A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of ThymoQuinone Formula (TQF) for Treating Outpatient SARS-CoV-2
et al., Pathogens, doi:10.3390/pathogens11050551, BOSS-001, NCT04914377, May 2022
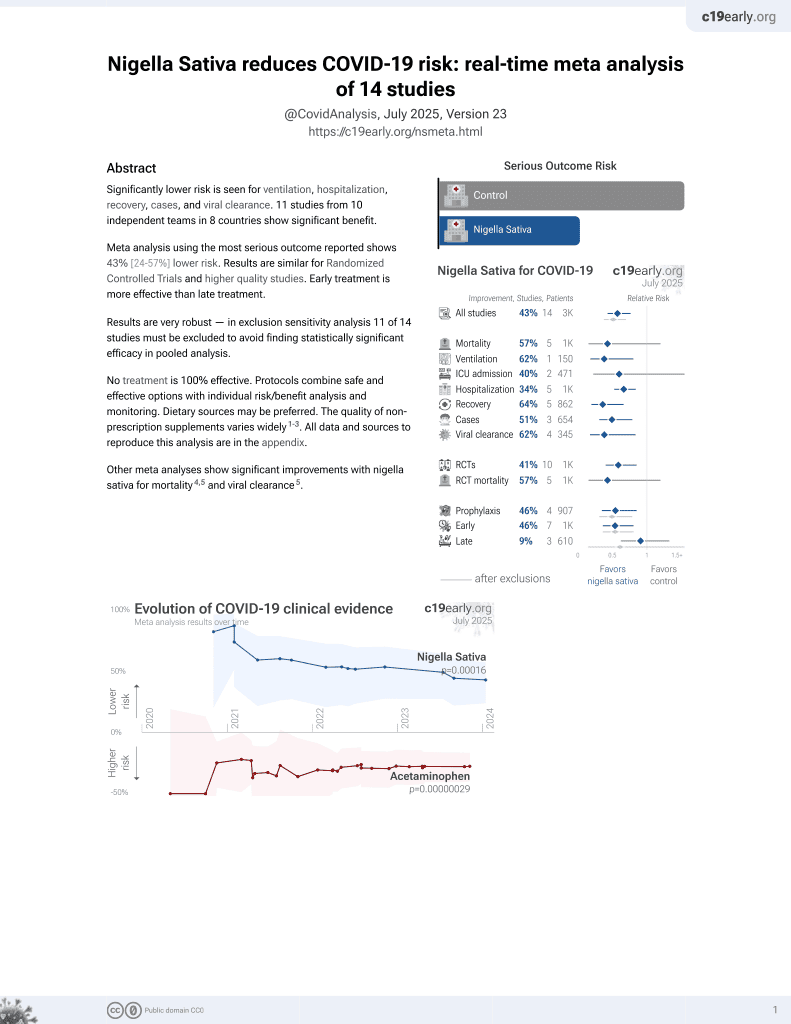
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
52 patient RCT in the USA with nigella sativa component thymoquinone, showing improved recovery with treatment. There was a significantly faster decline in the total symptom burden, and a significant increase in CD8+ and helper CD4+ central memory T lymphocytes. The treatment group contained 5 more vaccinated patients and 7 more overweight patients. Authors also present in vitro results showing an inhibitory effect with five SARS-CoV-2 variants including omicron.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 69.3% lower, RR 0.31, p = 0.44, treatment 0 of 29 (0.0%), control 1 of 23 (4.3%), NNT 23, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
time to sustained clinical response, 9.1% lower, HR 0.91, p = 0.78, treatment 28, control 23, inverted to make HR<1 favor treatment, Kaplan-Meier.
|
|
time to sustained clinical response, 35.5% lower, HR 0.65, p = 0.25, treatment 28, control 23, inverted to make HR<1 favor treatment, Kaplan-Meier, high-risk patients.
|
|
risk of no viral clearance, 43.5% lower, RR 0.57, p = 0.31, treatment 5 of 21 (23.8%), control 8 of 19 (42.1%), NNT 5.5, day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bencheqroun et al., 7 May 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, mean age 45.0, 25 authors, study period 27 May, 2021 - 27 September, 2021, trial NCT04914377 (history) (BOSS-001).
