Effectiveness of Azvudine and Nirmatrelvir-ritonavir in Kidney Transplant Recipients With COVID-19: A Retrospective Cohort Study
et al., Infections in the immunosuppressed and immunocompromised host, doi:10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A2917, Apr 2024
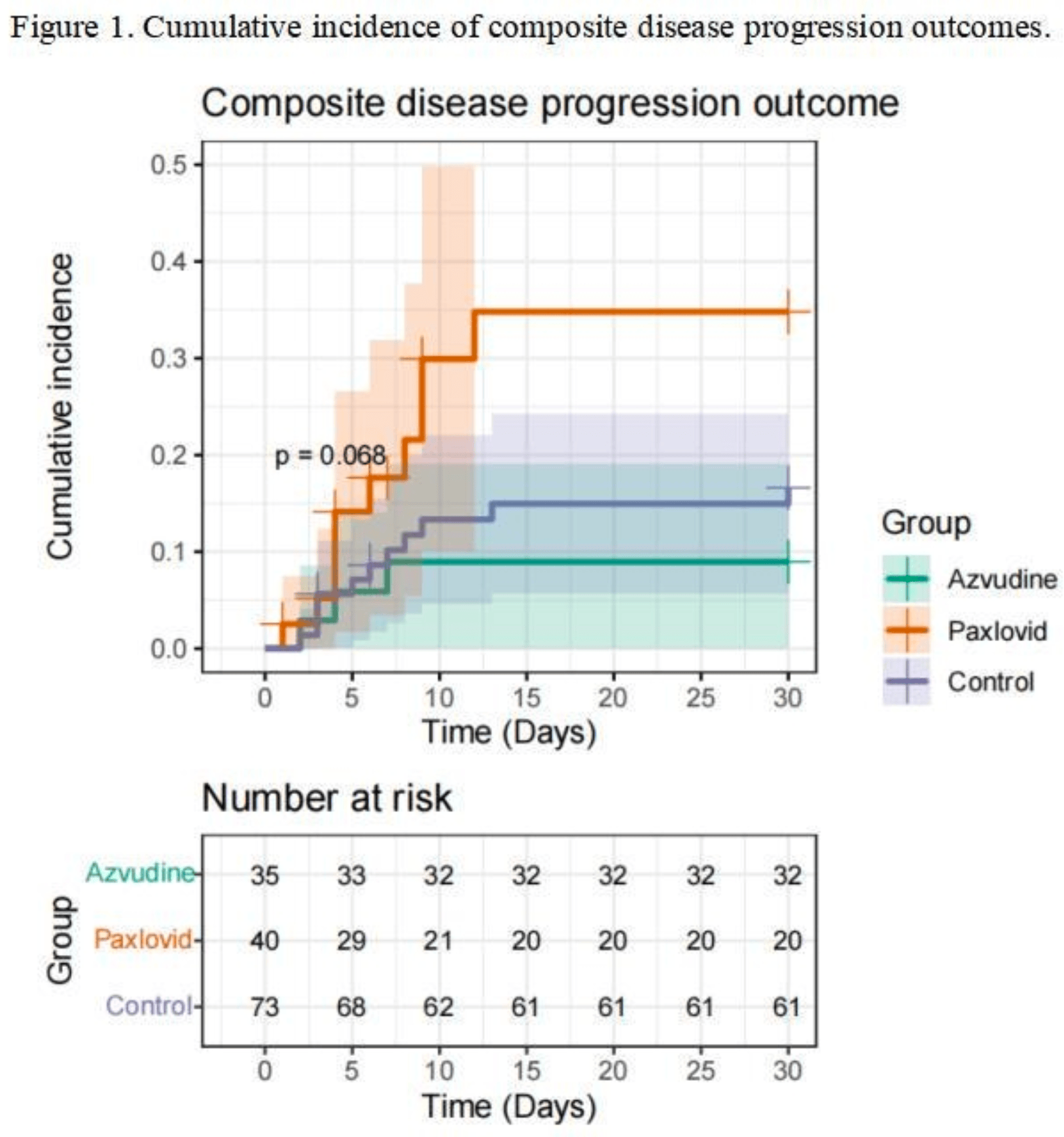
Retrospective 148 hospitalized kidney transplant patients with COVID-19 in China showing lower risk of disease progression with azvudine treatment compared, and higher risk with paxlovid treatment.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
Study covers azvudine and paxlovid.
|
risk of progression, 272.0% higher, HR 3.72, p = 0.046, propensity score matching, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Liu et al., 30 Apr 2024, retrospective, China, peer-reviewed, 3 authors, study period 1 December, 2022 - 19 January, 2023.
