
Molla, Abul, Azad, A review on antiviral effects of Nigella Sativa L, Pharmacologyonline
Ms, The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa, J Ethnopharmacol, doi:10.1016/S0378-8741(01)00216-1
Onifade, Jewell, Adedeji, Nigella sativa concoction induced sustained seroreversion in HIV patient, Afr J Tradit Complement Altern Med
Onifade, Jewell, Ajadi, Rahamon, Ogunrin, Effectiveness of a herbal remedy in six HIV patients in Nigeria, J Herb Med, doi:10.1016/j.hermed.2013.04.006
Oyero, Toyama, Mitsuhiro, Selective inhibition of hepatitis c virus replication by alpha-zam, a nigella sativa seed formulation, Afr J Tradit Complement Altern Med, doi:10.21010/ajtcam.v13i6.20
Razmpoosh, Safi, Abdollahi, The effect of Nigella sativa on the measures of liver and kidney parameters: a systematic review and meta-analysis of randomized-controlled trials, Pharmacol Res, doi:10.1016/j.phrs.2020.104767
Richardson, Hirsch, Narasimhan, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area, JAMA -J Am Med Assoc, doi:10.1001/jama.2020.6775
Sahebnasagh, Avan, Saghafi, Pharmacological treatments of COVID-19, Pharmacol Rep, doi:10.1007/s43440-020-00152-9
Salem, Hossain, Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection, Int J Immunopharmacol, doi:10.1016/S0192-0561(00)00036-9
Sayed, Aboonq, Rashedy, Promising preventive and therapeutic effects of TaibUVID nutritional supplements for COVID-19 pandemic: towards better public prophylaxis and treatment (a retrospective study), Am J Blood Res
Shirvani, Rostamkhani, Arabzadeh, Mohammadi, Mohammadi, Potential role of Nigella sativa supplementation with physical activity in prophylaxis and treatment of COVID-19: a contemporary review, Sport Sci Health, doi:10.1007/s11332-021-00787-y
Silveira, Prieto-Garcia, Boylan, COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy?, Front Pharmacol, doi:10.3389/fphar.2020.581840
Skipper, Pastick, Engen, Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial, Ann Intern Med, doi:10.7326/M20-4207
Suleyman, Fadel, Malette, Clinical Characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.12270
Sultan, Buttxs, Qayyum, Suleria, Immunity: plants as effective mediators, Crit Rev Food Sci Nutr, doi:10.1080/10408398.2011.633249
Sun, Wang, Cai, Cytokine storm intervention in the early stages of COVID-19 pneumonia, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2020.04.002
Ulasli, Gurses, Bayraktar, The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family, Mol Biol Rep, doi:10.1007/s11033-014-3019-7
Vijayvargiya, Garrigos, Almeida, Gurram, Stevens et al., Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof), Mayo Clin Proc, doi:10.1016/j.mayocp.2020.04.027
Wang, Jiang, Chen, Montaner, Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts, J Leukoc Biol, doi:10.1002/JLB.3COVR0520-272R
Whitcroft, Hummel, Olfactory dysfunction in COVID-19: diagnosis and management, JAMA -J Am Med Assoc, doi:10.1001/jama.2020.8391
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention, JAMA -J Am Med Assoc, doi:10.1001/jama.2020.2648
DOI record:
{
"DOI": "10.1016/j.ctim.2021.102769",
"ISSN": [
"0965-2299"
],
"URL": "http://dx.doi.org/10.1016/j.ctim.2021.102769",
"alternative-id": [
"S0965229921001102"
],
"article-number": "102769",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Complementary Therapies in Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ctim.2021.102769"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Koshak",
"given": "Abdulrahman E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Koshak",
"given": "Emad A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mobeireek",
"given": "Abdullah F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badawi",
"given": "Mazen A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5825-5642",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wali",
"given": "Siraj O.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1314-8031",