Contact: h.bencheqroun@respireresearch.com (corresponding author), r.fortunet@respireresearch.com, yasirahmed.med@gmail.com, cbarrera@ummc.care, mariya@ummc.care, mehmet.kocaktas@gmail.com, evilla@moraleshealthcare.com, emmanueli@tranquilconsulting.com, deborahb@tranquilconsulting.com, chineduo@tranquilconsulting.com, olaa@tranquilconsulting.com, karim@tranquilconsulting.com, spondell@ibtsolutions.us, amr.mohamed@uhhospitals.org, yimohamed@mdanderson.org, bgok@mdanderson.org, mkaseb1@gmail.com, okasseb@hotmail.com, mgocio@novatekpharmaceuticals.com, pt22064@comcast.net, danli@mdanderson.org, qma@mdanderson.org, jimmy_lu@codexbio.com, abdu94@yahoo.com, akaseb@mdanderson.org.
A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of ThymoQuinone Formula (TQF) for Treating Outpatient SARS-CoV-2
Pathogens, doi:10.3390/pathogens11050551
There is an urgent need for an oral drug for the treatment of mild to moderate outpatient SARS-CoV-2. Our preclinical and clinical study's aim was to determine the safety and preliminary efficacy of oral TQ Formula (TQF), in the treatment of outpatient SARS-CoV-2. In a double-blind, placebo-controlled phase 2 trial, we randomly assigned (1:1 ratio) non-hospitalized, adult (>18 years), symptomatic SARS-CoV-2 patients to receive oral TQF or placebo. The primary endpoints were safety and the median time-to-sustained-clinical-response (SCR). SCR was 6 days in the TQF arm vs. 8 days in the placebo arm (p = 0.77), and 5 days in the TQF arm vs. 7.5 days in the placebo arm in the high-risk cohort, HR 1.55 (95% CI: 0.70, 3.43, p = 0.25). No significant difference was found in the rate of AEs (p = 0.16). TQF led to a significantly faster decline in the total symptom burden (TSB) (p < 0.001), and a significant increase in cytotoxic CD8 + (p = 0.042) and helper CD4 + (p = 0.042) central memory T lymphocytes. TQF exhibited an in vitro inhibitory effect on the entry of five SARS-CoV-2 variants. TQF was well-tolerated. While the median time-to-SCR did not reach statistical significance; it was shorter in the TQF arm and preclinical/clinical signals of TQF activity across multiple endpoints were significant. Therefore, a confirmatory study is planned.
method and compared between the two arms of the study by the log-rank test. Median time-to-SCR was estimated through the Kaplan-Meier product-limit method. In comparing viral load (VL) distribution between the two arms of the study crosssectionally, the Wilcoxon-Mann-Whitney test, a non-parametric alternative to the twosample t-test, was used, where any measurement provided as '>25,000' was conservatively considered as 25,000 and the VL measurements of SARS-CoV-2-negative samples were considered as zero. For longitudinal modeling of VL, a random coefficient modeling approach through the SAS MIXED procedure was used with individual specific intercepts and slope estimates. The same approach was used for longitudinal individual symptom burden assessments, where both linear and quadratic changes over time were added to the model. We added symptom scores to obtain a measure of total symptom burden from days 1-14; similarly, subdomain scores were obtained as unweighted additions of relevant symptom scores. In all these longitudinal models, the primary interest was to investigate whether there was a significant change in these markers over time and whether such change depended on the treatment arm. For the T lymphocytes subsets, compared on day 1, day 7, and day 14, the abovementioned non-parametric testing approach was also used. As the flow-cytometry analysis was exploratory in nature, no multiplicity correction was carried out. Consequently, the widths of the intervals have..