Contact: liuyi200402@163.com.
Effectiveness of Azvudine and Nirmatrelvir-ritonavir in Kidney Transplant Recipients With COVID-19: A Retrospective Cohort Study
Rationale: The effectiveness of azvudine and nirmatrelvir-ritonavir in treating COVID-19 patients has been demonstrated. However, evidence regarding their real-world effectiveness in kidney transplant recipients remains limited.Methods: We conducted a single-centre retrospective cohort study, focusing on hospitalised kidney transplant patients with COVID-19 between December 1st, 2022 and anuary 19th, 2023 during the omicron BA.5.2 variant-dominant period. Patients were eligible for inclusion if they tested positive for SARS-CoV-2 within 3 days before or after hospital admission and were diagnosed with both COVID-19 and having undergone kidney transplant. Exclusion criteria encompassed the administration of other antiviral treatments within 10 days of a positive SARS-CoV-2 test, breastfeeding, pregnancy, or known allergies to nirmatrelvir-ritonavir or azvudine. The primary outcome was a composite outcome of all-cause mortality, disease progression to critical COVID-19, Intensive care unit admission or upgrade of respiratory support, as well as their separate events. Hazard ratios (HRs) was estimated using Cox regression models for each event outcome between the groups.Results: We identified 1761 hospitalised patients with COVID-19 during the study period. After exclusions and propensity score matching, a total of 148 kidney transplant patients were included, with 40 receiving azvudine, 73 receiving nirmatrelvirritonavir, and 35 not receiving antiviral treatment. Azvudine was associated with a lower risk of composite disease progression outcome than those with no antiviral treatment (HR, 1.82; 95% CI, 0.51 to 6.53), whereas risk of composite disease progression outcome was similar in nirmatrelvirritonavir groups (HR, 3.72; 95% CI, 1.02 to 13.55). There was no evidence indicating a reduction in all-cause death, disease progression or incidence of the Intensive care unit admission. Conclusions: During the Omicron BA.5.2 variant wave, azvudine improved the prognosis of hospitalised kidney transplant recipients with COVID-19. However, the findings are constrained by the small sample size and a short follow-up period.
DOI record:
{
"DOI": "10.1164/ajrccm-conference.2024.209.1_meetingabstracts.a2917",
"URL": "http://dx.doi.org/10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A2917",
"alternative-id": [
"10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A2917",
"10.1164/ajrccm-conference.2024.A107"
],
"author": [
{
"affiliation": [
{
"name": "Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Liu",
"given": "Y.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Zhang",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Liu",
"given": "D.",
"sequence": "additional"
}
],
"container-title": "A107. INFECTIONS IN THE IMMUNOSUPPRESSED AND IMMUNOCOMPROMISED HOST",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
4,
30
]
],
"date-time": "2024-04-30T20:10:21Z",
"timestamp": 1714507821000
},
"deposited": {
"date-parts": [
[
2024,
4,
30
]
],
"date-time": "2024-04-30T20:10:28Z",
"timestamp": 1714507828000
},
"event": "American Thoracic Society 2024 International Conference, May 17-22, 2024 - San Diego, CA",
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:34:05Z",
"timestamp": 1714523645357
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
4,
30
]
]
},
"member": "19",
"original-title": [],
"prefix": "10.1164",
"published": {
"date-parts": [
[
2024,
4,
30
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
30
]
]
},
"published-print": {
"date-parts": [
[
2024,
5
]
]
},
"publisher": "American Thoracic Society",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.atsjournals.org/doi/10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A2917"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effectiveness of Azvudine and Nirmatrelvir-ritonavir in Kidney Transplant Recipients With COVID-19: A Retrospective Cohort Study",
"type": "proceedings-article"
}
liu23