"affiliation": [],
"authenticated-orcid": false,
"family": "Malibary",
"given": "Husam M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Atwah",
"given": "Ali F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alhamdan",
"given": "Meshari M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almalki",
"given": "Reem A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madani",
"given": "Tariq A.",
"sequence": "additional"
}
],
"container-title": "Complementary Therapies in Medicine",
"container-title-short": "Complementary Therapies in Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
15
]
],
"date-time": "2021-08-15T13:31:47Z",
"timestamp": 1629034307000
},
"deposited": {
"date-parts": [
[
2022,
7,
6
]
],
"date-time": "2022-07-06T06:29:26Z",
"timestamp": 1657088966000
},
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T18:33:25Z",
"timestamp": 1712082805184
},
"is-referenced-by-count": 56,
"issued": {
"date-parts": [
[
2021,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T00:00:00Z",
"timestamp": 1630454400000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
12
]
],
"date-time": "2021-08-12T00:00:00Z",
"timestamp": 1628726400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0965229921001102?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0965229921001102?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "102769",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"author": "World Health Organization",
"key": "10.1016/j.ctim.2021.102769_bib0005",
"series-title": "Timeline of WHO’s response to COVID-19",
"year": "2020"
},
{
"author": "Saudi Center for Disease Prevention and Control",
"key": "10.1016/j.ctim.2021.102769_bib0010",
"series-title": "Daily updates",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.04.027",
"article-title": "Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof)",
"author": "Vijayvargiya",
"doi-asserted-by": "crossref",
"first-page": "1454",
"issue": "7",
"journal-title": "Mayo Clin Proc",
"key": "10.1016/j.ctim.2021.102769_bib0015",
"volume": "95",
"year": "2020"
},
{
"author": "World Health Organization",
"key": "10.1016/j.ctim.2021.102769_bib0020",
"series-title": "Clinical management of COVID-19",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA - J Am Med Assoc",
"key": "10.1016/j.ctim.2021.102769_bib0025",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.12270",
"article-title": "Clinical Characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit",
"author": "Suleyman",
"doi-asserted-by": "crossref",
"first-page": "e2012270",
"issue": "6",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.ctim.2021.102769_bib0030",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"issue": "20",
"journal-title": "JAMA - J Am Med Assoc",
"key": "10.1016/j.ctim.2021.102769_bib0035",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.4103/2225-4110.124335",
"article-title": "Antiviral natural products and herbal medicines",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "24",
"issue": "1",
"journal-title": "J Tradit Complement Med",
"key": "10.1016/j.ctim.2021.102769_bib0040",
"volume": "4",
"year": "2014"
},
{
"DOI": "10.3389/fphar.2020.581840",
"article-title": "COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy?",
"author": "Silveira",
"doi-asserted-by": "crossref",
"first-page": "1479",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ctim.2021.102769_bib0045",
"volume": "11",
"year": "2020"
},
{
"article-title": "Potential role of Nigella sativa supplementation with physical activity in prophylaxis and treatment of COVID-19: a contemporary review",
"author": "Shirvani",
"issue": "May",
"journal-title": "Sport Sci Health",
"key": "10.1016/j.ctim.2021.102769_bib0050",
"year": "2021"
},
{
"DOI": "10.1080/13880209.2021.1931353",
"article-title": "Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19",
"author": "Khazdair",