References
Akhtar, Riffat, Field trial of Saussurea lappa roots against nematodes and Nigella sativa seeds against cestodes in children, J. Pak. Med. Assoc
Barakat, El Wakeel, Hagag, Effects of Nigella sativa on outcome of hepatitis C in Egypt, World J. Gastroenterol, doi:10.3748/wjg.v19.i16.2529
Bergamaschi, Mescia, Turner, Hanson, Kotagiri et al., Longitudinal analysis reveals that delayed bystander CD8 + T cell activation and early immune pathology distinguish severe COVID-19 from mild disease, Immunity, doi:10.1016/j.immuni.2021.05.010
Bi, Wu, Mei, Ye, Zou et al., Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30287-5
Ciaglia, Vecchione, Puca, COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children, Front. Pediatr, doi:10.3389/fped.2020.00206
Fard, Zahrani, Bagheban, Mojab, Therapeutic Effects of Nigella Sativa Linn (Black Cumin) on Candida albicans Vaginitis, Arch. Clin. Infect. Dis, doi:10.5812/archcid.22991
Java, Apicelli, Liszewski, Coler-Reilly, Atkinson et al., The complement system in COVID-19: Friend and foe?, JCI Insight, doi:10.1172/jci.insight.140711
Kaseb, Mohamed, Malek, Raad, Altameemi et al., The Impact of Angiotensin-Converting Enzyme 2 (ACE2) Expression on the Incidence and Severity of COVID-19 Infection, Pathogens, doi:10.3390/pathogens10030379
Koshak, Koshak, Mobeireek, Badawi, Wali et al., Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial, Complement. Ther. Med, doi:10.1016/j.ctim.2021.102769
Kreutmair, Unger, Núñez, Ingelfinger, Alberti et al., Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia, Immunity, doi:10.1016/j.immuni.2021.05.002
Lechien, Chiesa-Estomba, Place, Van Laethem, Cabaraux et al., Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019, J. Intern. Med, doi:10.1111/joim.13089
Li, Li, Zhang, Wang, Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues, Infect. Dis. Poverty, doi:10.1186/s40249-020-00662-x
Lowery, Sariol, Perlman, Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19, Cell Host Microbe, doi:10.1016/j.chom.2021.05.004
Merad, Martin, Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages, Nat. Rev. Immunol, doi:10.1038/s41577-020-0331-4
Onifade, Jewell, Adedeji, Nigella sativa concoction induced sustained seroreversion in HIV patient, Afr. J. Tradit. Complement. Altern. Med, doi:10.4314/ajtcam.v10i5.18
Onifade, Jewell, Okesin, Seronegative conversion of an hiv positive subject treated with Nigella sativa and honey, Afr. J. Infect. Dis, doi:10.4314/ajid.v9i2.6
Rahman, Potential benefits of combination of Nigella sativa and Zn supplements to treat COVID-19, J. Herb. Med, doi:10.1016/j.hermed.2020.100382
Richard, Epsi, Pollett, Lindholm, Malloy et al., Performance of the inFLUenza Patient-Reported Outcome Plus (FLU-PRO Plus©) instrument in patients with COVID-19, Open Forum Infect. Dis, doi:10.1093/ofid/ofab517
Salem, Yar, Bamosa, Al-Quorain, Yasawy et al., Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia, Saudi J. Gastroenterol, doi:10.4103/1319-3767.65201
Seyedalinaghi, Abbasian, Solduzian, Yazdi, Jafari et al., Predictors of the prolonged recovery period in COVID-19 patients: A cross-sectional study, Eur. J. Med. Res, doi:10.1186/s40001-021-00513-x
Tenforde, Kim, Lindsell, Rose, Shapiro et al., Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network-United States, MMWR Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm6930e1
Tolossa, Wakuma, Gebre, Atomssa, Getachew et al., Time to recovery from COVID-19 and its predictors among patients admitted to treatment center of Wollega University Referral Hospital (WURH), Western Ethiopia: Survival analysis of retrospective cohort study, PLoS ONE, doi:10.1371/journal.pone.0252389
Wu, Li, Shi, Chen, Jiang et al., Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19), J. Intern. Med, doi:10.1111/joim.13063
Yu, Yan, Wang, Yang, Wang et al., Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients, Clin. Infect. Dis, doi:10.1093/cid/ciaa345
DOI record:
{
"DOI": "10.3390/pathogens11050551",
"ISSN": [
"2076-0817"
],
"URL": "http://dx.doi.org/10.3390/pathogens11050551",
"abstract": "<jats:p>There is an urgent need for an oral drug for the treatment of mild to moderate outpatient SARS-CoV-2. Our preclinical and clinical study’s aim was to determine the safety and preliminary efficacy of oral TQ Formula (TQF), in the treatment of outpatient SARS-CoV-2. In a double-blind, placebo-controlled phase 2 trial, we randomly assigned (1:1 ratio) non-hospitalized, adult (>18 years), symptomatic SARS-CoV-2 patients to receive oral TQF or placebo. The primary endpoints were safety and the median time-to-sustained-clinical-response (SCR). SCR was 6 days in the TQF arm vs. 8 days in the placebo arm (p = 0.77), and 5 days in the TQF arm vs. 7.5 days in the placebo arm in the high-risk cohort, HR 1.55 (95% CI: 0.70, 3.43, p = 0.25). No significant difference was found in the rate of AEs (p = 0.16). TQF led to a significantly faster decline in the total symptom burden (TSB) (p < 0.001), and a significant increase in cytotoxic CD8+ (p = 0.042) and helper CD4+ (p = 0.042) central memory T lymphocytes. TQF exhibited an in vitro inhibitory effect on the entry of five SARS-CoV-2 variants. TQF was well-tolerated. While the median time-to-SCR did not reach statistical significance; it was shorter in the TQF arm and preclinical/clinical signals of TQF activity across multiple endpoints were significant. Therefore, a confirmatory study is planned.</jats:p>",
"alternative-id": [
"pathogens11050551"