"doi-asserted-by": "crossref",
"first-page": "696",
"issue": "1",
"journal-title": "Pharm Biol",
"key": "10.1016/j.ctim.2021.102769_bib0055",
"volume": "59",
"year": "2021"
},
{
"DOI": "10.1016/S2221-1691(13)60075-1",
"article-title": "A review on therapeutic potential of Nigella sativa: a miracle herb",
"author": "Ahmad",
"doi-asserted-by": "crossref",
"first-page": "337",
"issue": "5",
"journal-title": "Asian Pac J Trop Biomed",
"key": "10.1016/j.ctim.2021.102769_bib0060",
"volume": "3",
"year": "2013"
},
{
"article-title": "Nigella sativa supplementation improves asthma control and biomarkers: a randomized, double-blind, placebo-controlled trial",
"author": "Koshak",
"issue": "January",
"journal-title": "Phyther Res",
"key": "10.1016/j.ctim.2021.102769_bib0065",
"year": "2017"
},
{
"DOI": "10.1016/j.phrs.2020.104767",
"article-title": "The effect of Nigella sativa on the measures of liver and kidney parameters: a systematic review and meta-analysis of randomized-controlled trials",
"author": "Razmpoosh",
"doi-asserted-by": "crossref",
"first-page": "104767",
"journal-title": "Pharmacol Res",
"key": "10.1016/j.ctim.2021.102769_bib0070",
"volume": "156",
"year": "2020"
},
{
"article-title": "A review on antiviral effects of Nigella Sativa L",
"author": "Molla",
"first-page": "47",
"journal-title": "Pharmacologyonline",
"key": "10.1016/j.ctim.2021.102769_bib0075",
"volume": "2",
"year": "2019"
},
{
"article-title": "Antiviral activity and mode of action of Dianthus caryophyllus L. and Lupinus termes L. seed extracts against in vitro herpes simplex and hepatitis A viruses infection",
"author": "Barakat",
"first-page": "23",
"issue": "3",
"journal-title": "J Microbiol Antimicrob",
"key": "10.1016/j.ctim.2021.102769_bib0080",
"volume": "2",
"year": "2010"
},
{
"DOI": "10.1007/s11033-014-3019-7",
"article-title": "The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family",
"author": "Ulasli",
"doi-asserted-by": "crossref",
"first-page": "1703",
"issue": "3",
"journal-title": "Mol Biol Rep",
"key": "10.1016/j.ctim.2021.102769_bib0085",
"volume": "41",
"year": "2014"
},
{
"DOI": "10.21608/jhiph.2019.29464",
"article-title": "Evaluation of antiviral and antioxidant activity of selected herbal extracts",
"author": "Dorra",
"doi-asserted-by": "crossref",
"first-page": "36",
"issue": "1",
"journal-title": "J High Inst Public Heal",
"key": "10.1016/j.ctim.2021.102769_bib0090",
"volume": "49",
"year": "2019"
},
{
"DOI": "10.21010/ajtcam.v13i6.20",
"article-title": "Selective inhibition of hepatitis c virus replication by alpha-zam, a nigella sativa seed formulation",
"author": "Oyero",
"doi-asserted-by": "crossref",
"first-page": "144",
"issue": "6",
"journal-title": "Afr J Tradit Complement Altern Med",
"key": "10.1016/j.ctim.2021.102769_bib0095",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1016/S0192-0561(00)00036-9",
"article-title": "Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection",
"author": "Salem",
"doi-asserted-by": "crossref",
"first-page": "729",
"issue": "9",
"journal-title": "Int J Immunopharmacol",
"key": "10.1016/j.ctim.2021.102769_bib0100",
"volume": "22",
"year": "2000"
},
{
"article-title": "Nigella sativa concoction induced sustained seroreversion in HIV patient",
"author": "Onifade",
"first-page": "332",
"issue": "5",
"journal-title": "Afr J Tradit Complement Altern Med",
"key": "10.1016/j.ctim.2021.102769_bib0105",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1016/j.hermed.2013.04.006",
"article-title": "Effectiveness of a herbal remedy in six HIV patients in Nigeria",
"author": "Onifade",
"doi-asserted-by": "crossref",
"first-page": "99",
"issue": "3",
"journal-title": "J Herb Med",
"key": "10.1016/j.ctim.2021.102769_bib0110",
"volume": "3",
"year": "2013"
},
{
"DOI": "10.3748/wjg.v19.i16.2529",
"article-title": "Effects of Nigella sativa on outcome of hepatitis C in Egypt",
"author": "Barakat",
"doi-asserted-by": "crossref",
"first-page": "2529",
"issue": "16",
"journal-title": "World J Gastroenterol",
"key": "10.1016/j.ctim.2021.102769_bib0115",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.1016/j.curtheres.2020.100602",
"article-title": "Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: a mini review of in silico studies",
"author": "Koshak",
"doi-asserted-by": "crossref",
"first-page": "100602",
"journal-title": "Curr Ther Res - Clin Exp",
"key": "10.1016/j.ctim.2021.102769_bib0120",
"volume": "93",
"year": "2020"
},
{
"DOI": "10.1080/10408398.2011.633249",
"article-title": "Immunity: plants as effective mediators",
"author": "Sultan",
"doi-asserted-by": "crossref",
"first-page": "1298",
"issue": "10",
"journal-title": "Crit Rev Food Sci Nutr",
"key": "10.1016/j.ctim.2021.102769_bib0125",
"volume": "54",
"year": "2014"
},
{
"article-title": "Nigella sativa oil as an immunostimulant adjuvant in H5 based DNA vaccine of H5N1 avian influenza virus",
"author": "Mady",
"first-page": "663",
"issue": "6",
"journal-title": "Glob Vet",
"key": "10.1016/j.ctim.2021.102769_bib0130",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1016/0162-3109(95)00016-M",
"article-title": "Nigella sativa: effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity",
"author": "Haq",
"doi-asserted-by": "crossref",
"first-page": "147",
"issue": "2",
"journal-title": "Immunopharmacology",
"key": "10.1016/j.ctim.2021.102769_bib0135",
"volume": "30",
"year": "1995"
},
{
"DOI": "10.1186/1472-6882-14-437",
"article-title": "Effect of Nigella sativa on immune response in treadmill exercised rat",
"author": "Gholamnezhad",
"doi-asserted-by": "crossref",
"first-page": "437",
"journal-title": "BMC Complement Altern Med",
"key": "10.1016/j.ctim.2021.102769_bib0140",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1016/j.intimp.2015.06.023",
"article-title": "Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review",
"author": "Majdalawieh",
"doi-asserted-by": "crossref",
"first-page": "295",
"issue": "1",
"journal-title": "Int Immunopharmacol",
"key": "10.1016/j.ctim.2021.102769_bib0145",
"volume": "28",
"year": "2015"
},
{
"article-title": "Comparative anti-inflammatory/immunomodulatory effect of different extracts of medicinal plant Nigella sativa",
"author": "Koshak",
"key": "10.1016/j.ctim.2021.102769_bib0150",
"series-title": "European Academy of Allergy and Clinical Immunology Congress",
"year": "2015"
},
{
"DOI": "10.1016/S0378-8741(01)00216-1",
"article-title": "The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa",
"author": "Al-Ghamdi",
"doi-asserted-by": "crossref",
"first-page": "45",
"issue": "1",
"journal-title": "J Ethnopharmacol",
"key": "10.1016/j.ctim.2021.102769_bib0155",
"volume": "76",
"year": "2001"
},
{
"article-title": "Bronchodilator, spasmolytic and calcium antagonist activities of Nigella sativa seeds (Kalonji): a traditional herbal product with multiple medicinal uses",
"author": "Gilani",
"first-page": "115",
"issue": "3",
"journal-title": "J Pak Med Assoc",
"key": "10.1016/j.ctim.2021.102769_bib0160",
"volume": "51",
"year": "2001"
},
{
"DOI": "10.1590/S1807-59322011000500027",
"article-title": "The effect of Nigella sativa extract on tracheal responsiveness and lung inflammation in ovalbumin-sensitized guinea pigs",
"author": "Boskabady",
"doi-asserted-by": "crossref",
"first-page": "879",
"issue": "5",
"journal-title": "Clinics",
"key": "10.1016/j.ctim.2021.102769_bib0165",
"volume": "66",
"year": "2011"
},
{
"DOI": "10.1002/ddr.21762",
"article-title": "Review of registered clinical trials for the treatment of COVID-19",
"author": "Babaei",
"doi-asserted-by": "crossref",
"first-page": "474",
"issue": "4",
"journal-title": "Drug Dev Res",
"key": "10.1016/j.ctim.2021.102769_bib0170",
"volume": "82",
"year": "2021"
},
{
"author": "Medscape Drugs & Diseases",
"key": "10.1016/j.ctim.2021.102769_bib0175",