],
"author": [
{
"affiliation": [],
"family": "Bencheqroun",
"given": "Hassan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Yasir",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3386-1734",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kocak",
"given": "Mehmet",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6200-0052",
"affiliation": [],
"authenticated-orcid": false,
"family": "Villa",
"given": "Enrique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barrera",
"given": "Cesar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohiuddin",
"given": "Mariya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fortunet",
"given": "Raul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iyoha",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bates",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Okpalor",
"given": "Chinedu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agbosasa",
"given": "Ola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohammed",
"given": "Karim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pondell",
"given": "Steven",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6962-3535",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mohamed",
"given": "Amr",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohamed",
"given": "Yehia I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gok Yavuz",
"given": "Betul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaseb",
"given": "Mohamed O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kasseb",
"given": "Osama O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gocio",
"given": "Michelle York",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tu",
"given": "Peter Tsu-Man",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Jianming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Selim",
"given": "Abdelhafez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Qing",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9491-0587",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kaseb",
"given": "Ahmed O.",
"sequence": "additional"
}
],
"container-title": "Pathogens",
"container-title-short": "Pathogens",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
9
]
],
"date-time": "2022-05-09T01:31:20Z",
"timestamp": 1652059880000
},
"deposited": {
"date-parts": [
[
2022,
5,
9
]
],
"date-time": "2022-05-09T02:20:51Z",
"timestamp": 1652062851000
},
"funder": [
{
"award": [
"ahmed kaseb"
],
"name": "novatek pharmaceuticals"
}
],
"indexed": {
"date-parts": [
[
2022,
5,
9
]
],
"date-time": "2022-05-09T02:40:49Z",
"timestamp": 1652064049521
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2022,
5,
7
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2022,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
7
]
],
"date-time": "2022-05-07T00:00:00Z",
"timestamp": 1651881600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-0817/11/5/551/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "551",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
5,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
5,
7
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.4103/1319-3767.65201",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"article-title": "Field trial of Saussurea lappa roots against nematodes and Nigella sativa seeds against cestodes in children",
"author": "Akhtar",
"first-page": "185",
"journal-title": "J. Pak. Med. Assoc.",
"key": "ref2",
"volume": "41",
"year": "1991"
},
{
"DOI": "10.5812/archcid.22991",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.3748/wjg.v19.i16.2529",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.4314/ajtcam.v10i5.18",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.4314/ajid.v9i2.6",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1016/j.hermed.2020.100382",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1016/j.ctim.2021.102769",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.15585/mmwr.mm6930e1",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1093/ofid/ofab517",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1172/jci.insight.140711",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1016/j.chom.2021.05.004",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.immuni.2021.05.010",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1016/j.immuni.2021.05.002",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1038/s41577-020-0331-4",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.3390/pathogens10030379",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.3389/fped.2020.00206",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1186/s40249-020-00662-x",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1111/joim.13089",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1111/joim.13063",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1093/cid/ciaa345",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/S1473-3099(20)30287-5",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1371/journal.pone.0252389",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1186/s40001-021-00513-x",
"doi-asserted-by": "publisher",
"key": "ref24"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-0817/11/5/551"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Immunology and Microbiology",
"Molecular Biology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of ThymoQuinone Formula (TQF) for Treating Outpatient SARS-CoV-2",
"type": "journal-article",
"volume": "11"
}