"series-title": "What are complications of patients with coronavirus disease 2019 (COVID-19)?",
"year": "2020"
},
{
"author": "International Severe Acute Respiratory and Emerging Infections Consortium",
"key": "10.1016/j.ctim.2021.102769_bib0180",
"series-title": "ISARIC COVID-19 report: 08 June 2020",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.8391",
"article-title": "Olfactory dysfunction in COVID-19: diagnosis and management",
"author": "Whitcroft",
"doi-asserted-by": "crossref",
"first-page": "2512",
"issue": "24",
"journal-title": "JAMA - J Am Med Assoc",
"key": "10.1016/j.ctim.2021.102769_bib0185",
"volume": "323",
"year": "2020"
},
{
"author": "U.S. Food and Drug Administration",
"key": "10.1016/j.ctim.2021.102769_bib0190"
},
{
"author": "U.S. Food and Drug Administration",
"key": "10.1016/j.ctim.2021.102769_bib0195",
"series-title": "FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. FDA-COVID-19-update",
"year": "2020"
},
{
"DOI": "10.7326/M20-4207",
"article-title": "Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial",
"author": "Skipper",
"doi-asserted-by": "crossref",
"first-page": "623",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.ctim.2021.102769_bib0200",
"volume": "173",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial",
"author": "Mitjà",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ctim.2021.102769_bib0205",
"year": "2020"
},
{
"article-title": "Pharmacological treatments of COVID-19",
"author": "Sahebnasagh",
"first-page": "3",
"journal-title": "Pharmacol Rep",
"key": "10.1016/j.ctim.2021.102769_bib0210",
"volume": "1",
"year": "2020"
},
{
"article-title": "Promising preventive and therapeutic effects of TaibUVID nutritional supplements for COVID-19 pandemic: towards better public prophylaxis and treatment (a retrospective study)",
"author": "El Sayed",
"first-page": "266",
"issue": "5",
"journal-title": "Am J Blood Res",
"key": "10.1016/j.ctim.2021.102769_bib0215",
"volume": "10",
"year": "2020"
},
{
"article-title": "Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): a multi-center placebo-controlled randomized clinical trial",
"author": "Ashraf",
"issue": "January",
"journal-title": "medRxiv",
"key": "10.1016/j.ctim.2021.102769_bib0220",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1016/j.cytogfr.2020.04.002",
"article-title": "Cytokine storm intervention in the early stages of COVID-19 pneumonia",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "38",
"journal-title": "Cytokine Growth Factor Rev",
"key": "10.1016/j.ctim.2021.102769_bib0225",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1002/JLB.3COVR0520-272R",
"article-title": "Cytokine storm and leukocyte changes in mild versus severe SARS‐CoV‐2 infection: review of 3939 COVID‐19 patients in China and emerging pathogenesis and therapy concepts",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "17",
"issue": "1",
"journal-title": "J Leukoc Biol",
"key": "10.1016/j.ctim.2021.102769_bib0230",
"volume": "108",
"year": "2020"
},
{
"article-title": "Revisiting pharmacological potentials of Nigella sativa seed: a promising option for COVID-19 prevention and cure",
"author": "Islam",
"first-page": "1",
"issue": "October",
"journal-title": "Phyther Res",
"key": "10.1016/j.ctim.2021.102769_bib0235",
"year": "2020"
},
{
"article-title": "Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: a hope to decelerate the COVID-19 pandemic",
"author": "Kulyar",
"first-page": "153277",
"issue": "July",
"journal-title": "Phytomedicine",
"key": "10.1016/j.ctim.2021.102769_bib0240",
"year": "2020"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0965229921001102"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Advanced and Specialized Nursing",
"Complementary and alternative medicine",
"Complementary and Manual Therapy"
],
"subtitle": [],
"title": "Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "61"
}